Abstract
The aim of this study was to evaluate the efficacy and safety of co-administration of oral S-1 and oxaliplatin (SOX) in combination with bevacizumab (bev) in patients with advanced recurrent colorectal cancer. A retrospective study of 36 patients with advanced recurrent colorectal cancer was performed, of whom 27 received first-line and 9 received second-line SOX+bev chemotherapy between 2010 and 2013 at the Hachioji Digestive Disease Hospital (Hachioji, Japan). The SOX+bev regimen consisted of administration of intravenous oxaliplatin (85 mg/m2) on days 1 and 14, bevacizumab (5 mg/kg) on day 1, and co-administration of oral S-1 twice daily on days 1–14. The drug regimen was repeated every 4 weeks. SOX+bev treatment was associated with a response rate of 45.2%, a disease control rate of 71%, and a median progression-free survival (PFS) and overall survival (OS) of 9.9 and 21.9 months, respectively. Patients who received first-line chemotherapy benefited from treatment in terms of prolonged PFS (13.8 months) and OS (28.2 months). Grade 3/4 adverse events were infrequent and included anaemia, thrombocytopenia, anorexia, diarrhea, sensory neuropathy, increased aspartate aminotransferase level and skin rash. In conclusion, SOX+bev therapy was found to be feasible and safe for patients with advanced and recurrent colorectal cancer.
Keywords: oxaliplatin, bevacizumab, S-1, SOX+bev, colorectal cancer
Introduction
The combination of leucovorin, 5-fluorouracil (5-FU), and oxaliplatin (FOLFOX) and bevacizumab (bev) has been extensively used as first-line treatment for metastatic colorectal cancer (1,2). However, infusion or injection of 5-FU with leucovorin has certain disadvantages, including increased cost, inconvenience and morbidity associated with the use of a portable infusion pump and central venous catheter (3). The use of oral anticancer drugs has helped to overcome problems associated with continuous infusion, infection and thrombosis. S-1 is an oral anticancer drug that combines the prodrug tegafur with two modulators of 5-FU metabolism, gimeracil and oteracil (4). Several phase III clinical trials on metastatic colorectal cancer have demonstrated that oral S-1 may substitute infusional 5-FU (5,6). Furthermore, a phase III trial of first-line treatments in patients with metastatic colorectal cancer (SOFT trial) demonstrated that the combination of S-1 and oxaliplatin with bevacizumab (SOX+bev) was as efficacious as FOLFOX6+bev with respect to progression-free survival (PFS) (7). Therefore, the aim of the present study was to evaluate the efficacy and safety of SOX+bev for patients with advanced recurrent colorectal cancer.
Patients and methods
Patients
A total of 36 patients with advanced recurrent colorectal cancer who were administered SOX+bev chemotherapy between January, 2010 and December, 2013 at the Department of Surgery, Hachioji Digestive Disease Hospital (Tokyo, Japan) were included in this retrospective study. All patients provided written informed consent prior to treatment initiation. All the patients had sufficient oral drug intake. The patient inclusion criteria were as follows: i) Colorectal cancer diagnosed by pathological examination or radiography; ii) unresectable, locally advanced disease, or metastatic/recurrent disease; iii) Eastern Cooperative Oncology Group performance status of 0–2; iv) at least one measurable lesion according to the Response Evaluation Criteria in Solid Tumours [RECIST (8)]; v) no limit of age and life expectancy; AND vi) adequate organ function, as indicated by white blood cell count ≥3,000/µl, haemoglobin levels of 8 g/dl, platelet count ≥100,000/mm3, total bilirubin levels ≤2.0 mg/dl, aspartate aminotransferase (AST) and alanine aminotransferase levels not exceeding three times the upper limit of normal, creatinine ≤1.5 mg/dl, blood urea nitrogen ≤25 mg/dl and creatinine clearance ≥50 ml/min. The exclusion criteria were as follows: i) Severe complications, including ACTIVE infection, cardiac or renal disease, marked pleural effusion or ascites; ii) severe drug hypersensitivity; iii) an active concomitant malignancy; and iv) pregnanCY or lactation.
Treatment
The SOX+bev regimen consisted of administration of intravenous oxaliplatin (85 mg/m2) on days 1 and 14, bevacizumab (5 mg/kg) on day 1 and co-administration of oral S-1 twice daily on days 1–14. The total daily S-1 dosage was calculated according to the body surface area (BSA): Patients with a BSA of <1.25 m2 received 80 mg/day, those with a BSA 1.25–1.5 m2 received 100 mg/day and those with a BSA ≥1.5 m2 received 120 mg/day. The drug regimen was repeated every 4 weeks. The tumour response was measured by computed tomography in accordance with the RECIST every two courses, or more frequently in patients with suspected disease progression. Toxicities associated with the drug regimen were evaluated according to the Common Terminology Criteria for Adverse Events [CTCAE; version 3.0 (9)].
Survival Analysis
Overall survival (OS) was calculated from the day of treatment initiation to either the date of the last patient follow-up or death, and PFS was calculated from the day of treatment initiation to the identification of disease progression, death or the censored date. Median OS and PFS were estimated using Kaplan-Meier analysis and compared using the log-rank test.
Results
Patient characteristics
A total of 36 patients were enrolled in this study. Patient characteristics are summarized in Table I. The median patient age was 67.4 years (range, 44–84 years) and the male:female ratio was 23:13. The primary tumour was located in the colon in 26 patients (72.2%) and the rectum in 10 patients (27.8%). Within this cohort, SOX+bev was the first-line chemotherapy regimen administered to 27 patients (75%), while only 9 patients (25%) had received previous chemotherapy. Of these 9 patients, 6 had received a course of S-1+irinotecan (IRIS) prior to SOX+bev, while the remaining 3 patients received courses of IRIS and FOLFOX prior to initiation of SOX+bev therapy.
Table I.
Characteristics of patients (n=36).
| CharacteristicS | Patients, n (%) |
|---|---|
| Age (years) | |
| Median (range) | 67.4 (44–86) |
| Gender | |
| Male | 23 (63.9) |
| Female | 13 (36.1) |
| Primary location (%) | |
| Colon | 26 (72.2) |
| Rectum | 10 (27.8) |
| Previous chemotherapy | |
| No | 27 (75.0) |
| Yes | 9 (25.0) |
| Metastatic sites | |
| Liver | 8 |
| Peritoneum | 7 |
| Local recurrence | 4 |
| Lung | 4 |
| Lung/lymph nodes | 4 |
| Liver/lymph nodes | 3 |
| Lymph nodes | 2 |
| Other | 4 |
| Number of treatment courses, MEAN (range) | 5.7 (1–18) |
| Response rate (%) | 45.2 |
Efficacy
The median number of chemotherapy courses was 5.7 (range, 1–18). Although a response to chemotherapy could not be determined in 5 patients, 14/31 patients achieved a partial response to SOX+bev treatment, resulting in a response rate (RR) of 45.2%. Stable disease was observed in an additional 8 patients, resulting in a disease control rate (DCR) of 71%. The RR and DCR of patients who received SOX+bev as first-line chemotherapy were the same as those calculated for the complete cohort.
Survival
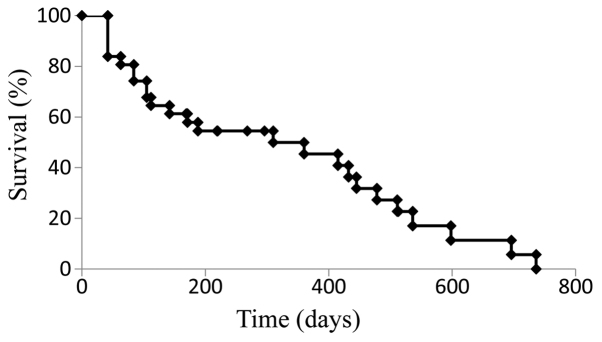
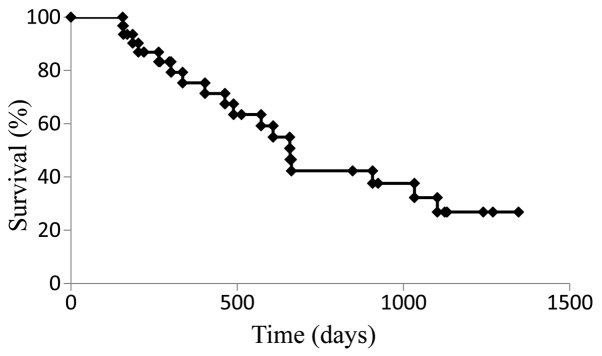
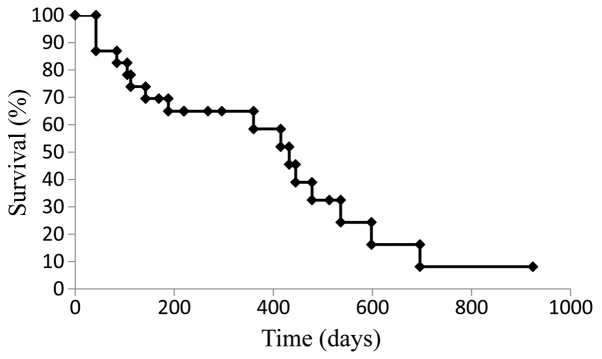
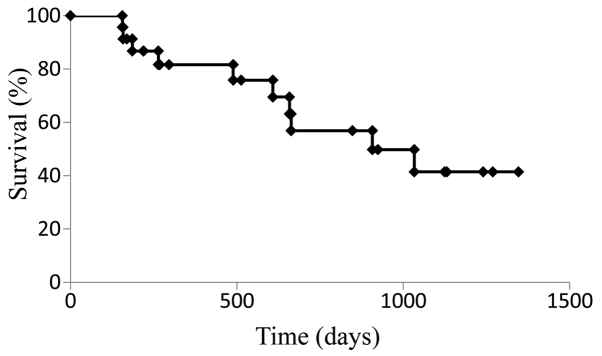
The median PFS for the cohort treated with SOX+bev was 9.9 months (Fig. 1), while the median OS was 21.9 months (Fig. 2). For patients who received SOX+bev exclusively as first-line treatment, the median PFS (13.8 months) (Fig. 3) and the median OS (28.2 months) (Fig. 4) were increased compared with those for the entire cohort.
Figure 1.
Kaplan-Meier curve for progression-free survival in all patients (n=36).
Figure 2.
Kaplan-Meier curve for overall survival in all patients (n=36).
Figure 3.
Kaplan-Meier curve for progression-free survival in patients who received first-line treatment with oral S-1, oxaliplatin and bevacizumab (n=27).
Figure 4.
Kaplan-Meier curve for overall survival in patients who received first-line treatment with oral S-1, oxaliplatin and bevacizumab (n=27).
Toxicity
Adverse events, which were potentially due to drug-related toxicity of SOX+bev, are summarized in Table II. Certain adverse events were frequently observed, including sensory neuropathy (n=22; 61.1%), thrombocytopenia (n=13; 36.1%), anorexia (n=9; 25%) and leukopenia (n=7; 19.4%). More severe toxicities (grade 3 and 4), including anaemia, thrombocytopenia, anorexia, diarrhea, sensory neuropathy, increased AST levels, rash and infusion reaction, were observed in 8 patients. No adverse events were observed in patients who had completed other chemotherapies prior to the SOX+bev regimen.
Table II.
Adverse events in the ORAL S-1, oxaliplatin and bevacizumab group.
| Grade (n) | |||||
|---|---|---|---|---|---|
| Adverse eventS | 1 | 2 | 3 | 4 | Patients with grade 3/4 toxicity, n (%) |
| Leukopenia | 3 | 4 | 0 | 0 | 0 (0.0) |
| Anemia | 1 | 2 | 1 | 0 | 1 (2.8) |
| Thrombocytopenia | 9 | 4 | 0 | 1 | 1 (2.8) |
| Anorexia | 8 | 2 | 1 | 0 | 1 (2.8) |
| Diarrhea | 3 | 3 | 1 | 0 | 1 (2.8) |
| Sensory neuropathy | 7 | 14 | 1 | 0 | 1 (2.8) |
| Nausea | 0 | 2 | 0 | 0 | 0 (0.0) |
| Alopecia | 1 | 0 | 0 | 0 | 0 (0.0) |
| Increased alanine aminotransferase | 0 | 3 | 0 | 0 | 0 (0.0) |
| Increased aspartate aminotransferase | 0 | 1 | 2 | 0 | 2 (5.5) |
| Stomatitis | 1 | 0 | 0 | 0 | 0 (0.0) |
| Rash | 1 | 0 | 1 | 0 | 1 (2.8) |
| Elevated blood pressure | 2 | 0 | 0 | 0 | 0 (0.0) |
Discussion
A combinatorial approach to chemotherapy using targeted agents and complementary drug combinations, such as oxaliplatin and 5-FU, is widely used for THE first-line treatment of patients with advanced and recurrent colorectal cancer (10,11). Combinations of folinic acid and 5-FU (either bolus or infusional) with either oxaliplatin or irinotecan (FOLFOX and FOLFIRI, respectively), are considered the gold standard regimens for the first-line treatment of advanced and recurrent colorectal cancer (12–14). In Japan, oral S-1 has been widely used for the treatment of gastrointestinal cancers. In phase II studies, S-1 alone elicited an RR of 19–40% with tolerable adverse events in patients with metastatic colorectal cancer (15–17). Furthermore, several trials using SOX regimens have reported RRs of 47–54% and DCRs of 81–90% (6,18,19). In thOse studies, the median PFS of patients receiving SOX regimens was 6.5–8.5 months, while the median OS was 21.2–27.2 months, highlighting the efficacy and feasibility of SOX as first-line chemotherapy for metastatic colorectal cancer (6,18,19). As mentioned above, the SOFT trial reported that the SOX+bev combination was as effective as MFOLFOX6+bev in terms of PFS in patients with metastatic colorectal cancer (7). To determine whether the continuous 5-FU infusion in the FOLFOX regimen can be replaced with oral S-1, the present clinical study was performed with the SOX+bev regimen administered TO patients with advanced and recurrent colorectal cancer. In the present study, the dose of intravenous oxaliplatin on days 1 and 14 and that of bevacizumab was based on the FOLFOX6 regimen PRIOR TO the SOFT trial. In the cohort of the present study, an RR of 45.2%, a DCR of 71%, a median PFS of 9.9 months and a median OS of 21.9 months were observed. The increased median PFS of 13.8 months and median OS of 28.2 months in patients undergoing first-line chemotherapy with SOX+bev suggests that this regimen may effective as a first-line chemotherapeutic regimen. The RR, DCR, median PFS and median OS observed in the present study were similar to those obtained with the combination of oxaliplatin and S-1. Furthermore, OUR SOX+bev first-line chemotherapy DATA were similar to those presented in the SOFT trial (7). However, it should be mentioned that the 27 patients who received SOX+bev as first-line chemotherapy subsequently received second-line chemotherapy with IRIS+bev, indicating that our data require further evaluation. In addition, third-line and subsequent treatments included the addition of experimental molecular-targetED agents (cetuximab+irinotecan), which may have contributed to the survival prolongation observed in our cohort.
The SOFT trial also reported a significantly lower incidence of grade ≥3 neutropenia and leukopenia with SOX+bev, compared with mFOLFOX6+bev treatment (7). Other studies also reported grade 3–4 leukopenia in 0–2% patients following administration of SOX (6,7,18). Thrombocytopenia is one of the main haematological abnormalities caused by oxaliplatin, with 13–28% of patients exhibiting grade ≥3 thrombocytopenia in previous SOX therapy studies (6,7,18). It has been suggested that oxaliplatin-induced thrombocytopenia may be associated with bone marrow suppression, as well as liver damage due to sinusoidal obstruction syndrome (20–22). In addition, it has been reported that 0–10% of patients treated with SOX experienced grade ≥3 diarrhea; however, grade 3–4 thrombocytopenia and diarrhea were only observed in 2.8% of our cohort at the SOX dose administered. Of note, the most frequently observed adverse event in trials using SOX was sensory neuropathy, which occurred at a frequency of 75–91%, with high-grade neuropathy occurring in 0–10% of the patients (6,7,18). In the present study, sensory neuropathy of any grade was also the most frequent adverse event observed, occurring at a frequency of 61.1%, while grade 3–4 sensory neuropathy occurred in 2.8% of the patients. The reduced frequency of sensory neuropathy in the present study compared with that in previous studies may be the result of the revised criteria for dose reduction.
In conclusion, the results of the present study suggested that SOX+bev combination chemotherapy is safe and feasible for patients with advanced recurrent colorectal cancer.
References
- 1.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 2.Tournigand C, Andrú T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe K, Kawahara H, Enomoto H, Toyama Y, Akiba T, Yanaga K. Feasibility study of oxaliplatin with oral S-1 or capecitabine as first-line therapy for patients with metastases from colorectal cancer. Anticancer Res. 2013;33:4029–4032. [PubMed] [Google Scholar]
- 4.Shirasaka T. Development history and concept of an oral anticancer agent S-1 (TS-1): Its clinical usefulness and future vistas. J Clin Oncol. 2009;39:2–15. doi: 10.1093/jjco/hyn127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muro K, Boku N, Shimada Y, Tsuji A, Sameshima S, Baba H, Satoh T, Denda T, Ina K, Nishina T, et al. Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: A randomised phase 2/3 non-inferiority study (FIRIS study) Lancet Oncol. 2010;11:853–860. doi: 10.1016/S1470-2045(10)70181-9. [DOI] [PubMed] [Google Scholar]
- 6.Hong YS, Park YS, Lim HY, Lee J, Kim TW, Kim KP, Kim SY, Baek JY, Kim JH, Lee KW, et al. S-1 plus oxaliplatin versus capecitabine plus oxaliplatin for first-line treatment of patients with metastatic colorectal cancer: A randomised, non-inferiority phase 3 trial. Lancet Oncol. 2012;13:1125–1132. doi: 10.1016/S1470-2045(12)70363-7. [DOI] [PubMed] [Google Scholar]
- 7.Yamada Y, Takahari D, Matsumoto H, Baba H, Nakamura M, Yoshida K, Yoshida M, Iwamoto S, Shimada K, Komatsu Y, et al. Leucovorin, fluorouracil and oxaliplatin plus bevacizumab versus S-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): An open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2013;14:1278–1286. doi: 10.1016/S1470-2045(13)70490-X. [DOI] [PubMed] [Google Scholar]
- 8.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Glabbeke MV, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Center Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 9.Shimoyama M. The Japanese edition of the National Cancer Institute-common toxicity criteria. Jpn J Cancer Chemother. 1999;26:1084–1144. (In Japanese) [Google Scholar]
- 10.Cassidy J, Tabernero J, Twelves C, Brunet R, Butts C, Conroy T, Debraud F, Figer A, Grossmann J, Sawada N, et al. XELOX (capecitabine plus oxaliplatin): Active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol. 2004;22:2084–2091. doi: 10.1200/JCO.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 11.deGramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, CortesFunes H, Cervantes A, Freyer G, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 12.Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y, Coudert B, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136–147. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]
- 13.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/S0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 14.Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476–487. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 15.Ohtsu A, Baba H, Sakata Y, Mitachi Y, Horikoshi N, Sugimachi K, Taguchi T. Phase II study of S-1, a novel oral fluoropyrimidine derivative, in patients with metastatic colorectal carcinoma. S-1 Cooperative Colorectal Carcinoma Study Group. Br J Cancer. 2000;83:141–145. doi: 10.1054/bjoc.2000.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van den Brande J, Schöffski P, Schellens JH, Roth AD, Duffaud F, Weigang-Köhler K, Reinke F, Wanders J, de Boer RF, Vermorken JB, et al. EORTC Early Clinical Studies Group early phase II trial of S-1 in patients with advanced or metastatic colorectal cancer. Br J Cancer. 2003;88:648–653. doi: 10.1038/sj.bjc.6600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shirao K, Ohtsu A, Takada H, Mitachi Y, Hirakawa K, Horikoshi N, Okamura T, Hirata K, Saitoh S, Isomoto H, et al. Phase II study of oral S-1 for treatment of metastatic colorectal carcinoma. Cancer. 2004;100:2355–2361. doi: 10.1002/cncr.20277. [DOI] [PubMed] [Google Scholar]
- 18.Zang DY, Lee BH, Park HC, Song HH, Kim HJ, Jung JY, Kim JH, Kim HY, Kwon JH, Hwang SW, et al. Phase II study with oxaliplatin and S-1 for patients with metastatic colorectal cancer. Ann Oncol. 2009;20:892–896. doi: 10.1093/annonc/mdn721. [DOI] [PubMed] [Google Scholar]
- 19.Yamada Y, Tahara M, Miya T, Satoh T, Shirao K, Shimada Y, Ohtsu A, Sasaki Y, Tanigawara Y. Phase I/II study of oxaliplatin with oral S-1 as FIRST-line therapy for patients with metastatic colorectal cancer. Br J Cancer. 2008;98:1034–1038. doi: 10.1038/sj.bjc.6604271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Overman MJ, Maru DM, Charnsangavej C, Loyer EM, Wang H, Pathak P, Eng C, Hoff PM, Vauthey JN, Wolff RA, et al. Oxaliplatin-mediated increase in spleen size as a biomarker for the development of hepatic sinusoidal injury. J Clin Oncol. 2010;28:2549–2555. doi: 10.1200/JCO.2009.27.5701. [DOI] [PubMed] [Google Scholar]
- 21.RubbiaBrandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane O, Chaussade S, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 22.Tournigand C, Cervantes A, Figer A, Lledo G, Flesch M, Buyse M, Mineur L, Carola E, Etienne PL, Rivera F, et al. OPTIMOX1: A randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal canceR - A GERCOR study. J Clin Oncol. 2006;24:394–400. doi: 10.1200/JCO.2005.03.0106. [DOI] [PubMed] [Google Scholar]