Abstract
Epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI)-induced interstitial lung disease (ILD) may be a life-threatening condition that may develop during treatment of lung cancer patients harboring EGFR mutations. We herein present the case of a 41-year-old female patient diagnosed with lung adenocarcinoma with an EGFR mutation (exon 19 deletion). The patient was treated with gefitinib followed by erlotinib and developed ILD induced by both EGFR-TKIs; furthermore, the patient acquired resistance to EGFR-TKI treatment. A repeat biopsy revealed a T790M mutation, which is associated with resistance to first-generation EGFR-TKIs, along with an exon 19 deletion identified by cytology of the pleural fluid. Treatment with afatinib and prednisolone resulted in tumor shrinkage, without worsening of the ILD. The present case demonstrated that combination treatment with afatinib and a glucocorticoid may be effective for the treatment of lung cancer patients who develop EGFR-TKI-induced ILD.
Keywords: gefitinib, erlotinib, interstitial lung disease, afatinib, non-small-cell lung cancer
Introduction
Detection of epidermal growth factor receptor (EGFR) mutations in lung cancer patients is vital in order to predict the therapeutic response to EGFR-tyrosine kinase inhibitors (TKIs) (1). While patients harboring EGFR mutations conferring sensitivity to EGFR-TKIs often exhibit a rapid and marked response to these drugs, resistance frequently develops after ~1 year. Mutations conferring resistance to EGFR-TKIs, such as EGFR-T790M, represent the major mechanism underlying the development of resistance to first-generation EGFR-TKIs. Approximately half of EGFR-TKI-resistant cases are due to the EGFR-T790M mutation. Recent advances in drug research have led to the development of several novel EGFR-TKIs, including the pan-HER inhibitor afatinib and the EGFR mutation-specific inhibitor osimertinib (2). However, even with the emergence of these novel drugs, tumor heterogeneity remains an important issue when treating EGFR-TKI-resistant patients. Although the tumor characteristics are similar between the primary site and metastatic lesions (3), tumor heterogeneity has been reported even within the original tumor, or between primary and metastatic sites. Therefore, the same patient may develop EGFR-TKI resistance through different mechanisms at different sites (4).
EGFR-TKI-induced interstitial lung disease (ILD) represents a major issue with this type of treatment, and its incidence among Japanese patients is higher compared with that in other ethnicities (5). There is currently no established treatment for lung cancer patients with EGFR-TKI-induced ILD and EGFR-TKI resistance.
The present study reports a case of a lung cancer patient with an EGFR mutation conferring sensitivity to EGFR-TKIs, who was treated with gefitinib followed by erlotinib, resulting in EGFR-TKI-induced ILD. Treatment with afatinib and a glucocorticoid effectively controlled the primary tumor, as well as the EGFR-TKI-induced ILD. Therefore, afatinib in combination with a glucocorticoid may be an effective treatment option for patients with EGFR-TKI-induced ILD.
Case report
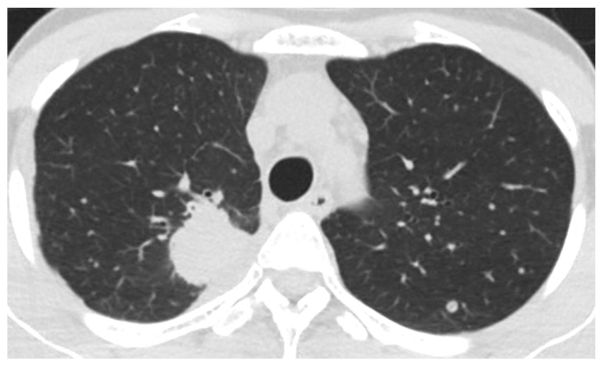
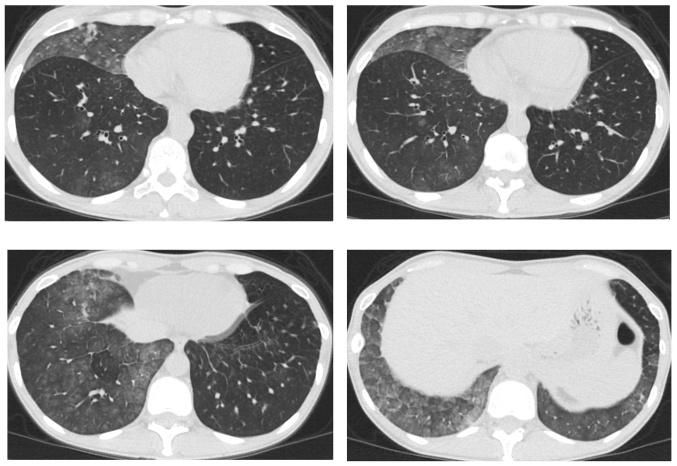
A 41-year-old female patient visited Keio University Hospital due to a chronic cough. A chest radiograph and computed tomography (CT) scan revealed a mass in the right lung and multiple lung metastatic lesions (Fig. 1). The patient was diagnosed with lung adenocarcinoma by transbronchial lung biopsy, and an activating EGFR mutation (exon 19 deletion) was detected. A 5-month treatment course with gefitinib achieved a partial response. However, a CT scan revealed bilateral ground-glass opacities (GGO) (Fig. 2), which improved following cessation of gefitinib treatment (Fig. 3A). The patient was diagnosed with gefitinib-induced ILD. One month after cessation of gefitinib, lung cancer progression was detected. The patient was then treated with carboplatin and pemetrexed for two cycles, but the treatment was not successful. Due to the increased risk of ILD, the patient was subsequently switched to erlotinib (25 mg/day) with informed consent. The size of the multiple lung metastases was reduced with erlotinib. However, a CT scan revealed new GGO regions, suggesting erlotinib-induced ILD (Fig. 3B); furthermore, a left pleural effusion was positive for EGFR exon 19 deletion and T790M. Following cessation of erlotinib, chest drainage and pleurodesis with talc were performed. Within 2 weeks, the right lung tumor and lymphangitis had markedly progressed (Fig. 4A). The patient opted to receive a molecular-targeted agent. Due to the previous positive response to erlotinib, apart from the worsening of the pleural effusion, the patient was treated with afatinib (20 mg/day) and prednisolone (30 mg/day) with informed consent. During this treatment, the primary tumor decreased in size and the carcinomatous lymphangitis improved (Fig. 4B). The patient has been continuously receiving afatinib and prednisolone for >3 months. The last follow-up took place 3 months later. Informed consent was obtained from the patient for publication of the present case report.
Figure 1.
Chest computed tomography scan prior to gefitinib treatment revealed a mass in the right lung and multiple lung metastases.
Figure 2.
Computerized tomography scan at the central area of the chest of the patient who developed interstitial lung disease, following 5 months of treatment with gefitinib. Bilateral ground-glass opacities were observed.
Figure 3.
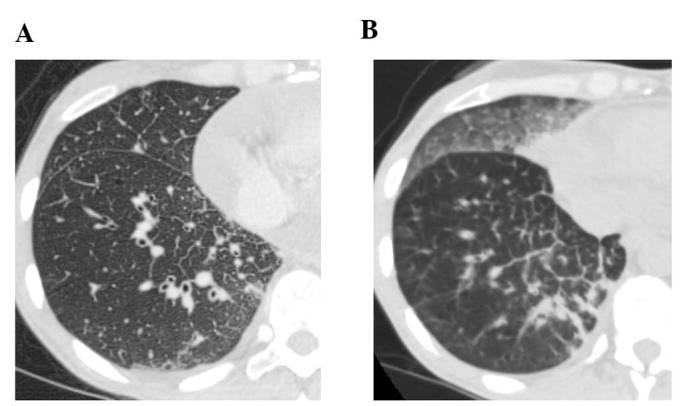
Chest computed tomography scan (A) 1 month after discontinuation of gefitinib, showing improvement of ground-glass opacities (GGO), and (B) 1 month after initiation of erlotinib, showing new GGO and carcinomatous lymphangitis.
Figure 4.
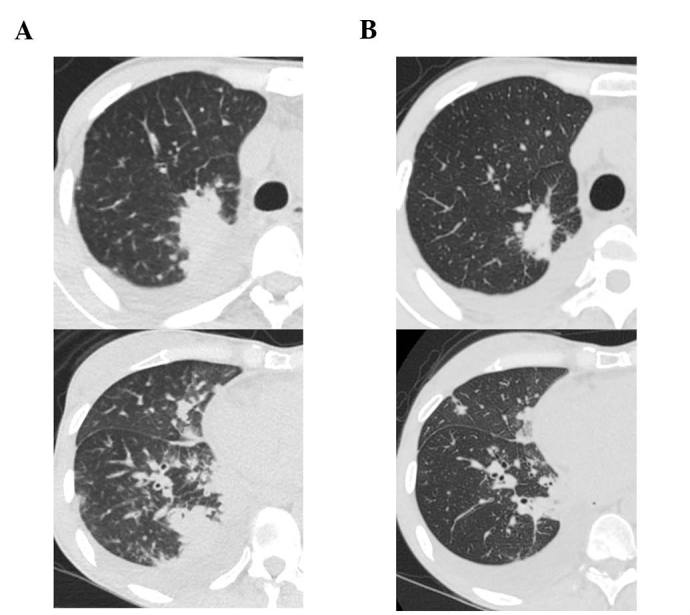
Chest computed tomography scan (A) 1 month after discontinuation of erlotinib and prior to the initiation of afatinib and prednisolone and (B) after combination treatment with afatinib and predonisolone. The treatment decreased the size of the primary tumor (upper panel) and improved carcinomatous lymphangitis (lower panel).
Discussion
The present case demonstrated that combined treatment with afatinib and a glucocorticoid was effective in the management of a lung cancer patient who developed gefitinib-and erlotinib-induced ILD. The frequency of EGFR-TKI-induced ILD was reported to be ~2.8–3.5% in the Japanese population (5–7). In a study by Endo et al (8), the average mortality rate of EGFR-TKI-induced ILD was reported to be 44.3%, whereas that of ILD with a diffuse alveolar damage (DAD) pattern was 75%. Due to the risk of life-threatening adverse events, EGFR-TKI rechallenge should not be considered for such patients, particularly when they present with the DAD pattern of ILD. Rechallenge with erlotinib in combination with a glucocorticoid was reported to be successful in a patient with EGFR-TKI-induced ‘non-DAD pattern’ ILD (9). In the present case, ILD induced by gefitinib and erlotinib exhibited a non-DAD pattern without respiratory symptoms. Treatment with afatinib and prednisolone induced a partial response of the tumor, without worsening of the ILD. To the best of our knowledge, this is the first reported case of a patient treated with afatinib for non-small-cell lung cancer after developing EGFR-TKI-induced ILD.
The present case provides an example regarding treatment selection for EGFR-TKI-resistant lung cancer from the perspective of tumor heterogeneity. The second-generation EGFR-TKI afatinib was effective in treating the primary tumor and the carcinomatous lymphangitis, notwithstanding the T790M mutation detected from cytological examination of the pleural effusion. The pleural effusion was manageable following pleurodesis, but the amount of the fluid increased despite shrinkage of the primary right lung tumor. Tumor heterogeneity is a recently reported phenomenon (4). In the present case, we hypothesized that the majority of the cells in the primary right lung tumor did not harbor a T790M mutation due to the repeated response to EGFR-TKIs and the non-parallel response of the left pleural effusion, where the T790M mutation was detected.
In conclusion, treatment with afatinib and a glucocorticoid may be an effective treatment option for lung cancer patients following development of EGFR-TKI-induced ILD.
References
- 1.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 2.Hirano T, Yasuda H, Tani T, Hamamoto J, Oashi A, Ishioka K, Arai D, Nukaga S, Miyawaki M, Kawada I, et al. In vitro modeling to determine mutation specificity of EGFR tyrosine kinase inhibitors against clinically relevant EGFR mutants in non-small-cell lung cancer. Oncotarget. 2015;6:38789–38803. doi: 10.18632/oncotarget.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naoki K, Soejima K, Okamoto H, Hamamoto J, Hida N, Nakachi I, Yasuda H, Nakayama S, Yoda S, Satomi R, et al. The PCR-invader method (structure-specific 5′ nuclease-based method), a sensitive method for detecting EGFR gene mutations in lung cancer specimens; comparison with direct sequencing. Int J Clin Oncol. 2011;16:335–344. doi: 10.1007/s10147-011-0187-5. [DOI] [PubMed] [Google Scholar]
- 4.Hata A, Katakami N, Yoshioka H, Kaji R, Masago K, Fujita S, Imai Y, Nishiyama A, Ishida T, Nishimura Y. Spatiotemporal T790M heterogeneity in individual patients with EGFR-mutant non-small-cell lung cancer after acquired resistance to EGFR-TKI. J Thorac Oncol. 2015;10:1553–1559. doi: 10.1097/JTO.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 5.Kudoh S, Kato H, Nishiwaki Y, Fukuoka M, Nakata K, Ichinose Y, Tsuboi M, Yokota S, Nakagawa K, Suga M, et al. Interstitial lung disease in Japanese patients with lung cancer. A cohort and nested case-control study. Am J Respir Crit Care Med. 2008;177:1348–1357. doi: 10.1164/rccm.200710-1501OC. [DOI] [PubMed] [Google Scholar]
- 6.Shi L, Tang J, Tong L, Liu Z. Risk of interstitial lung disease with gefitinib and erlotinib in advanced non-small cell lung cancer: A systematic review and meta-analysis of clinical trials. Lung Cancer. 2014;83:231–239. doi: 10.1016/j.lungcan.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Ando M, Okamoto I, Yamamoto N, Takeda K, Tamura K, Seto T, Ariyoshi Y, Fukuoka M. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2006;24:2549–2556. doi: 10.1200/JCO.2005.04.9866. [DOI] [PubMed] [Google Scholar]
- 8.Endo M, Johkoh T, Kimura K, Yamamoto N. Imaging of gefitinib-related interstitial lung disease: Multi-institutional analysis by the West Japan Thoracic Oncology Group. Lung Cancer. 2006;52:135–140. doi: 10.1016/j.lungcan.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Arakawa N, Tsujita A, Saito N, Ishikawa S, Ohno S. Successful erlotinib rechallenge after both gefitinib- and erlotinib-induced interstitial lung diseases. Respirol Case Rep. 2013;1:17–19. doi: 10.1002/rcr2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]