Abstract
In a hollow-fiber model, we mimicked the drug exposures achieved in the lungs of humans treated with standard amikacin, clarithromycin, and cefoxitin combination therapy for Mycobacterium abscessus infection. At optimal dosing, a kill rate of −0.09 (95% confidence interval, −0.04 to 0.03) log10 CFU per ml/day was achieved over the first 14 days, after which there was regrowth due to acquired drug resistance. Thus, the standard regimen quickly failed. A new regimen is needed.
TEXT
Clinicians treat Mycobacterium abscessus pulmonary disease with a combination of amikacin, clarithromycin, and cefoxitin for 1 to 2 months, followed by an oral maintenance regimen, usually with a fluoroquinolone, based on the recommendations of experts (1). However, the use of this approach “cures” only 50% even with adjunctive surgery, most of whom relapse or die (2). We have developed a hollow-fiber model of M. abscessus (HFS-M. abscessus) and evaluated the effect of monotherapy of amikacin and moxifloxacin in the system. Each drug failed dramatically, and acquired drug resistance (ADR) developed (3, 4). Since these drugs are given as combination therapy in patients, they could still work in combination. Here, we evaluated the performance of the standard combination regimen of amikacin, cefoxitin, and clarithromycin in the HFS-M. abscessus to determine potential synergy, and even more importantly, whether the combination therapy could prevent ADR.
Antibiotics were purchased from the Baylor University Medical Center Pharmacy (Dallas, TX) and from Sigma-Aldrich (St. Louis, MO). Antibiotics were dissolved in water-methanol (clarithromycin), sterile filtered, and diluted to the desired concentrations in Middlebrook 7H9 broth (here broth; Remel, Lenexa, KS). Stock solutions of M. abscessus ATCC 19977 (ATCC, Manassas, VA) were grown to logarithmic-growth phase in the broth. MICs were measured using broth microdilution (5). The amikacin, cefoxitin, and clarithromycin MICs were 32, 16, and 8 mg/liter, respectively.
The peripheral compartments of six HFS-M. abscessus systems were inoculated with 20 ml of 6.0 log10 CFU/ml M. abscessus, as previously described (3). Three systems were immediately treated with a combination of amikacin, cefoxitin, and clarithromycin, while three replicate systems were nontreated controls. Amikacin, cefoxitin, and clarithromycin were administered via syringe pumps for 28 days, as in patients. We mimicked free-drug area under the concentration-time curve from 0 to 24 h (fAUC0–24), peak concentration (fCmax), and time to maximum concentration achieved in the lungs of humans treated with amikacin and clarithromycin administered once daily, at 1,000 mg, and cefoxitin administered at 2 g four times a day (6, 7). Different half-lives in each HFS-M. abscessus system were achieved, as described by us in the past (8). Antibiotic concentrations achieved in all the systems were validated by sampling from the central compartment of each system at seven time points over 24 h postdose, after which concentrations were assayed in a validated multiplexed assay using liquid chromatography-tandem mass spectrometry in our laboratory at the Baylor University Medical Center. We achieved an AUC0–24/MIC of 5.3 mg · h/liter for clarithromycin, a percentage of time above the MIC of 100% for cefoxitin, and an fCmax/MIC of 4.0 of amikacin, so that each drug was at optimal free-drug exposure (3). These exposures are actually better than those achieved in most patients with standard dosing, based on prior work (3, 6, 7, 9).
We sampled each system's peripheral compartment on days 0, 1, 2, 3, 5, 7, 10, 14, 21, and 28 of treatment, washed the cultures in saline to avoid antibiotic carryover, serially diluted the cultures, and cultured them on antibiotic-free Middlebrook 7H10 agar. The size of the resistant subpopulation was quantified by culturing on Middlebrook agar plates containing 3 times the MIC of each antibiotic. The day-to-day bacterial burdens with standard therapy and in the controls are shown in Fig. 1a. The combination therapy killed at the low rate of −0.09 (95% confidence interval [CI], −0.04 to 0.03) log10 CFU/ml/day, to achieve 1.22 log10 CFU/ml kill on day 14 compared to the day 0 bacterial burden. After day 14, regrowth was observed. Thus, the combination regimen failed quickly, despite optimal exposures. The proportions of amikacin- and cefoxitin-resistant subpopulations to total population remained at a low level in the untreated control throughout the experiment, as shown in Fig. 1b. However, in the systems exposed to combination therapy, the cefoxitin-resistant subpopulation increased considerably after day 14, while the amikacin-resistant subpopulation proportion did not change (Fig. 1c). Clarithromycin resistance could not be reliably assessed because of inducible macrolide resistance in M. abscessus (10).
FIG 1.
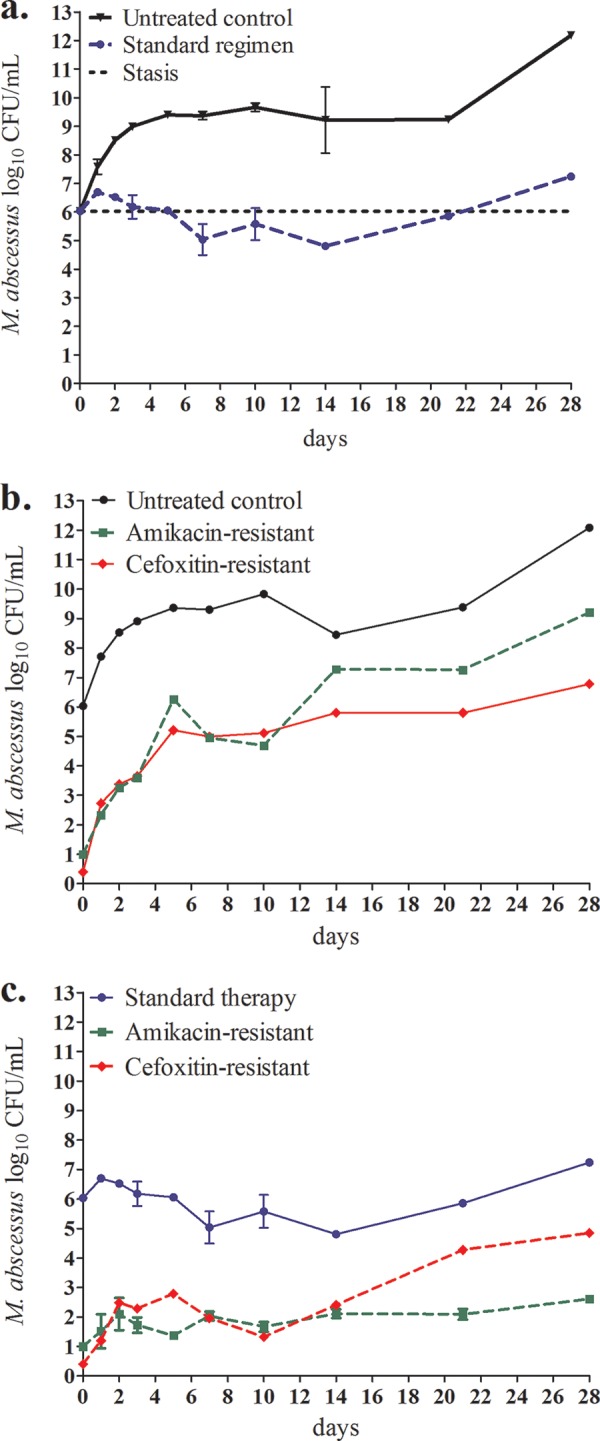
Efficacy of standard combination regimen against M. abscessus and resistance emergence during 1 month of treatment. (a) The initial very slow decline, slower than the doubling time of the bacteria, was reversed after 14 days, and all treated systems started to grow. (b) Changes in bacterial burden and the emergence of acquired drug resistance with time during repetitive sampling in nontreated controls. Total M. abscessus population in untreated control (solid line) and amikacin- or cefoxitin-resistant subpopulation (dashed lines) over the course of 28 days. The amikacin- and cefoxitin-resistant proportion was stable in the untreated control. (c) Changes in bacterial burden and the emergence of acquired drug resistance with time during repetitive sampling in systems treated with standard therapy. Starting with no CFU per milliliter resistant to 3 times the cefoxitin MIC, there developed a higher cefoxitin-resistant subpopulation of M. abscessus in the systems exposed to standard therapy, coincident with cessation of effect of the triple regimen.
The standard regimen tested failed to effectively kill M. abscessus in the 28-day evaluation in the HFS-M. abscessus model that mimicked lung disease. Cefoxitin seemed to be the driving force of the regimen, as the HFS-M. abscessus shows that while there was initial kill, this was abrogated by ADR to cefoxitin. In the nude-mouse M. abscessus disseminated disease model, cefoxitin has played a key role as well, as the activity of the triple regimen was equal to that of cefoxitin alone (11). Mouse studies, while useful, however, do not allow for repetitive sampling that would allow for an assessment of the evolution of ADR with time in the same mouse. Even though the proportion of bacteria resistant to 3 times the MIC of amikacin did not change, the biphasic nature of response on the current amikacin-containing regimen suggests resistance as well, albeit with <3-fold increases in MIC. This type of low-level resistance, which rapidly emerges within days, has been shown to be due to evolutionarily conserved efflux pumps in mycobacteria that are part of the “antibiotic resistance arrow of time” (12, 13).
Even at above-optimal amikacin and cefoxitin exposures, the regimen still failed. The situation observed here resembles the poor outcome often faced when patients are treated with the standard combined regimen; if they tolerate it, they may initially respond, but in the end, many simply fail to respond at all, and some respond and then relapse. In other words, it is going nowhere fast. The propensity to develop multiple drug resistances quickly is in part why M. abscessus has been termed the “antibiotic resistance nightmare” (14). Since dosing to achieve greater-than-optimal exposure would not result in any greater effectiveness, the solution is a de novo building of new regimens that are more bactericidal and can suppress the emergence of ADR. One of the alternatives could be to use tigecycline as an anchor drug in such a regimen, which has been shown to be effective (15, 16). However, tigecycline will need to be combined with other effective antibiotics, and the search for such antibiotics is ongoing.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (grant R56 AI111985 to T.G.) and a Doctoral Fellowship from the Instituto Colombiano para el Desarrollo de la Ciencia y la Tecnología Francisco José de Caldas (COLCIENCIAS).
T.G. is a consultant for L.E.A.F. Pharmaceuticals and LuminaCare solutions; also, he founded Jacaranda Biomed, Inc.
REFERENCES
- 1.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K, ATS Mycobacterial Diseases Subcommittee, American Thoracic Society, Infectious Diseases Society of America. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 2.Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. 2011. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis 52:565–571. doi: 10.1093/cid/ciq237. [DOI] [PubMed] [Google Scholar]
- 3.Ferro BE, Srivastava S, Deshpande D, Sherman CM, Pasipanodya JG, van Soolingen D, Mouton JW, van Ingen J, Gumbo T. 2015. Amikacin pharmacokinetics/pharmacodynamics in a novel hollow-fiber Mycobacterium abscessus disease model. Antimicrob Agents Chemother 60:1242–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferro BE, Srivastava S, Deshpande D, Pasipanodya JG, van Soolingen D, Mouton JW, van Ingen J, Gumbo T. 2016. Moxifloxacin's limited efficacy in the hollow-fiber model of Mycobacterium abscessus disease. Antimicrob Agents Chemother 60:3779–3785. doi: 10.1128/AAC.02821-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2011. Susceptibility testing of mycobacteria, Nocardiae and other aerobic actinomycetes, 2nd ed; approved standard CLSI document M24-A2. Clinical and Laboratory Standards Institute, Wayne PA. [PubMed] [Google Scholar]
- 6.Dull WL, Alexander MR, Kasik JE. 1979. Bronchial secretion levels of amikacin. Antimicrob Agents Chemother 16:767–771. doi: 10.1128/AAC.16.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santré C, Georges H, Jacquier JM, Leroy O, Beuscart C, Buguin D, Beaucaire G. 1995. Amikacin levels in bronchial secretions of 10 pneumonia patients with respiratory support treated once daily versus twice daily. Antimicrob Agents Chemother 39:264–267. doi: 10.1128/AAC.39.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. 2011. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J Infect Dis 204:1951–1959. doi: 10.1093/infdis/jir658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Ingen J, Egelund EF, Levin A, Totten SE, Boeree MJ, Mouton JW, Aarnoutse RE, Heifets LB, Peloquin CA, Daley CL. 2012. The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment. Am J Respir Crit Care Med 186:559–565. doi: 10.1164/rccm.201204-0682OC. [DOI] [PubMed] [Google Scholar]
- 10.Bastian S, Veziris N, Roux AL, Brossier F, Gaillard JL, Jarlier V, Cambau E. 2011. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob Agents Chemother 55:775–781. doi: 10.1128/AAC.00861-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lerat I, Cambau E, Roth Dit BR, Gaillard JL, Jarlier V, Truffot C, Veziris N. 2014. In vivo evaluation of antibiotic activity against Mycobacterium abscessus. J Infect Dis 209:905–912. doi: 10.1093/infdis/jit614. [DOI] [PubMed] [Google Scholar]
- 12.Schmalstieg AM, Srivastava S, Belkaya S, Deshpande D, Meek C, Leff T, van Oers NS, Gumbo T. 2012. The antibiotic resistance arrow of time: efflux pump induction is a general first step in the evolution of mycobacterial drug resistance. Antimicrob Agents Chemother 56:4806–4815. doi: 10.1128/AAC.05546-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gumbo T. 2013. Biological variability and the emergence of multidrug-resistant tuberculosis. Nat Genet 45:720–721. doi: 10.1038/ng.2675. [DOI] [PubMed] [Google Scholar]
- 14.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. 2012. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 67:810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 15.Ferro BE, Srivastava S, Deshpande D, Pasipanodya JG, van Soolingen D, Mouton JW, van Ingen J, Gumbo T. 2016. Tigecycline is highly efficacious in Mycobacterium abscessus pulmonary disease. Antimicrob Agents Chemother 60:2895–2900. doi: 10.1128/AAC.03112-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace RJ Jr, Dukart G, Brown-Elliott BA, Griffith DE, Scerpella EG, Marshall B. 2014. Clinical experience in 52 patients with tigecycline-containing regimens for salvage treatment of Mycobacterium abscessus and Mycobacterium chelonae infections. J Antimicrob Chemother 69:1945–1953. doi: 10.1093/jac/dku062. [DOI] [PMC free article] [PubMed] [Google Scholar]


