Abstract
Visceral leishmaniasis is a fatal parasitic disease, and there is an emergent need for development of effective drugs against this neglected tropical disease. We report here the development of a novel spirooxindole derivative, N-benzyl-2,2′α-3,3′,5′,6′,7′,7α,α′-octahydro-2methoxycarbonyl-spiro[indole-3,3′-pyrrolizidine]-2-one (compound 4c), which inhibits Leishmania donovani topoisomerase IB (LdTopIB) and kills the wild type as well as drug-resistant parasite strains. This compound inhibits catalytic activity of LdTopIB in a competitive manner. Unlike camptothecin (CPT), the compound does not stabilize the DNA-topoisomerase IB cleavage complex; rather, it hinders drug-DNA-enzyme covalent complex formation. Fluorescence studies show that the stoichiometry of this compound binding to LdTopIB is 2:1 (mole/mole), with a dissociation constant of 6.65 μM. Molecular docking with LdTopIB using the stereoisomers of compound 4c produced two probable hits for the binding site, one in the small subunit and the other in the hinge region of the large subunit of LdTopIB. This spirooxindole is highly cytotoxic to promastigotes of L. donovani and also induces apoptosis-like cell death in the parasite. Treatment with compound 4c causes depolarization of mitochondrial membrane potential, formation of reactive oxygen species inside parasites, and ultimately fragmentation of nuclear DNA. Compound 4c also effectively clears amastigote forms of wild-type and drug-resistant parasites from infected mouse peritoneal macrophages but has less of an effect on host macrophages. Moreover, compound 4c showed strong antileishmanial efficacies in the BALB/c mouse model of leishmaniasis. This compound potentially can be used as a lead for developing excellent antileishmanial agents against emerging drug-resistant strains of the parasite.
INTRODUCTION
DNA topoisomerases are an important group of enzymes that maintain the topological state of the DNA in the cell by transesterification reactions and in that way help the cellular processes of replication, transcription, etc. (1). This group of enzymes is divided into two categories according to the number of strands they cleave, type I (cleaves one strand) and type II (cleaves two strands) (2). Because of their importance in cellular functioning, topoisomerases are exploited as targets of anticancer, antitumor, and antibacterial agents. The inhibitors targeting topoisomerases are classified into two categories, topoisomerase poisons (class I) and catalytic inhibitors (class II). Class I inhibitors or poisons trap the DNA-enzyme covalent complex (cleavable complex) and slow down further religation of cleaved DNA strands (3). Inhibitors that hamper other steps of topoisomerase catalytic cycle but do not trap the DNA-enzyme cleavable complex are known as class II or catalytic inhibitors (4).
Leishmania donovani, an intramacrophage protozoan parasite transmitted by female sandflies, causes the deadly disease visceral leishmaniasis (kala-azar). This fatal parasitic disease affects millions of people in tropical and subtropical countries such as India, Bangladesh, Nepal, Sudan, Brazil, and Ethiopia (5). The lack of a proper vaccine (6), toxicity, side effects, high cost of available drugs (7, 8), and emergence of drug-resistant strains (9) have made the discovery of novel chemotherapeutic targets and agents a pressing need. Recent advances in molecular and cell biology have provided many excellent molecular targets for chemotherapy. DNA topoisomerase enzymes of Leishmania have been found to be excellent targets for antileishmanial chemotherapy (10).
The type IB topoisomerases of kinetoplastid parasites have an unusual heterodimeric architecture, and this was first reported in L. donovani by Villa et al. (11) and in Trypanosoma brucei by Bodley et al. (12). This unique bisubunit topoisomerase IB of the kinetoplastid parasites is a very attractive chemotherapeutic target because of its difference in structure from human topoisomerase I (13). Several L. donovani topoisomerase IB (LdTopIB) poisons which can stabilize the DNA-LdTopIB cleavable complex and kill Leishmania parasite have been reported in literature, viz., camptothecin (14), diospyrin (15), 3,3′-diindolyl methane (DIM) (16), baicalein, luteolin, and quercetin (17), niranthin (18), lyoniside and saracoside (19), etc. Catalytic inhibitors of LdTopIB are not well studied, and only a few, such as dihydrobetulinic acid (DHBA) (20) and betulin and dihydrobetulin derivatives (21), have been reported. Catalytic inhibitors of type IB topoisomerases may affect topoisomerase function in two ways, either by preventing the enzyme from binding to substrate DNA or by inhibiting the formation of the covalent cleavage complex (22, 23). Thus, they virtually deplete topoisomerase IB inside the cell by blocking the cellular function and affect proliferation of the cell (4). Although topoisomerase IB poisons are of great potency, sometimes they do show dose-limiting toxicities and side effects in humans (24). Therefore, detailed studies are required to discover and establish novel LdTopIB “catalytic inhibitors” with minimum dose-limiting toxicities and side effects.
Oxindoles are aromatic heterocyclic organic compounds that have a tryptophan-like structure in the core scaffold (25). Spirocyclic oxindole (spirooxindole) constitutes a vast family of natural products (e.g., alkaloids) and other synthetic compounds that are of great pharmaceutical value (26, 27). Spirooxindoles exhibit a wide range of biological activities, including insecticidal, antitumor, anthelmintic, and antibacterial properties and use for treatment of hyponatremia (26, 28, 29, 30). Paraherquamide A is a spirooxindole isolated from cultures of Penicillium paraherquei, and it shows potent antiparasitic activity and antinematodal properties (31). Most importantly, spirooxindole-containing small molecules are used as a new class of potent inhibitors of the MDM2-p53 interaction and show anticancer properties (25, 32, 33). Spirooxindoles are also effective antiprotozoal agents. Rottmann et al. established spiroindolone NITD609 as a potent antimalarial drug candidate (34). Recently, another group reported that C-3 monofunctionalized oxindoles and spiro[cyclohexanone-oxindoles] can stop proliferation of both promastigote and axenic amastigote forms of Leishmania infantum in a dose-dependent manner (35). Therefore, preparation of new spirooxindole derivatives or C-3 functionalized oxindoles is of utmost interest, as these compounds could serve as potent antileishmanial agents (35).
Here we report a novel spirooxindole, N-benzyl-2,2′α-3,3′,5′,6′,7′,7α,α′-octahydro-2methoxycarbonyl-spiro[indole-3,3′-pyrrolizidine]-2-one (compound 4c), as a catalytic inhibitor of the unusual bisubunit DNA topoisomerase IB of L. donovani. In silico molecular docking studies were performed to provide a possible explanation of the in vitro LdTopIB-inhibitory activity of compound 4c. The ability of this compound to kill the L. donovani wild-type AG83 strain as well as drug-resistant strain GE1 and miltefosine-resistant (MILr) and camptothecin-resistant (CPTr) cells (promastigotes and amastigotes) and its strong antileishmanial efficacy in the BALB/c mouse model of leishmaniasis with relatively lesser cytotoxicity toward host macrophages make it a good candidate for development of novel antileishmanial therapeutic agents.
MATERIALS AND METHODS
Chemicals.
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) was purchased from Invitrogen Life Technologies. Dimethyl sulfoxide (DMSO) and camptothecin were purchased from Sigma Chemicals (St. Louis, MO, USA). All drugs were dissolved in 100% DMSO at a concentration of 20 mM and stored at −20°C. Recombinant human topoisomerase I was purchased from Topogen Inc. (Buena Vista, CO, USA).
Representative procedures for synthesis of spirooxindoles (compounds 4a to f) and bis-spirooxindoles (compounds 6a to f).
The spirooxindoles 4a to f were synthesized by a modified routes and the representative procedure for synthesis is as follows. To a solution of isatin (compound 1a) (147 mg, 1 mmol) in CH3CN (5 ml) with stirring, l-proline (compound 2a) (115.06 mg, 1 mmol) was added, followed by the addition of 4-Å molecular sieves (MS) (200 mg), and the solution was left with stirring at room temperature (28°C) for 30 min. A deep green reaction mass appeared, to which methyl acrylate (compound 3a) (84 μl, 1 mmol) was added, and the stirring was continued at room temperature (28°C). The progress of the reaction was monitored by thin-layer chromatography (TLC) by using 40% ethyl acetate in petroleum ether as the solvent system. After 6 h, the starting materials completely disappeared, and then the 4-Å MS were filtered off over a thin pad of celite and the filtrate was evaporated in a rotary evaporator. The residue was then diluted with water (15 ml) and extracted with ethyl acetate (3 times, 25 ml). The organic layer was separated, washed with brine, and then dried over anhydrous Na2SO4. Removal of solvent resulted in a sticky brownish mass which was chromatographed over silica gel (60-120 mesh) using petroleum ether with an increasing proportion of ethyl acetate as the eluent. Elution with 25% ethyl acetate in petroleum ether gave the desired compound 4a (259 mg, 91%) as a white solid, which was crystallized in chloroform.
The representative procedure for synthesis of compounds 6a to f is as follows. To a solution of N-benzyl isatin (compound 1b) (118.5 mg, 0.5mmol) in CH3CN (3 ml) with stirring, l-proline (compound 2a) (57.5 mg, 0.5mmol) was added followed by the addition of 4-Å MS (100 mg), and the solution was left with stirring at room temperature (30°C) for 30 min. A light yellow reaction mass appeared, and to the reaction mass dipolarophile (compound 5a) (93.5 mg, 0.5mmol) was added and subjected to stirring at room temperature (30°C). The progress of the reaction was monitored by TLC by using 50% ethyl acetate in petroleum ether solvent system. After 6 h of reaction, there was very little starting material, which was not consumed on further continuation of the reaction. The MS were then filtered off over a thin pad of celite, and the filtrate was evaporated in a rotary evaporator. The residue was then diluted with water (15 ml) and extracted with ethyl acetate (3 times, 25 ml). The organic layer was separated, washed with brine, and then dried over anhydrous Na2SO4. Removal of solvent resulted in a sticky solid which was chromatographed over silica gel (60-120 mesh) using petroleum ether with an increasing proportion of ethyl acetate as the eluent. Elution with 30% ethyl acetate in petroleum ether gave compound 6a (202 mg, 85%) as a gray solid.
Overexpression and purification of recombinant LdTopIB by affinity column chromatography.
L. donovani topoisomerase type IB (LdTopIB) is a bisubunit enzyme. The larger subunit (LdTOP1L, 73 kDa) was cloned in pET16b, and the smaller subunit (LdTOP1S, 29 kDa) was cloned in pET16b. Both subunits were purified by methodology described previously (13). Escherichia coli BL21(DE3)/pLysS cells harboring pET16b-LdTOP1L and pET16b-LdTOP1S were separately induced at an optical density at 600 nm (OD600) of 0.6 with 0.5 mM IPTG (isopropyl-β-d-thiogalactoside) at 22°C for 12 h. Cells harvested from 1 liter of culture were separately lysed by lysozyme and sonication, and the proteins were purified through an Ni2+-nitriloacetate (Ni-NTA) agarose column (Qiagen, Hilden, Germany) followed by a phosphocellulose column (P11 cellulose; Whatman, Maidstone, Kent, United Kingdom) as described previously (13). Finally, the purified proteins LdTOP1L and LdTOP1S were stored at −70°C. The concentrations of each protein were quantified by using Bradford reagent (Pierce, Thermo Fisher Scientific Inc., Rockford, IL, USA).
Purified LdTOP1L was mixed with purified LdTOP1S at a molar ratio of 1:1 at a total protein concentration of 0.5 mg/ml in reconstitution buffer (50 mM potassium phosphate [pH 7.5], 0.5 mM dithiothreitol [DTT], 1 mM EDTA, 0.1 mM phenylmethylsulfonyl fluoride [PMSF] and 10% [vol/vol] glycerol). The mixture was dialyzed overnight at 4°C, and dialyzed fractions were used for plasmid relaxation activity (13).
Plasmid relaxation assay.
Six spirooxindole derivatives (compounds 4a to f) and four bis-spirooxindole derivatives (compounds 6a to d) were screened for their Leishmania DNA topoisomerase IB inhibition activity in a plasmid relaxation assay.
The type I DNA topoisomerases were assayed by decreased mobility of the relaxed isomers of supercoiled pBluescript SK(+) [pBS SK(+)] DNA in an agarose gel. The relaxation assay was carried out as described previously with LdTopIB (17) serially diluted in the relaxation buffer (25 mM Tris-HCl [pH 7.5], 5% glycerol, 0.5 mM DTT, 10 mM MgCl2, 50 mM KCl, 25 mM EDTA, and 150 μg/ml bovine serum albumin [BSA]) and supercoiled plasmid pBS SK(+) DNA (85 to 95% were negatively supercoiled, with the remainder being nicked circles). All the relaxation experiments were carried out at 37°C for 15 min.
For all kinetic studies, the reaction mixtures containing the buffer and DNA were heated to 37°C before addition of the enzymes. The reactions were rapidly quenched using stop solution and kept on ice. The gels were stained with ethidium bromide (EtBr) (0.5 μg/ml), and the amount of supercoiled monomer DNA band fluorescence was quantified by integration using Gel Doc 2000 under UV illumination (Bio-Rad Quantity One software) as described previously (18). Initial velocities (nanomolar DNA base pairs relaxed per minute) were calculated using the following equation: initial velocity = [supercoiled DNA]0 − (Intt [supercoiled DNA]0/Int0)/t, where [supercoiled DNA]0 is the initial concentration of supercoiled DNA, Int0 is the area under the supercoiled DNA band at time zero, and Intt is the area at reaction time t (36). The effect of DNA concentration on the kinetics of relaxation was examined over a range of 8 to 60 nM supercoiled pBS SK(+) DNA (0.16 to 2.4 μg/25 μl of reaction mixture) at constant concentration of 10 mM MgCl2 and 0.98 nM enzyme (LdTOP1LS) at 37°C for 1 min. The data were analyzed by a Lineweaver-Burk plot. The intercept of the y axis is 1/Vmax, and turnover number = Vmax/enzyme concentration (plasmid molecules relaxed per minute per molecule of enzyme.
Plasmid cleavage assay.
Cleavage assay was carried out as described previously (18). Briefly, 50 fmol of pHOT1 supercoiled DNA (containing the topoisomerase I cleavage site) and 100 fmol of reconstituted LdTopIB were incubated in a standard reaction mixture (50 μl) containing 50 mM Tris-HCl (pH 7.5), 100 mM KCl, 10 mM MgCl2, 0.5 mM DTT, 0.5 mM EDTA, and 30 μg/ml BSA in the presence of various concentrations of compound 4c at 37°C for 30 min. The reactions were terminated by adding 1% SDS and 150 μg/ml proteinase K, and the mixtures were further incubated for 1 h at 37°C. DNA samples were electrophoresed in 1% agarose gel containing 0.5 μg/ml EtBr to resolve more slowly migrating nicked product (form II) from the supercoiled molecules (form I).
Immunoband depletion assay.
Leishmania cells (2 × 107) were cultured for 12 h at 22°C with or without drugs. Nuclear fractions were isolated as described previously (37). Briefly, cells were suspended in hypo-osmotic buffer (10 mM Tris-HCl [pH 7.5], 1 mM EDTA, 0.1 mM EGTA. 1 mM PMSF, 1 mM benzamidine hydrochloride, and 5 mM DTT) and homogenized. The homogenate was centrifuged at 10,000 × g for 10 min at 4°C. The pellet was washed and used as the source of the nuclear fraction. The nuclear fractions after lysis with 1% SDS were subjected to SDS-PAGE, and the proteins were electrophoretically transferred on to nitrocellulose membranes. Immunoblotting of immobilized proteins was carried out using a rabbit antibody raised against LdTOP1S (17).
Job plot.
The binding stoichiometry for the inhibitor with LdTopIB was determined using the method of continuous variation (38, 39). Several mixtures of compound 4c and LdTopIB were prepared by continuously varying the concentrations of recombinant LdTopIB and compound 4c in the mixtures while keeping the total concentration of inhibitor plus recombinant LdTopIB constant at 1.25 μM as described previously (17). Reaction mixtures were incubated for 10 min at 25°C, and the quenching of tryptophan fluorescence was recorded at 350 nm upon excitation at 295 nm on PerkinElmer LS55 luminescence spectrometer. The excitation and emission slit widths were 5 nm and 10 nm, respectively. Values for appropriate blanks corresponding to the buffer were subtracted to eliminate background fluorescence (<5%).
Spectrofluorimetric binding assay.
LdTopIB (200 nM) was incubated in fluorescence buffer (20 mM Tris-HCl, 50 mM NaCl, and 10 mM MgCl2) with various concentrations of compound 4c (0 to 11 μM) at 25°C for 10 min as described previously (21). Briefly, the fluorescence intensities were measured at the emission range of 320 to 400 nm upon excitation at 295 nm. Excitation and emission slit widths were 5 and 10 nm, respectively. The measurements of the fluorescence values in the presence of continuously increasing concentrations of inhibitors were performed in triplicate. The fraction of binding sites (B) occupied by inhibitor was determined by the equation B = (F0 − F)/Fmax, where F0 is the fluorescence intensity at 350 nm of LdTopIB alone in the absence of any inhibitors, F is the fluorescence intensity at 350 nm of LdTopIB in the presence of inhibitor, and Fmax is obtained from the plot of 1/(F0 − F) versus 1/[X] and by extrapolating 1/[X] to zero, where [X] is the concentration of compound 4c. The dissociation constant (KD) was determined as described previously (21) using the equation Fmax/(F0 − F) = 1 + KD/Lf, where Lf denotes the free concentration of inhibitor (Lf = C − B[J], where C is the total concentration of inhibitor and [J] is the molar concentration of ligand-binding sites using a stoichiometry from the Job plot).
Molecular docking procedure.
Structural information for L. donovani topoisomerase 1 (LdTopIB) was obtained from the Protein Data Bank (PDB ID 2B9S). Missing side chain residues were modeled in AutoDockTools4 (40), followed by steepest descend energy minimization in Desmond. Chemical structures of compounds 4a and 4c were drawn on Avogadro (41), and side chain orientations were optimized by systemic rotor search followed by geometry optimized using the B3LYP/6-311+G(2d,p) level of density functional theory in Gaussian 09. Molecular docking was performed using different algorithms: AutoDock4 (40), AutoDock Vina (42), PatchDock (43)/FireDock (44), and SwissDock (45). Docking was performed without DNA, and the whole protein was put into the search space. Genetic algorithm (GA) was used in AutoDock4. In order to obtain a statistical validation, we ran the GA 1,000 times, and each time GA was set to terminate after a maximum of 25 million binding energy evaluations. For PatchDock, the top 1,000 solutions were further refined by FireDock.
Parasite maintenance and cultures.
The sodium antimony gluconate (SAG)-sensitive (Sbs) MHOM/IN/1983/AG83 (AG83) strain of L. donovani, the laboratory-grown SAG-resistant (Sbr) strain GE1, laboratory-grown miltefosine-resistant (MILr) cells, and camptothecin-resistant (CPTr) cells (raised in hamsters) were used (46, 47). Amastigotes obtained from the spleens of infected hamsters were cultured at 22°C to obtain promastigotes and cultured in M199 containing 20% (vol/vol) heat-inactivated fetal bovine serum (FBS) supplemented with 100 IU/ml of penicillin and 100 mg/ml of streptomycin at 22°C to obtain promastigotes. Promastigotes were further grown in 10% (vol/vol) heat-inactivated FBS for 3 to 5 days at 22°C before use.
Measurement of Leishmania promastigote cell viability.
The L. donovani Ag83 wild-type strain, laboratory-grown SAG-resistant (Sbr) GE1, laboratory-grown miltefosine-resistant (MILr) cells, and camptothecin-resistant (CPTr) cells (3.0 × 106 cells/ml) were individually incubated with 50 μM concentrations of 10 different spirooxindole derivatives (compounds 4a to f and compounds 6a to d) or 10 different concentrations of compound 4c (0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.25, and 2.5 μM) for 12 h, following which the survival percentage was estimated by MTT assay. Yellow MTT, a tetrazole, is reduced to purple formazan in the mitochondria of living cells. The formazan is then solubilized and the concentration determined by optical density at 570 nm. Metabolically active cells convert MTT to formazan, thereby generating a quantitative measure of viability and cytotoxicity.
Double staining with annexin V and PI.
Externalization of phosphatidyl serine on the outer membranes of untreated promastigotes and promastigotes treated with 0.2% DMSO and compound 4c (1 μM, 1.5 μM, 2 μM, and 5 μM) was measured by binding of fluorescein isothiocyanate (FITC)-annexin V and propidium iodide (PI) using an annexin V staining kit (Invitrogen Ltd.). Flow cytometry was carried out for treated and untreated parasites. The gating was done so that the FL-1 channel denotes the mean intensity of FITC-annexin V, whereas the FL-2 channel denotes the mean intensity of PI. The data represented here means from three experiments.
Measurement of ROS.
Intracellular levels of reactive oxygen species (ROS) were measured in compound 4c-treated and untreated parasites. Promastigotes (2 × 107 cells/ml) were treated with 0.5, 1, 1.5, 2, and 5 μM compound 4c for 8 h. Parasites treated with 0.2% DMSO served as controls. After different treatments, parasites were washed and resuspended in 500 μl of phosphate-buffered saline (PBS) (without phenol red) and loaded with the cell-permeant dye H2DCFDA for 1 h (47). The green fluorescence of 2′,7′-dichlorofluorescein (DCF) was measured at 530 nm using a flow cytometer.
Measurement of ΔΨm.
Mitochondrial membrane potential (ΔΨm) was investigated using JC-1 dye. This dye accumulates in the mitochondrial matrix under the influence of ΔΨm, where it reversibly forms monomers (green) with characteristic absorption and emission spectra (37). In brief, after different treatments with compound 4c (1, 2, and 5 μM), leishmanial cells were harvested and washed with PBS (1×). Cells were then incubated at 37°C for 1 h with a final concentration of JC-1 dye at 5 μg/μl. Cells were then analyzed by flow cytometry and fluorescence measurement with a spectrofluorometer using 507 and 530 nm as excitation and emission wavelengths, respectively (for green fluorescence), and 507 and 590 nm as excitation and emission wavelengths, respectively (for red fluorescence) to analyze ΔΨm. The spectrofluorometric data presented here are representative of three experiments. The ratio of the reading at 590 nm to the reading at 530 nm was considered to be the relative ΔΨm value.
TUNEL assay.
A two-color terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay (APO-BrdU kit; BD Pharmingen, catalog number 556405) was performed for labeling DNA breaks and total cellular DNA to confirm that compound 4c induced apoptotic cell death and genomic DNA fragmentation. Leishmania promastigotes were treated with 2 and 5 μM compound 4c for 2, 6, and 8 h, and TUNEL assay was performed as described in the manufacturer's protocol. In brief, leishmanial cells were fixed in paraformaldehyde (1 h, 4°C). Cells were then incubated with bromolated dUTP (Br-dUTP) and terminal deoxynucleotidyl transferase (TdT) for DNA labeling (1 h, 37°C). After the Br-dUTP labeling, the terminal sites (3′-OH ends) of double- and single-stranded DNA were identified using flow cytometry by staining cells with FITC-labeled antibromodeoxyuridine (anti-BrdU) antibody (30-min incubation in the dark) and propidium iodide-RNase A solution for counterstaining the total DNA.
Ethics.
BALB/c mice, originally obtained from Jackson Laboratories, Bar Harbor, ME, and reared in the Indian Institute of Chemical Biology (IICB) animal facilities, were used for experimental purposes with prior approval of the animal ethics committee. The studies and animal handling were approved by IICB Animal Ethical Committee (registration no. 147/1999), registered with Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA), Government of India.
In vitro macrophage infection.
Macrophages were isolated from BALB/c mice (female, 4 to 6 weeks old) at 36 to 48 h after injection (intraperitoneal) with 2% (wt/vol) hydrolyzed starch by peritoneal lavage with ice-cold phosphate-buffered saline. In vitro infection with promastigotes of the AG83 wild-type strain, laboratory-grown SAG-resistant (Sbr) strain GE1, the laboratory-grown miltefosine-resistant (MILr) strain, and the camptothecin-resistant (CPTr) strain were carried out as described previously (18). Compound 4c was added at different concentrations (0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, and 2 μM) to infected macrophages and left for another 24 h. Cells were then fixed in methanol and stained with 2% Giemsa stain. Percentages of infected cells and total number of intracellular parasites were determined by manual counting of at least 200 cells using a light microscope.
Measurement of dose-dependent cytotoxic effect of compound 4c on cultured murine peritoneal macrophages by MTT assay.
Macrophages isolated from mice were seeded on a 96-well tissue culture plate (approximately 105 cells/well) in RPMI complete medium supplemented with 10% fetal calf serum (FCS) and left to adhere for 48 h at 37°C under 5% CO2. Macrophages were then treated with increasing concentrations of compound 4c (1, 5, 10, 20, 50, and 100 μM) for 24 h. The percentage of viable macrophages was measured by MTT assay.
Infection of mice and treatment with compound 4c.
For experimental visceral infections, female BALB/c mice (4 to 6 weeks old, 20 to 25 g each) were divided into six groups (uninfected control group, infected control group, and four treatment groups), each group consisting of 5 animals. The animals were injected via the intracardiac route with 2 × 107 hamster spleen-transformed L. donovani promastigotes (suspended in 200 μl of 0.02 M PBS per mouse). At 3 weeks postinfection, compound 4c was administered to infected animals via the intraperitoneal route at 1, 2.5, 5, and 10 mg/kg body weight separately twice a week for a period of 3 weeks. Visceral infection was determined by Giemsa-stained impression smears of spleens and livers from 6-week-infected mice and reported as Leishman-Donovan units (LDU), calculated as the number of parasites per 1,000 nucleated cells times organ weight in milligrams (48).
Statistical analysis.
Data are provided as means ± standard errors of the means (SEM) or means ± standard deviations (SD) from the number of independent experiments performed. Statistical analysis and graphical representation were performed with GraphPad Prism version 5.00 (GraphPad Software, San Diego, CA, USA).
RESULTS
Compound 4c inhibits catalytic activity of LdTopIB.
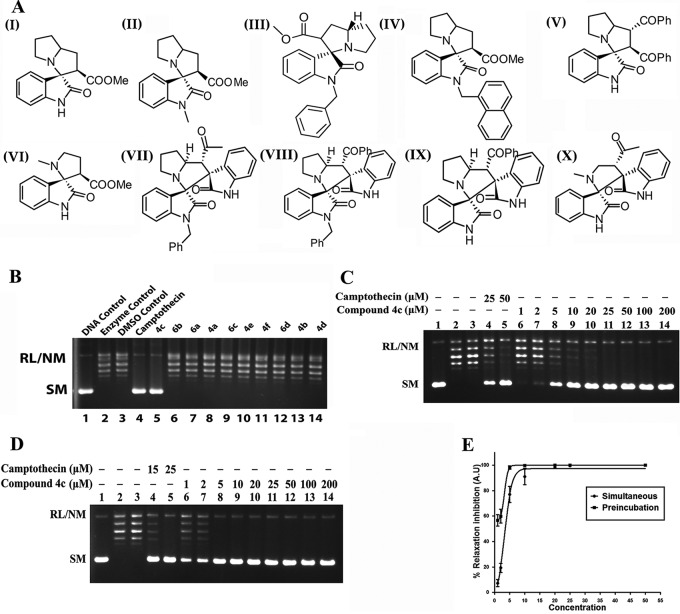
Screening of six spirooxindole and four bis-spirooxindoles derivatives (compounds 4a to f and compounds 6a to d) (Fig. 1A, panels I to X; see Data S1 and Tables S1 to S3 in the supplemental material) showed that only compound 4c inhibits L. donovani topoisomerase IB (LdTopIB) activity (Fig. 1B). To study the dose-dependent inhibition pattern of compound 4c, increasing concentrations of the compound were added to the relaxation assay mixture under simultaneous and preincubation assay conditions (Fig. 1C and D). Under the simultaneous assay condition, substrate plasmid DNA, LdTopIB, and compound 4c were added to the reaction mixture simultaneously (Fig. 1C). To investigate the interaction of compound 4c with the enzyme, a relaxation experiment was carried out under the preincubation condition, and under this condition LdTopIB was preincubated separately with compound 4c at different concentrations for 5 min at 37°C prior to addition of substrate plasmid DNA (Fig. 1D). The percentage of relaxation inhibition was plotted against the concentration of the compound for simultaneous and preincubation assay conditions (Fig. 1E). The 50% inhibitory concentrations (IC50s) (calculated using the variable-slope model for finding the 50% effective concentration [EC50] in Prism, version 5.0 [GraphPad Software, San Diego, CA]) of compound 4c in simultaneous and preincubation DNA relaxation assays were 3.29 μM and 0.91 μM, respectively. Therefore, compound 4c is much more potent inhibitor of LdTopIB under the preincubation assay condition. Under both assay conditions, camptothecin (CPT) was used as a positive control.
FIG 1.
Chemical structures of six spirooxindole and four bis-spirooxindole compounds and inhibition of catalytic activity of LdTopIB by compound 4c. (A) Chemical structures of the compounds. Panels: I, compound 4a, 2,2′α-3,3′,5′,6′,7′,7α,α′-octahydro-2-methoxycarbonyl-spiro[indole-3,3′-pyrrolizidine]-2-one; II, compound 4b, N-methyl-2,2′α 3,3′,5′,6′,7′,7α,α′-octahydro-2methoxycarbonyl-spiro[indole-3,3′-pyrrolizidine]-2-one; III, compound 4c, N-benzyl-2,2′α-3,3′,5′,6′,7′,7α,α′-octahydro-2-methoxycarbonyl-spiro[indole-3,3′-pyrrolizidine]-2-one; IV, compound 4d, N-naphthyl-2,2′α-3,3′,5′,6′,7′,7α,α′-octahydro-2-methoxycarbonyl-spiro[indole-3,3′-pyrrolizidine]-2-one; V, compound 4e, 2,2′α-3,3′,5′,6′,7′,7α,α′-octahydro-2,3-dibenzoyl-spiro[indole-3,3′-pyrrolizidine]-2-one; VI, compound 4f, 2-methoxycarbonyl-spiro[indole-3,3′-N-methylpyrrolizidine]-2-one; VII, compound 6a, N-benzyl-spiro[2,3′]oxindolespiro-[3,3′]-oxindole-4-acetyl-pyrrolizidine; VIII, compound 6b, N-benzyl-spiro[2,3′]oxindolespiro-[3,3′]-oxindole-4-benzoyl-pyrrolizidine; IX, compound 6c, spiro[2,3′]-oxindolespiro[3,3′]-oxindole-4-benzoyl pyrrolizidine; X, compound 6d, spiro[2,3′]-oxindolespiro[3,3′]-oxindole-4-acetyl-N-methylpyrrolidine. (B) Screening of 10 compounds (compounds 4a to f and compounds 6a to d) for anti-LdTopIB activity. Relaxation of supercoiled pBS SK(+) DNA with reconstituted LdTopIB at a molar ratio of 3:1 at 37°C for 15 min was performed. Lane 1, 90 fmol of pBS SK(+) DNA; lanes 2 and 3, same as lane 1 but simultaneously incubated with 30 fmol of LdTopIB with reaction buffer and 2% DMSO, respectively; lane 4, same as lane 2 but in the presence of 50 μM camptothecin; lanes 5 to 14, same as lane 2 but in the presence of 50 μM compound 4c, compound 6b, compound 6a, compound 4a, compound 6c, compound 4e, compound 4f, compound 6d, compound 4b, and compound 4d, respectively. (C) Inhibition of LdTopIB-mediated plasmid relaxation by compound 4c under the simultaneous condition. Relaxation of supercoiled pBS SK(+) DNA with reconstituted LdTOP1LS at a molar ratio of 3:1 at 37°C for 15 min was performed. Lane 1, 90 fmol of pBS SK(+) DNA; lane 2, same as lane 1 but simultaneously incubated with 30 fmol of LdTopIB for 15 min at 37°C; lane 3, same as lane 2 but in the presence of 2% (vol/vol) DMSO; lanes 4 and 5, same as lane 2 but in the presence of 25 and 50 μM CPT, respectively; lanes 6 to 14, same as lane 2 but in the presence of 1, 2, 5, 10, 20, 25, 50, 100, and 200 μM compound 4c, respectively. (D) Preincubation of LdTopIB with compound 4c followed by addition of DNA. Lane 1, 90 fmol of pBS SK(+) DNA; lane 2, same as lane 1 but DNA was added after preincubation of 30 fmol LdTopIB with reaction buffer for 5 min at 37°C; lane 3, same as lane 2 but with 2% DMSO; lanes 4 and 5, same as lane 2 but the enzyme was preincubated with 15 and 25 μM CPT, respectively; lanes 6 to 14, same as lane 2 but the enzyme was preincubated with 1, 2, 5, 10, 20, 25, 50, 100, and 200 μM compound 4c, respectively. (E) Quantitative representation of enzyme inhibition as a function of inhibitor concentrations in the presence of compound 4c in simultaneous and preincubation relaxation experiments. The fitted lines (sigmoidal) from these data points (n = 3) have r2 values of 0.9951 and 1.00, respectively.
Further, two stereoisomers (see Data S2 in the supplemental material) of compound 4c (compound 4c-1 and compound 4c-2) were used to study the dose-dependent inhibition pattern of LdTopIB. The isomers of compound 4c showed similar inhibition patterns, with IC50s of 7.57 μM and 5.79 μM, respectively (see Fig. S1 in the supplemental material).
We also performed a time course plasmid relaxation assay with LdTopIB in the absence or presence of camptothecin (50 μM) and compound 4c (25 μM) (see Fig. S3 in the supplemental material). Camptothecin-mediated inhibition of LdTopIB is reversed with time, whereas compound 4c-mediated inhibition of LdTopIB is not reversed up to 30 min (see Fig. S3 in the supplemental material).
Compound 4c inhibits LdTopIB-mediated DNA cleavable complex formation by abrogating topoisomerase IB-DNA interaction both in vitro and in vivo.
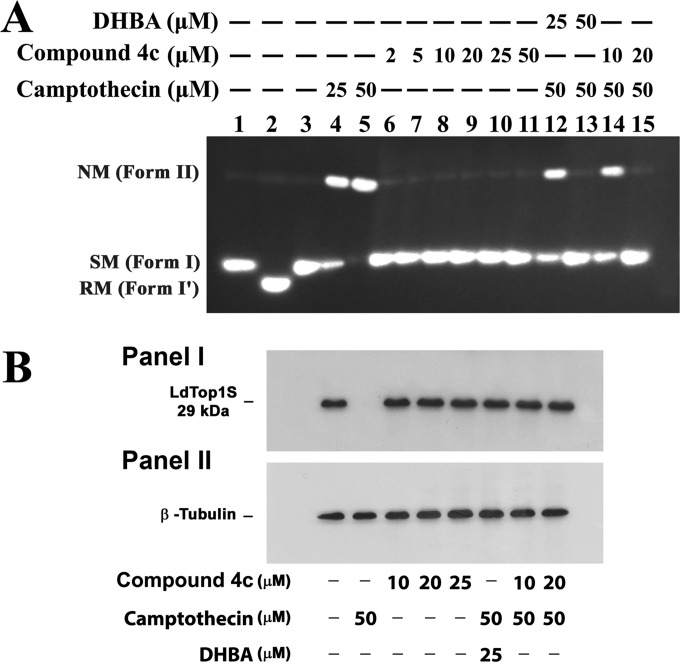
Topoisomerase inhibition can be achieved by prevention of enzyme-DNA binary complex formation or by stabilizing the enzyme-DNA cleavable complex. To find out whether compound 4c can form a covalent complex with topoisomerase IB and DNA, a plasmid cleavage reaction was performed under equilibrium conditions in the presence of increasing concentrations of compound 4c with camptothecin as a positive control under standard assay conditions. As shown in Fig. 2A, camptothecin converted closed circular DNA (form I) to nicked circular DNA (form II) by stabilization of the “cleavable complex.” When the cleavage assay was performed at increasing concentrations (2, 5, 10, 20, 25, and 50 μM) of compound 4c using 100 fmol of LdTopIB, no obvious nicked products were observed (Fig. 2A, lanes 6 to 11). Moreover, when 100 fmol of LdTopIB was preincubated with 10 and 20 μM concentrations of compound 4c before addition of 50 μM CPT (Fig. 2A, lanes 14 and 15), the CPT-mediated cleavage complex stabilization was inhibited drastically with increasing concentrations of this compound. These results not only depict the inability of compound 4c to trap the topoisomerase IB-mediated cleavable complex but also highlight the antagonistic nature of this compound against camptothecin-mediated stabilization of the DNA-topoisomerase IB cleavage complex. This is a clear indication of the fact that this compound inhibits the binding of enzyme to substrate DNA and inhibits cleavable complex formation. This conclusion was further justified by using dihydrobetulinic acid (DHBA) as a positive control, because DHBA binds with the free enzyme and prevents the stabilization of cleavable complex formation by camptothecin, as described previously (20).
FIG 2.
Compound 4c does not stabilize LdTopIB-mediated DNA cleavage complex and abrogates CPT-mediated DNA cleavage complex stabilization. (A) Effect of compound 4c treatment on LdTopIB-mediated cleavage of plasmid DNA. The plasmid cleavage reaction and agarose gel electrophoresis were performed as described in Materials and Methods. Lane 1, 50 fmol of pHOT1 DNA; lane 2, same as lane 1 but with 100 fmol LdTopIB; lane 3, same as lane 2 but with SDS-proteinase K treatment; lanes 4 and 5, same as lane 3 but in the presence of 25 and 50 μM CPT, respectively, as controls; lanes 6 to 11, same as lane 3 but in the presence of 2, 5, 10, 20, 25, and 50 μM compound 4c, respectively; lanes 12 and 13, same as lane 2 but the enzyme was preincubated with 25 and 50 μM dihydrobetulin acid (DHBA) before addition of camptothecin (50 μM) and DNA; lanes 14 and 15, same as lane 2 but the enzyme was preincubated with 10 and 20 μM compound 4c before addition of camptothecin (50 μM) and DNA. Positions of supercoiled monomer (SM) (form I), nicked monomer (NM) (form II), and relaxed molecules (form I′) are indicated. The figure represents two independent experiments. (B) Compound 4c does not stabilize the LdTopIB-mediated DNA cleavage complex inside L. donovani cells. Stabilization of the topoisomerase-mediated cleavable complex was analyzed by the immunoband depletion assay. Panel I, immunoband depletion of L. donovani topoisomerase I using an antibody raised against the LdTopIB small subunit. Leishmanial cells were treated with 0.2% DMSO alone (lane 1), with 10, 20, and 25 μM compound 4c (lanes 3, 4, and 5, respectively), 50 μM CPT (lane 2), 25 μM DHBA before treatment with CPT (lane 6), or 10 and 20 μM compound 4c before treatment with CPT (lanes 7 and 8, respectively). Panel II, β-tubulin served as a loading control.
In vitro studies indicated that compound 4c inhibits L. donovani topoisomerase IB-mediated DNA cleavable complex formation by abrogating topoisomerase-DNA interaction. In order to find out whether compound 4c performs similarly inside parasites, we carried out immunoband depletion experiments with L. donovani AG83 promastigotes. Cellular fractions were prepared from untreated as well as drug-treated promastigotes and subjected to SDS-PAGE. If a topoisomerase forms a covalent complex with genomic DNA inside cells, the topoisomerase-DNA covalent complex cannot penetrate into the gel. On the other hand, if the topoisomerase does not form a complex with DNA and remains free, it will enter into the gel. The presence of topoisomerase IB in the cell lysate was detected by immunoblotting against anti-LdTOP1S raised in rabbit as described in Materials and Methods. The immunoband depletion experiment showed the same results as the in in vitro experiment; i.e., compound 4c inhibits L. donovani topoisomerase IB-mediated DNA cleavable complex stabilization by inhibiting topoisomerase IB-DNA interaction (Fig. 2B).
Compound 4c acts as a competitive inhibitor of LdTopIB.
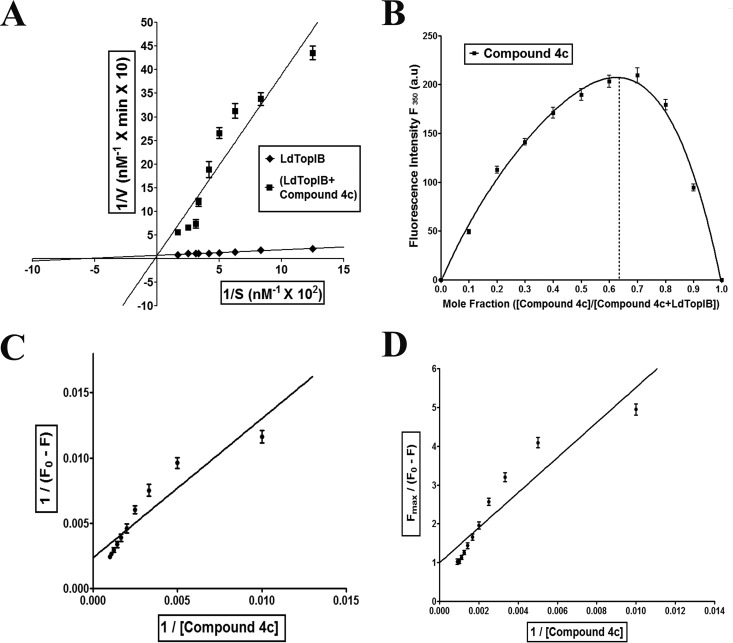
The preincubation relaxation assay and abrogation of cleavage complex formation by compound 4c suggested that the compound interacts with the free enzyme LdTopIB. To confirm this, a time course relaxation experiment was performed under standard relaxation assay conditions at 37°C, where the concentration of supercoiled substrate pBS SK(+) DNA was varied over a range of 8 to 60 nM. The enzyme/DNA ratio was kept within the steady-state assumption. As the velocity of the enzyme remains linear for the first 5 min of reaction, all the subsequent velocities for this kinetic study were measured for the time point up to 1 min, which falls within the linear range for the velocity examined. The initial velocities for each substrate concentration were plotted on a Lineweaver-Burk plot (Fig. 3A). The maximal velocity (Vmax) for the LdTopIB was 6.37 × 10−8 M base pairs of supercoiled DNA relaxed/min/0.98 nM enzyme, which corresponds to a turnover number of about 72 plasmid molecules relaxed/min/molecule of enzyme, and it remained unchanged after treatment with compound 4c. However, the Km increased approximately 16-fold after compound 4c treatment. Compound 4c competed for the DNA binding, and the affinity of LdTopIB toward DNA decreased drastically.
FIG 3.
Compound 4c-mediated LdTopIB inhibition kinetics and compound 4c-LdTopIB interaction study. (A) Lineweaver–Burk representation of the kinetics of relaxation of negatively supercoiled pBS SK(+) DNA by LdTopIB (◆) alone or with compound 4c (■). Data are represented as mean ± SD from three independent experiments. (B) Job plot of LdTopIB binding to compound 4c. Data are represented as mean ± SD from three independent experiments. (C) Double-reciprocal plot of compound 4c binding to LdTopIB. Data are represented as mean ± SD from three independent experiments. (D) Linear plot of compound 4c binding to LdTopIB. Data are represented as mean ± SD from three independent experiments.
Two molecules of compound 4c bind to one molecule of LdTopIB.
To find out the nature of the enzyme-inhibitor interaction, i.e., the interaction of compound 4c with LdTopIB, we measured the compound 4c-mediated quenching of intrinsic tryptophan fluorescence of LdTopIB. The stoichiometry of the ligand-protein interaction was measured using the Job plot (38, 39) as described in Materials and Methods. The results of such an experiment are shown in Fig. 3B. The stoichiometry of binding calculated using this method of continuous variation was found to be 2:1 for LdTopIB, which suggests that there are two binding sites for compound 4c in the enzyme. The dissociation constant was calculated from Fig. 3C and D. Figure 3C emphasizes the quenching profile of a fixed amount of LdTopIB (200 nM) with various concentrations of compound 4c (0 to 11 μM). The dissociation constant (KD) of the ligand-receptor interaction was determined to be 6.65 μM.
Compound 4c has probable binding sites in the small subunit and the hinge region of the large subunit of LdTopIB.
In order to get in-depth insight into the binding sites of compound 4c on LdTopIB, we carried out computational analysis. The molecular docking was carried out with R and S stereoisomers of compound 4c and also with compound 4a as negative control. The binding sites of the least-energy conformers along with the binding energies are listed in Table 1. The interaction of 4a was found to be energetically less favorable. Compound 4a does not inhibit the enzyme activity as well. From Table 1, it emerges that there are two most probable binding sites for 4c: site one is in the small subunit, and site two is in the hinge region of the large subunit, which corroborates with the binding stoichiometry analysis and fluorescence quenching studies (Fig. 3B, C, and D).
TABLE 1.
Binding sites and binding energies of compounds 4c and 4a as obtained from different docking algorithms
| Compounda | AutoDock4 |
AutoDock Vina |
PatchDock/FireDock |
SwissDock |
||||
|---|---|---|---|---|---|---|---|---|
| Energy (kJ/mol) | Site | Energy (kJ/mol) | Site | Energy (kJ/mol) | Site | Energy (kJ/mol) | Site | |
| 4c-1 | −28.33 | Small subunit | −30.96 | Hinge | −31.27 | Small subunit | −32.09 | Hinge |
| 4c-2 | −26.48 | Small subunit | −33.47 | Hinge | −37.89 | Small subunit | −32.80 | Hinge |
| 4a | −21.51 | Small subunit | −27.61 | Hinge | −28.48 | Small subunit | −28.20 | Hinge |
Compounds 4c-1 and 4c-2 are stereoisomers separated from compound 4c (racemic).
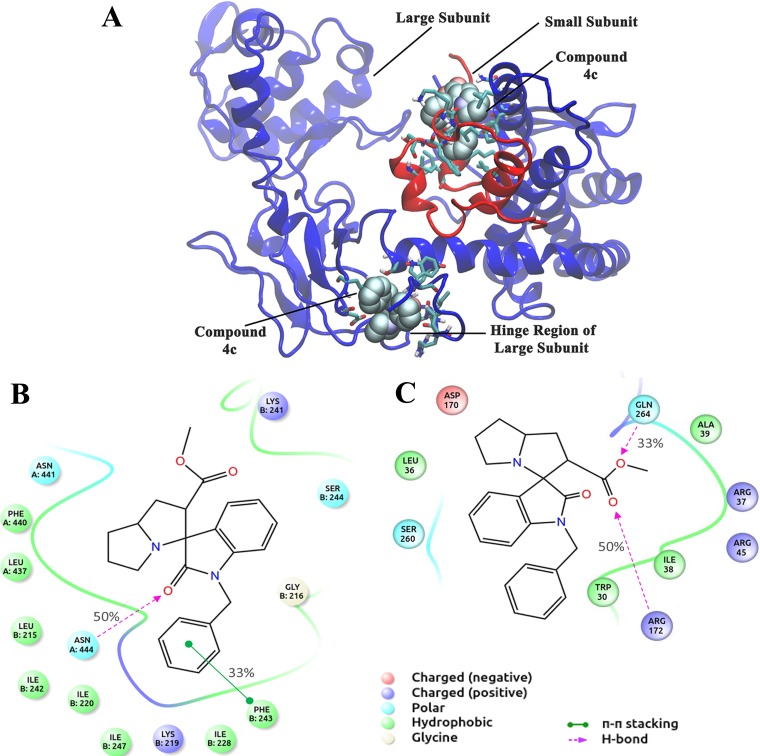
Molecular models of compound 4c complexed with LdTopIB are shown in Fig. 4A. Figures 4B and C show the detailed interactions of the amino acid residues of the two binding sites with compound 4c. A consensus of binding site residues was obtained by comparing the best binding modes of compound 4c with LdTopIB and is shown as the interaction fraction in Fig. 4B and C. Leu-215, Gly-216, Lys-219, Ile-220, Ile-228, Lys-241, Ile-242, Phe-243, and Ile-247 of the small subunit were found to be the most important amino acids in the interaction with compound 4c. Leu-437, Phe-440, Asn-441, and Asn-444 of the large subunit were also involved during this binding to the small subunit of LdTopIB. Hydrophobic, hydrogen-bonding, polar, and electrostatic interactions were the major driving forces in this binding. Important amino acids that interact with compound 4c in the hinge region of LdTopIB include Trp-30, Leu-36, Arg-37, Ala-39, Arg-45, Asp-170, Arg-172, Ser-260, and Gln-264. Hydrophobic, electrostatic, polar, and hydrogen-bonding interactions were the major forces involved.
FIG 4.
Binding analysis of compound 4c with LdTopIB. (A) Binding sites of compound 4c on LdTopIB. Protein is shown as cartoon and the ligand in CPK model. Interacting residues are shown as sticks. (B) Interaction of compound 4c with the small subunit of LdTopIB. Residues with more than a 30% interaction fraction (i.e., common for at least 3 stereoisomers) are shown. (C) Interaction diagram of compound 4c with the hinge region of LdTopIB. Residues with more than a 30% interaction fraction are shown.
Compound 4c inhibits human topoisomerase I at much higher concentrations.
For development of compound 4c as a potent antileishmanial agent targeting LdTopIB, it was necessary to study the effect of this compound on human topoisomerase I. The relaxation experiment was performed under standard assay conditions. It was noteworthy that when incubated simultaneously, compound 4c inhibited human topoisomerase I at much higher concentrations (IC50, 47.11 μM) than in case of LdTopIB (3.29 μM) (data not shown).
Compound 4c shows cytotoxicity against wild-type AG83 as well as against drug-resistant L. donovani promastigotes.
The cytotoxic potentials of these 6 spirooxindole compounds (compounds 4a to f) and four bis-spirooxindoles derivatives (compounds 6a to d) against Leishmania promastigotes were checked using the MTT assay. Treatment of promastigotes with these compounds (100 μM for 12 h) showed that only compound 4c has a cytotoxic effect on the leishmanial parasites (data not shown).
Again promastigotes of the L. donovani wild-type AG83 strain, SAG-resistant GE1 strain, miltefosine-resistant strain, and camptothecin-resistant strain (3.0 × 106 cells/ml) were incubated with 10 different concentrations of compound 4c (0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.25, and 2.5 μM) for 12 h, following which the survival percentage was estimated by MTT assay. Treatment of promastigotes of all the strains with compound 4c demonstrated a dose-dependent cytotoxic effect. The EC50 values of compound 4c against all 4 strains (AG83, GE1, and the MILr and CPTr strains) of L. donovani were calculated using the variable-slope model for finding the EC50 in Prism (version 5.0; GraphPad Software, San Diego, CA), and these are presented in Table 2. Miltefosine was used as a positive control.
TABLE 2.
EC50 and EC90 values for the effect of compound 4c on Leishmania promastigotesa
| Promastigotes | Compound 4c |
Miltefosine EC50 (μM) | Compound 4c selectivity index | |
|---|---|---|---|---|
| EC50 (μM) | EC90 (μM) | |||
| Ag83 | 1.249 ± 0.0010 | 2.007 ± 0.0011 | 19.713 ± 0.0009 | 9.45 |
| GE1 | 1.34 ± 0.0011 | 2.394 ± 0.0012 | 20.218 ± 0.0013 | 8.81 |
| MILr | 1.413 ± 0.0011 | 2.735 ± 0.0012 | >100 | 8.35 |
| CPTr | 1.294 ± 0.0010 | 2.098 ± 0.0011 | 19.831 ± 0.0012 | 9.11 |
All EC50 and EC90 values are presented as mean ± standard error (n = 3).
Next, the mode of cell death in compound 4c-treated wild-type AG83 parasites was investigated. Externalization of phosphatidyl serine (stained by annexin V-FITC) and presence of and impermeant cell membrane (negative PI staining) (see Fig. S2A in the supplemental material), formation of reactive oxygen species (ROS) causing cellular oxidative stress (see Fig. S2B in the supplemental material), loss of mitochondrial membrane potential (ΔΨm) (see Fig. S2C and D in the supplemental material), and fragmentation of genomic DNA (see Fig. S2E in the supplemental material) confirmed that compound 4c-treated cells die via the apoptosis-like pathway.
Compound 4c reduces the presence of intracellular amastigotes of AG83 and other resistant Leishmania strains in cultured murine peritoneal macrophage cells.
Primary macrophage cells were infected with early-passage promastigotes of the L. donovani wild-type AG83 strain, the SAG-resistant GE1 strain, the miltefosine-resistant strain, and the camptothecin-resistant strain in vitro. Infected macrophages were incubated with different concentrations (0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, and 2 μM) of compound 4c for 24 h, and intracellular amastigotes were counted. The EC50s of compound 4c against intracellular AG83, GE1, MILr, and CPTr amastigotes of L. donovani were calculated using the variable-slope model for finding the EC50 in Prism (version 5.0; GraphPad Software, San Diego, CA), and these are given in Table 3. Miltefosine was used as a positive control.
TABLE 3.
EC50 and EC90 values for the effect of compound 4c on Leishmania intracellular amastigotesa
| Amastigotes | Compound 4c |
Miltefosine EC50 (μM) | Compound 4c selectivity index | |
|---|---|---|---|---|
| EC50 (μM) | EC90 (μM) | |||
| Ag83 | 0.513 ± 0.0013 | 1.453 ± 0.0015 | 11.409 ± 0.0016 | 23 |
| GE1 | 0.858 ± 0.0011 | 1.606 ± 0.0018 | 12.543 ± 0.0011 | 13.75 |
| MILr | 0.908 ± 0.0012 | 1.715 ± 0.0019 | >60 | 12.99 |
| CPTr | 0.570 ± 0.0011 | 1.491 ± 0.0013 | 10.934 ± 0.0014 | 20.70 |
All EC50 and EC90 values are presented as mean ± standard error (n = 3).
The effects of compound 4c on cultured murine peritoneal macrophages were determined using the MTT assay. Compound 4c kills macrophages at much higher concentrations. The EC50 and EC90 values were found to be 11.80 μM and 41.95 μM.
Intraperitoneal administration of compound 4c reduces the liver and spleen parasite burdens in a BALB/c mouse model of experimental visceral leishmaniasis.
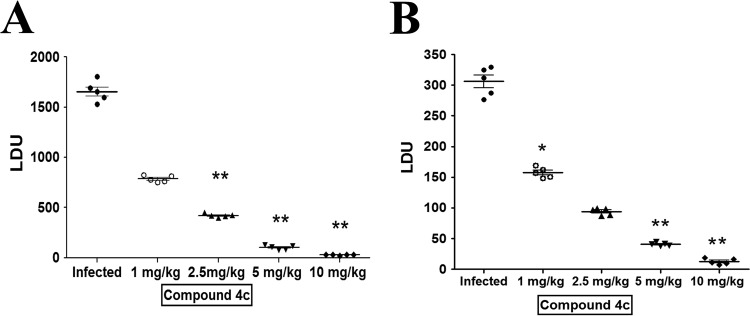
We also studied the effect of compound 4c on the in vivo animal model of L. donovani infection to confirm the in vitro antileishmanial activity of this spirooxindole. After intraperitoneal administration of compound 4c at 1 and 2.5 mg/kg, there were almost 52% and 74% reductions of liver parasite burden (Fig. 5A and B) and 48.5% and 69.3% reductions of splenic parasite load (Fig. 5B), respectively. The leishmanicidal effect was more prominent with 5 and 10 mg/kg of compound 4c, as there were 94% and 98% reductions in hepatic burden and 86.7% and 96% reductions in splenic parasite burden compared to infected mouse controls (Fig. 5A and B). During the study, all mice remained alive and healthy and showed no in vivo toxicity (see Fig. S4 in the supplemental material) and no remarkable change in body weight (data not shown).
FIG 5.
In vivo antileishmanial effect of compound 4c. In vivo leishmanicidal efficacy of compound 4c in BALB/c mice infected with AG83 promastigotes via intracardiac route was determined. Compound 4c was given at a dosage of 1, 2.5, 5, and 10 mg/kg of body weight intraperitoneally for 3 weeks (2 times per week) starting on day 21 after infection. Animals were sacrificed 10 days after treatment, and liver (A) and splenic (B) parasite loads were determined for all groups. Untreated infected mice were used as controls. Liver and spleen parasite burdens were determined by the stamp-smear method and expressed as LDU. Data represent mean ± SEM (n = 5 mice per group). *, P < 0.01; **, P < 0.001 (by Student's t test for different compound 4c treatment groups compared to the infection control).
DISCUSSION
In the present study, we have shown for the first time that a novel spirooxindole derivative, compound 4c, inhibits L. donovani topoisomerase IB (LdTopIB) and effectively kills both promastigote and amastigote forms of wild-type as well as drug-resistant strains of the parasite.
Spirooxindoles constitute a privileged heterocyclic moiety that forms the core of a large number of synthetic and natural compounds (27). Many attempts toward the synthesis of isatin-derived polycyclic compounds having fused pyrrolidine rings have been made (49). Multicomponent 1,3-dipolar cycloaddition reactions are very fruitful synthetic procedures for the construction of these fused five-member heterocyclic ring systems (50). These methodologies offer superior advantages allowing the construction of complex molecular frameworks from easily available starting materials in a single synthetic procedure without the necessity for the isolation of intermediates (51, 52). Synthesis of spirooxindole and bis-spirooxindole derivatives is still a great challenge because of their unnatural fused ring system and structural diversity (53). Here we report the synthesis of six spirooxindoles (among the six compounds, compound 4a, 2,2′α3,3′,5′,6′,7′,7α,α′-octahydro-2-methoxycarbonyl-spiro[indole-3,3′-pyrrolizidine]-2-one, was reported earlier [54]) and four bis-spirooxindoles in a modified route (55) by reacting isatin and l-proline with electron-deficient dipolarophile in acetonitrile at room temperature for 5 to 8 h in the presence of 4-Å MS as an additive (see Data S1 and Tables S1 to S3 in the supplemental material).
Most commonly reported LdTopIB inhibitors (such as camptothecin) stabilize the LdTopIB-DNA cleavage complex (14) and inhibit this enzyme in a noncompetitive manner. The synthesized compound 4c inhibits the relaxation activity of L. donovani topoisomerase IB more effectively under the preincubation condition (Fig. 1D) than under the simultaneous assay condition (Fig. 1C) in vitro. The mode of inhibition by compound 4c suggests that there is some kind of interaction between free LdTopIB and the compound, unlike with CPT. In accordance with the preincubation assay, an enzyme kinetics study revealed that the inhibition mode is of competitive the type; i.e., the compound indeed binds to the free enzyme, whereas CPT binds only to the enzyme-DNA complex. The increase in Km suggests that the affinity of the enzyme toward DNA decreases when enzyme is bound to inhibitor. A topoisomerase reaction has three general mechanistic steps (56), viz., (i) binding of the enzyme to the substrate DNA, (ii) cleavage of one strand by trans-esterification reaction followed by strand rotation (57), leading to a change in linking number by one or more than one, and (iii) strand religation and turnover of the enzyme. The in vitro plasmid cleavage experiment made it clear that this spirooxindole compound 4c does not stabilize the LdTopIB-DNA cleavage complex but rather abrogates camptothecin-mediated cleavable complex formation (Fig. 2A). Cellular studies, i.e., immunoband depletion experiments with nuclear fractions of L. donovani promastigotes using specific antibody raised against LdTopIB, revealed that compound 4c does not induce stabilization of the covalent topoisomerase I-DNA cleavable complex inside parasite cells (Fig. 2B). Further intrinsic fluorescence quenching analysis established an interaction (with KD values in the range of ∼10 to 6 M for LdTopIB) between the free enzyme and compound 4c (Fig. 3C and D). Finally, Job plot analysis established that 2 molecules of compound 4c bind to 1 molecule of LdTopIB; i.e., there is a 2:1 interaction of this inhibitor with LdTopIB (Fig. 3B). Thus, from our biochemical studies, compound 4c can be classified as a “catalytic inhibitor” or a class II-type drug, and it abrogates topoisomerase IB-DNA interaction (i.e., the first step of topoisomerization).
An in silico docking experiment was performed with compound 4c and compound 4a to further support our in vitro biochemical observations and to detect possible binding sites of compound 4c on LdTopIB. Indeed, docking studies revealed two binding sites on the bisubunit enzyme LdTopIB. One possible hit for binding sites is in the small subunit and the other is in the hinge region of the large subunit of LdTopIB. Compared to compound 4a, compound 4c has a benzyl moiety, which gave it inhibitory activity. When compound 4c binds to site one, the benzyl group penetrates the hydrophobic groove between the large and small subunits and forms a pi-stacking interaction with Phe-243 of the small subunit. It also forms strong H bonding with Asn-444 of the large subunit, thus interfering with the relative dynamics of the two subunits. On the other hand, the interaction of compound 4a with site one is relatively weak (Table 1).
The hinge region of topoisomerase IB has important role in opening and closing of the enzyme around DNA and in turn helping the enzyme in attaining precleavage formation (56, 58). Near binding site two (hinge region), there is a hydrophobic cluster formed by three Trp residues (Trp-30 and -31 of the large subunit and Trp-253 of the small subunit) that is critical for the DNA binding and strand rotation step of the catalytic cycle of LdTopIB (58, 59, 60, 61). The benzyl group of compound 4c interacts with this Trp cluster through Trp-30. Therefore, the benzyl moiety of compound 4c appears to be important for its inhibitory actions. Site-directed mutagenesis of the important interacting amino acid residues in both the small subunit and the hinge region of the large subunit can give us further insight into the reaction mechanism by which compound 4c inhibits LdTopIB.
It is widely reported that topoisomerase poisons cause apoptosis-like cell death in the parasite by inducing double-strand breaks in the DNA (62). Treatment with catalytic inhibitors of topoisomerases also sometimes may cause apoptosis-like cell death (47). Catalytic inhibitors bind directly with topoisomerases and prevent them from binding to DNA and executing their cellular functions, i.e., helping in replication and transcription processes. This kind of phenomenon may generate cellular stress and increase cytosolic ROS and may ultimately trigger an apoptosis-like phenomenon (63, 64, 65, 66). The same may be the case with this novel spirooxindole compound 4c, which is a catalytic inhibitor of LdTopIB. Though this compound does not stabilize the LdTopIB-DNA cleavage complex, it is highly cytotoxic and induces apoptosis-like cell death in the parasite. Treatment of the parasite with compound 4c causes depolarization of mitochondrial membrane potential and formation of reactive oxygen species, which triggers cell death inside the parasite. Externalization of phosphatidyl serine (annexin V positive staining) and genomic DNA fragmentation upon treatment with compound 4c supported apoptosis-like cell death in the parasite.
With the recent emergence of L. donovani strains that are resistant to commonly used leishmanicidal agents, viz., SAG and miltefosine, development of better chemotherapeutic agents is important. Compound 4c effectively killed SAG- and miltefosine-resistant parasites with EC50s comparable to those for wild-type AG83 strains. This compound also is highly cytotoxic against parasite strains that are resistant to the topoisomerase-targeting drug camptothecin. Finally, the antileishmanial therapeutic potential of compound 4c was established in the BALB/c mouse model of visceral leishmaniasis. The mouse model mimics all the immunological features of human visceral leishmaniasis within 3 to 4 weeks postinfection and is considered a good model (67). Compound 4c treatment at dosages of 5 and 10 mg/kg nearly eliminated the parasite burdens in the liver and spleen.
From all these data it can be concluded that spirooxindole compound 4c is a potent L. donovani topoisomerase IB catalytic inhibitor and that this compound may be used as a lead to develop more effective and potent leishmanicidal agents against emerging resistant strains.
Supplementary Material
ACKNOWLEDGMENTS
We thank Samit Chattopadhyay, Director of the CSIR-Indian Institute of Chemical Biology, for his interest in this work. We also acknowledge Kshudiram Naskar for help during mouse handling.
This work was supported by the Department of Biotechnology (DBT), Government of India (BT/PR4456/MED/29/355/2012 to H.K.M and P.J.), and the Department of Science and Technology (DST) Indo-Brazil project (DST/INT/Brazil/RPO-01/2009/2) and Council of Scientific and Industrial Research (CSIR), New Delhi, India, in the form of Network Project CSC-0108 (to P.J.). S.S. is financially supported by a senior research fellowship from Council of Scientific and Industrial Research, Government of India (CSIR-SRF).
We have no conflicts of interest to declare.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00352-16.
REFERENCES
- 1.Wang JC. 1996. DNA topoisomerases. Annu Rev Biochem 65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- 2.Wang JC. 2002. Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol 3:430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- 3.Pommier Y, Leo E, Zhang H, Marchand C. 2010. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol 17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen AK, Escargueil AE, Skladanowski A. 2003. Catalytic topoisomerase II inhibitors in cancer therapy. Pharmacol Ther 99:167–181. doi: 10.1016/S0163-7258(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 5.Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, Alvar J, Boelaert M. 2007. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol 5:873–882. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 6.Nagill R, Kaur S. 2011. Vaccine candidates for leishmaniasis: a review. Int Immunopharmacol 11:1464–1488. doi: 10.1016/j.intimp.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Wortmann G, Zapor M, Ressner R, Fraser S, Hartzell J, Pierson J, Weintrob A, Magill A. 2010. Lipsosomal amphotericin B for treatment of cutaneous leishmaniasis. Am J Trop Med Hyg 83:1028–1033. doi: 10.4269/ajtmh.2010.10-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahman M, Ahmed BN, Faiz MA, Chowdhury MZ, Islam QT, Sayeedur R, Rahman MR, Hossain M, Bangali AM, Ahmad Z, Islam MN, Mascie-Taylor CG, Berman J, Arana B. 2011. Phase IV trial of Miltefosine in adults and children for treatment of visceral leishmaniasis (kala-azar) in Bangladesh. Am J Trop Med Hyg 85:66–69. doi: 10.4269/ajtmh.2011.10-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashutosh Sundar S, Goyal N. 2007. Molecular mechanisms of antimony resistance in Leishmania. J Med Microbiol 56:143–153. doi: 10.1099/jmm.0.46841-0. [DOI] [PubMed] [Google Scholar]
- 10.Das A, Sengupta T, Dasgupta A, Majumder HK. 2004. Topoisomerases of kinetoplastid parasites as potential chemotherapeutic targets. Trends Parasitol 20:381–387. doi: 10.1016/j.pt.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Villa H, Otero-Marcos AR, Reguera RM, Balaña-Fouce R, García-Estrada C, Pérez-Pertejo Y, Tekwani BL, Myler PJ, Stuart KD, Bjornsti MA, Ordóñez D. 2003. A novel active DNA topoisomerase I in Leishmania donovani. J Biol Chem 278:3521–3526. doi: 10.1074/jbc.M203991200. [DOI] [PubMed] [Google Scholar]
- 12.Bodley A, Chakraborty AK, Xie S, Burri C, Shapiro TA. 2003. An unusual type IB topoisomerase from African trypanosomes. Proc Natl Acad Sci U S A 100:7539–7544. doi: 10.1073/pnas.1330762100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das BB, Sen N, Ganguly A, Majumder HK. 2004. Reconstitution and functional characterization of the unusual bi-subunit type I DNA topoisomerase from Leishmania donovani. FEBS Lett 565:81–88. doi: 10.1016/j.febslet.2004.03.078. [DOI] [PubMed] [Google Scholar]
- 14.Bodley AL, Shapiro TA. 1995. Molecular and cytotoxic effects of camptothecin a topoisomerase I inhibitor, on trypanosomes and Leishmania. Proc Natl Acad Sci U S A 92:3726–3730. doi: 10.1073/pnas.92.9.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ray S, Hazra B, Mittra B, Das A, Majumder HK. 1998. Diospyrin, a bisnaphthoquinone: a novel inhibitor of type I DNA topoisomerase of Leishmania donovani. Mol Pharmacol 54:994–999. [DOI] [PubMed] [Google Scholar]
- 16.Roy A, Das BB, Ganguly A, Bose Dasgupta S, Khalkho NV, Pal C, Dey S, Giri VS, Jaisankar P, Dey S, Majumder HK. 2008. An insight into the mechanism of inhibition of unusual bi-subunit topoisomerase I from Leishmania donovani by 3,3′-di-indolylmethane, a novel DNA topoisomerase I poison with a strong binding affinity to the enzyme. Biochem J 409:611–622. doi: 10.1042/BJ20071286. [DOI] [PubMed] [Google Scholar]
- 17.Das BB, Sen N, Roy A, Dasgupta SB, Ganguly A, Mohanta BC, Dinda B, Majumder HK. 2006. Differential induction of Leishmania donovani bi-subunit topoisomerase I-DNA cleavage complex by selected flavones and camptothecin: activity of flavones against camptothecin-resistant topoisomerase I. Nucleic Acids Res 34:1121–1132. doi: 10.1093/nar/gkj502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowdhury S, Mukherjee T, Mukhopadhyay R, Mukherjee B, Sengupta S, Chattopadhyay S, Jaisankar P, Roy S, Majumder HK. 2012. The lignan niranthin poisons Leishmania donovani topoisomerase IB and favours a Th1 immune response in mice. EMBO Mol Med 4:1126–1143. doi: 10.1002/emmm.201201316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saha S, Mukherjee T, Chowdhury S, Mishra A, Chowdhury SR, Jaisankar P, Mukhopadhyay S, Majumder HK. 2013. The lignan glycosides lyoniside and saracoside poison the unusual type IB topoisomerase of Leishmania donovani and kill the parasite both in vitro and in vivo. Biochem Pharmacol 86:1673–1687. doi: 10.1016/j.bcp.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Chowdhury AR, Mandal S, Goswami A, Ghosh M, Mandal L, Chakraborty D, Ganguly A, Tripathi G, Mukhopadhyay S, Bandyopadhyay S, Majumder HK. 2003. Dihydrobetulinic acid induces apoptosis in Leishmania donovani by targeting DNA topoisomerase I and II: implications in antileishmanial therapy. Mol Med 9:26–36. [PMC free article] [PubMed] [Google Scholar]
- 21.Chowdhury S, Mukherjee T, Sengupta S, Chowdhury SR, Mukhopadhyay S, Majumder HK. 2011. Novel betulin derivatives as antileishmanial agents with mode of action targeting type IB DNA topoisomerase. Mol Pharmacol 80:694–703. doi: 10.1124/mol.111.072785. [DOI] [PubMed] [Google Scholar]
- 22.Akerman KJ, Fagenson AM, Cyril V, Taylor M, Muller MT, Akerman MP, Munro OQ. 2014. Gold(III) macrocycles: nucleotide-specific unconventional catalytic inhibitors of human topoisomerase I. J Am Chem Soc 136:5670–5682. doi: 10.1021/ja412350f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu N, Wu XW, Agama K, Pommier Y, Du J, Li D, Gu LQ, Huang ZS, An LK. 2010. A novel DNA topoisomerase I inhibitor with different mechanism from camptothecin induces G2/M phase cell cycle arrest to K562 cells. Biochemistry 49:10131–10136. doi: 10.1021/bi1009419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pommier Y. 2013. Drugging topoisomerases: lessons and challenges. ACS Chem Biol 8:82–95. doi: 10.1021/cb300648v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown CJ, Lain S, Chandra S, Fersht AR, Lane DP. 2009. Awakening guardian angels: drugging the p53 pathway. Nature Rev Cancer 9:862–873. doi: 10.1038/nrc2763. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Wang N, Li C, Jia X. 2012. Multicomponent reaction to construct spirocyclic oxindoles with a Michael (triple Michael)/cyclization cascade sequence as the key step. Chemistry 18:9645–9650. doi: 10.1002/chem.201104071. [DOI] [PubMed] [Google Scholar]
- 27.Lashgari N, Ziarani GM. 2012. Synthesis of heterocyclic compounds based on isatin through 1,3-dipolar cycloaddition reactions. Arch Org Chem 1:277–320. [Google Scholar]
- 28.Greshock TJ, Grubbs AW, Jiao P, Wicklow DT, Gloer JB, Williams RM. 2008. Isolation, structure elucidation, and biomimetic total synthesis of versicolamide B, and the isolation of antipodal (−)-stephacidin A and (+)-notoamide B from Aspergillus versicolor NRRL 35600. Angew Chem Int Ed Engl 47:3573–3577. doi: 10.1002/anie.200800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venkatesan H, Davis MC, Altas Y, Snyder JP, Liotta DC. 2001. Total synthesis of SR 121463 A, a highly potent and selective vasopressin v(2) receptor antagonist. J Org Chem 66:3653–3661. doi: 10.1021/jo0004658. [DOI] [PubMed] [Google Scholar]
- 30.Monteiro Â, Gonçalves LM, Santos MM. 2014. Synthesis of novel spiropyrazoline oxindoles and evaluation of cytotoxicity in cancer cell lines. Eur J medicinal chemistry 79:266–272. doi: 10.1016/j.ejmech.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 31.Williams RM, Cao J, Tsujishima H. 2000. Asymmetric, stereocontrolled total synthesis of paraherquamide A. Angew Chem Int Ed Engl 39:2540–2544. doi:. [DOI] [PubMed] [Google Scholar]
- 32.Ding K, Lu Y, Nikolovska-Coleska Z, Ding Y, Gao W, Stuckey J, Krajewski K, Roller PP, Tomita Y, Parrish DA, Deschamps JR, Wang S. 2005. Structure-based design of potent non-peptide MDM2 inhibitors. J Am Chem Soc 127:10130–10131. doi: 10.1021/ja051147z. [DOI] [PubMed] [Google Scholar]
- 33.Kumar A, Gupta G, Bishnoi AK, Saxena R, Saini KS, Konwar R, Kumar S, Dwivedi A. 2015. Design and synthesis of new bioisosteres of spirooxindoles (MI-63/219) as anti-breast cancer agents. Bioorg Med Chem 23:839–848. doi: 10.1016/j.bmc.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 34.Rottmann M, McNamara C, Yeung BK, Lee MC, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J, Cohen SB. 2010. Spiroindolones, a potent compound class for the treatment of malaria. Science 329:1175–1180. doi: 10.1126/science.1193225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scala A, Cordaro M, Grassi G, Piperno A, Barberi G, Cascio A, Risitano F. 2014. Direct synthesis of C3-mono-functionalized oxindoles from N-unprotected 2-oxindole and their antileishmanial activity. Bioorg Med Chem 22:1063–1069. doi: 10.1016/j.bmc.2013.12.039. [DOI] [PubMed] [Google Scholar]
- 36.Osheroff N, Shelton ER, Brutlag DL. 1983. DNA topoisomerase II from Drosophila melanogaster: relaxation of supercoiled DNA. J Biol Chem 258:9536–9543. [PubMed] [Google Scholar]
- 37.Sen N, Das BB, Ganguly A, Mukherjee T, Tripathi G, Bandyopadhyay S, Rakshit S, Sen T, Majumder HK. 2004. Camptothecin induced mitochondrial dysfunction leading to programmed cell death in unicellular hemoflagellate Leishmania donovani. Cell Death Differ 11:924–936. doi: 10.1038/sj.cdd.4401435. [DOI] [PubMed] [Google Scholar]
- 38.Huang CY. 1982. Determination of binding stoichiometry by the continuous variation method: the Job plot. Methods Enzymol 87:509–525. doi: 10.1016/S0076-6879(82)87029-8. [DOI] [PubMed] [Google Scholar]
- 39.Ward LD. 1985. Measurement of ligand binding to proteins by fluorescence spectroscopy. Methods Enzymol 117:400–414. doi: 10.1016/S0076-6879(85)17024-2. [DOI] [PubMed] [Google Scholar]
- 40.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. 2009. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR. 2012. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminformatics 4:17. doi: 10.1186/1758-2946-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trott O, Olson AJ. 2010. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ. 2005. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res 33:W363–W367. doi: 10.1093/nar/gki481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mashiach E, Schneidman-Duhovny D, Andrusier N, Nussinov R, Wolfson HJ. 2008. FireDock: a web server for fast interaction refinement in molecular docking. Nucleic Acids Res 36:W229–W232. doi: 10.1093/nar/gkn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grosdidier A, Zoete V, Michielin O. 2011. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res 39:W270–W277. doi: 10.1093/nar/gkr366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basu R, Bhaumik S, Basu JM, Naskar K, De T, Roy S. 2005. Kinetoplastid membrane protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and -resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: evidence for mixed Th1- and Th2-like responses in visceral leishmaniasis. J Immunol 174:7160–7171. doi: 10.4049/jimmunol.174.11.7160. [DOI] [PubMed] [Google Scholar]
- 47.Chowdhury S, Mukherjee T, Chowdhury SR, Sengupta S, Mukhopadhyay S, Jaisankar P, Majumder HK. 2014. Disuccinyl betulin triggers metacaspase-dependent endonuclease G-mediated cell death in unicellular protozoan parasite Leishmania donovani. Antimicrob Agents Chemother 58:2186–2201. doi: 10.1128/AAC.02193-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stauber AL. 1956. Resistance to the Khartoum strain of Leishmania donovani. Rice Inst Pam 45:80–96. [Google Scholar]
- 49.Galliford CV, Scheidt KA. 2007. Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents. Angew Chem Int Ed Eng 46:8748–8758. doi: 10.1002/anie.200701342. [DOI] [PubMed] [Google Scholar]
- 50.Marti C, Careira EM. 2003. Construction of spiro[pyrrolidine-3,3′-oxindoles]—recent applications to the synthesis of oxindole alkaloids. Eur J Org Chem 2003:2209. doi: 10.1002/ejoc.200300050. [DOI] [Google Scholar]
- 51.Mamari AK, Ennajih H, Zouihri H, Bouhfid R, Ng SW, Essassi EM. 2012. Synthesis of novel dispiro-oxindoles via 1,3-dipolar cycloaddition reactions of azomethine ylides. Tetrahedron Lett 53:2328–2331. doi: 10.1016/j.tetlet.2012.02.097. [DOI] [Google Scholar]
- 52.Padwa A. (ed). 1984. 1,3-Dipolar cycloaddition chemistry, vol 1 and 2. Wiley, New York, NY. [Google Scholar]
- 53.Waldmann H. 1995. Amino acid esters: versatile chiral auxiliary groups for the asymmetric synthesis of nitrogen heterocycles. Synlett 1995:133–141. doi: 10.1055/s-1995-4882. [DOI] [Google Scholar]
- 54.Ardill H, Dorrity MJR, Grigg R, Leon-Ling MS, Malone JF, Sridharan V, Thianpatanagul S. 1990. X=Y-ZH compounds as potential 1,3-dipoles. 28. The iminium ion route to azomethine ylides. Background and reaction of amines with bifunctional ketones Tetrahedron 46:6433–6448. [Google Scholar]
- 55.Babu ARS, Raghunathan R. 2007. Ultrasonic assisted-silica mediated [3+2] cycloaddition of azomethine ylides—a facile multicomponent one-pot synthesis of novel dispiroheterocycles. Tetrahedron Lett 48:6809–6813. doi: 10.1016/j.tetlet.2007.07.085. [DOI] [Google Scholar]
- 56.Stewart L, Redinbo MR, Qiu X, Hol WG, Champoux JJ. 1998. A model for the mechanism of human topoisomerase I. Science 279:1534–1541. doi: 10.1126/science.279.5356.1534. [DOI] [PubMed] [Google Scholar]
- 57.Koster DA, Croquette V, Dekker C, Shuman S, Dekker NH. 2005. Friction and torque govern the relaxation of DNA supercoils by eukaryotic topoisomerase IB. Nature 434:671–674. doi: 10.1038/nature03395. [DOI] [PubMed] [Google Scholar]
- 58.Das BB, Sen N, Dasgupta SB, Ganguly A, Majumder HK. 2005. N-terminal region of the large subunit of Leishmania donovani bisubunit topoisomerase I is involved in DNA relaxation and interaction with the smaller subunit. J Biol Chem 280:16335–16344. doi: 10.1074/jbc.M412417200. [DOI] [PubMed] [Google Scholar]
- 59.Lisby M, Olesen JR, Skouboe C, Krogh BO, Straub T, Boege F, Velmurugan S, Martensen PM, Andersen AH, Jayaram M, Westergaard O, Knudsen BR. 2001. Residues within the N-terminal domain of human topoisomerase I play a direct role in relaxation. J Biol Chem 276:20220–20227. doi: 10.1074/jbc.M010991200. [DOI] [PubMed] [Google Scholar]
- 60.Frohlich RF, Andersen FF, Westergaard O, Andersen AH, Knudsen BR. 2004. Regions within the N-terminal domain of human topoisomerase I exert important functions during strand rotation and DNA binding. J Mol Biol 336:93–103. doi: 10.1016/j.jmb.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Frohlich RF, Veigaard C, Andersen FF, McClendon AK, Gentry AC, Andersen AH, Osheroff N, Stevnsner T, Knudsen BR. 2007. Tryptophane-205 of human topoisomerase I is essential for camptothecin inhibition of negative but not positive supercoil removal. Nucleic Acids Res 35:6170–6180. doi: 10.1093/nar/gkm669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pommier Y. 2006. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer 6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 63.Ganguly A, Das B, Roy A, Sen N, Dasgupta SB, Mukhopadhayay S, Majumder HK. 2007. Betulinic acid, a catalytic inhibitor of topoisomerase I, inhibits reactive oxygen species mediated apoptotic topoisomerase I-DNA cleavable complex formation in prostate cancer cells but does not affect the process of cell death. Cancer Res 67:11848–11858. doi: 10.1158/0008-5472.CAN-07-1615. [DOI] [PubMed] [Google Scholar]
- 64.Li CJ, Averboukh L, Pardee AB. 1993. β-Lapachone, a novel DNA topoisomerase I inhibitor with a mode of action different from camptothecin. J Biol Chem 268:22463–22468. [PubMed] [Google Scholar]
- 65.Bentle MS, Reinicke KE, Bey EA, Spitz DR, Boothman DA. 2006. Calcium-dependent modulation of poly(ADPribose) polymerase-1 alters cellular metabolism and DNA repair. J Biol Chem 281:33684–33696. doi: 10.1074/jbc.M603678200. [DOI] [PubMed] [Google Scholar]
- 66.Capranico G, Marinello J, Baranello L. 2010. Dissecting the transcriptional functions of human DNA topoisomerase I by selective inhibitors: implications for physiological and therapeutic modulation of enzyme activity. Biochim Biophys Acta 1806:240–250. doi: 10.1016/j.bbcan.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Melby PC, Chandrasekar B, Zhao W, Coe JE. 2001. The hamster as a model of human visceral leishmaniasis: progressive disease and impaired generation of nitric oxide in the face of a prominent Th1-like cytokine response. J Immunol 166:1912–1920. doi: 10.4049/jimmunol.166.3.1912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.