Abstract
Daptomycin (DAP) is being used more frequently to treat infections caused by vancomycin-resistant enterococcus (VRE). DAP tends to be less active against enterococci than staphylococci and may require high doses or combination therapy to be bactericidal. Fosfomycin (FOF) has activity against VRE and has demonstrated synergistic bactericidal activity with DAP in vitro. The objective of this study was to evaluate the activity of DAP alone and in combination with FOF against VRE in an in vitro pharmacokinetic/pharmacodynamic (PK/PD) model. The activity of DAP at 8 and 12 mg/kg of body weight/day (DAP 8 and DAP 12, respectively) and FOF of 40 mg/kg intravenously every 8 h, alone and in combination, were evaluated against 2 vancomycin-resistant Enterococcus faecium strains (8019 and 5938) and 2 vancomycin-resistant E. faecalis strains (V583 and R7302) in an in vitro PK/PD model over 72 h. Cell surface charge in the presence and absence of FOF was evaluated by zeta potential analysis. Daptomycin-boron-dipyrromethene (bodipy) binding was assessed by fluorescence microscopy. The addition of FOF to DAP 8 and DAP 12 resulted in significantly increased killing over DAP alone at 72 h for 8019, V583, and R7302 (P < 0.05). Therapeutic enhancement was observed with DAP 12 plus FOF against 8019, V583, and R7302. Cell surface charge became more negative after exposure to FOF by ∼2 to 8mV in all 4 strains. Daptomycin-bodipy binding increased by 2.6 times in the presence of fosfomycin (P < 0.0001). The combination of DAP plus FOF may provide improved killing against VRE (including DAP-resistant strains) through modulation of cell surface charge. Further studies to clarify the role of intravenous FOF are warranted.
INTRODUCTION
Daptomycin (DAP) is a cationic cyclic lipopeptide demonstrating rapid bactericidal activity against Gram-positive bacteria, including vancomycin-resistant Enterococcus (VRE), through disruption of electrochemical membrane potential (1). Fosfomycin (FOF) is a phosphonic acid derivative that inhibits peptidoglycan synthesis in both Gram-negative and Gram-positive organisms. It displays activity against methicillin-resistant Staphylococcus aureus (MRSA), multidrug-resistant Streptococcus pneumoniae, Enterococcus spp., including vancomycin-resistant strains, and Gram-negative bacteria, including extended-spectrum β-lactamase (ESBL)-producing Enterobacter (2–4). The following MIC interpretive criteria have been established by the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST): Enterococcus faecalis (urinary isolates only) at ≤64, 128, and ≥256 mg/liter as susceptible (S), intermediate (I), and resistant (R), respectively, by CLSI; Staphylococcus spp. at ≤32 and ≥32 mg/liter as S and R by EUCAST (2, 5; http://www.eucast.org). It primarily undergoes renal elimination and is indicated for treatment of uncomplicated urinary tract infection (UTI) caused by Escherichia coli and Enterococcus faecalis (2, 6). Although an oral preparation approved for urinary tract infections is the only fosfomycin product available in the United States, the intravenous formulation has been used outside the United States for treatment of severe systemic infections (7). Fosfomycin doses of 3 g orally have been well established to treat UTIs, and bloodstream infections have been treated with fosfomycin at 50 mg/kg of body weight three to four times daily in combination with a β-lactam agent (4, 8, 9). Other studies have utilized fosfomycin doses of 2 g every 6 or 8 h (10, 11). Developed in 1969, fosfomycin has drawn increasing attention for use in multidrug-resistant bacterial infections as combination therapy due to the increase in antimicrobial resistance and lack of novel antimicrobial development (6, 12, 13). While most of the data on fosfomycin-based antimicrobial combinations come from small in vitro studies, clinical trials are ongoing in Europe to evaluate the combination of daptomycin and intravenous fosfomycin for treatment of MRSA bacteremia (11).
VRE has been associated with increased clinical failure rates, and reports of clinical failure or resistance to newer antimicrobial agents, including daptomycin, are emerging (14–19). One large, retrospective cohort study found 2% of isolates developed nonsusceptibility to daptomycin, even when doses of >6 mg/kg/day were administered (20). Daptomycin and fosfomycin appear to be a promising combination for eradication of resistant Gram-positive infections. Several in vitro studies have demonstrated synergism. One study evaluated clinical strains by checkerboard and subsequently time-kill methods and found synergism against Enterococcus spp. (21). An additional study demonstrated significantly improved killing in vitro when combined with daptomycin against clinical isolates of vancomycin-resistant E. faecium than with either agent alone (22).
The mechanism of synergy between these agents is unknown. Synergy between daptomycin and beta-lactams is believed to be mediated through beta-lactam-induced alterations in surface charge that facilitate an increase in daptomycin binding, leading to enhanced membrane depolarization (23–28). Since fosfomycin also disrupts cell wall synthesis, it could be theorized that there is a similar mechanism that drives the synergy that has been observed with daptomycin and fosfomycin.
The purpose of this study was to evaluate the potential for synergistic effects of fosfomycin in combination with high-dose daptomycin against strains of vancomycin-resistant E. faecium and E. faecalis in an in vitro pharmacokinetic/pharmacodynamic (PK/PD) model simulating clinically relevant drug exposures.
(This study was presented in part at the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 2012, and the 23rd European Congress of Clinical Microbiology, Berlin, Germany, 2013.)
MATERIALS AND METHODS
Bacterial strains.
Four vancomycin-resistant enterococcus strains were evaluated: a clinical isogenic E. faecium strain pair (1 daptomycin-resistant [5939] and 1 daptomycin-susceptible [8019] strain) and 2 daptomycin-susceptible E. faecalis strains (V583 and R7203).
Antimicrobials and media.
DAP and FOF analytical powder were commercially purchased (Sigma-Aldrich, St. Louis, MO). Due to the calcium-dependent mechanism of DAP, Mueller-Hinton broth II supplemented to contain 50 μg/ml calcium and 12.5 μg/ml magnesium (SMHB) (Difco, Detroit, MI) was used for PK/PD models with DAP and FOF. For FOF-containing simulations, SMHB was supplemented with 25 μg/ml glucose-6-phosphate (G-6-P; Sigma-Aldrich, St. Louis, MO), which is a necessary cofactor for active transmembrane transport of FOF. Colony counts were determined using brain heart infusion agar (BHIA; Difco, Detroit, MI) plates. BHIA supplemented with 50 μg/ml of calcium or 25 μg/ml of G-6-P was used for antimicrobial resistance screening plates containing DAP or FOF, respectively. Mueller-Hinton agar (MHA) supplemented with 25 μg/ml of G-6-P was used for FOF bioassays.
Susceptibility testing.
MICs of DAP and FOF were determined in duplicate by Etest methodology at ∼1.5 × 108 CFU/ml according to the manufacturer's recommendations. All samples were incubated at 37°C for 18 to 24 h. Development of nonsusceptibility was evaluated at 72 h for all simulated regimens. Samples (100 μl) were plated on FOF- or DAP-containing agar plates at 3× the MIC.
In vitro PK/PD model.
An in vitro, one-compartment PK/PD model with a 250-ml capacity and inflow and outflow ports was used. The apparatus was prefilled with medium, and antimicrobials were administered as boluses over a 72-h time period. Prior to each experiment, bacterial lawns from an overnight growth on BHI were suspended and added to each model to obtain a starting inoculum of ∼108 CFU/ml. The model apparatus was maintained at 37°C throughout the experiment, and a magnetic stir bar was placed in the medium for thorough mixing of the drug in the model. Fresh medium was continuously supplied and removed from the compartment along with the drug via a peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, IL) at an appropriate rate to simulate the average human half-lives of the antimicrobials. A total of 5 simulated regimens were evaluated on each isolate for 72 h: daptomycin at 8 mg/kg (termed DAP 8) every 24 h (q24h) (maximum concentration of free drug in serum [fCmax], 9.86 mg/liter; average half-life [t1/2], 8 h; protein binding, 92%) (29), DAP at 8 mg/kg q24h plus FOF at 40 mg/kg q8h (fCmax, 260 mg/liter; average t1/2, 2.3 h) (30), daptomycin at 12 mg/kg (DAP 12) q24h (fCmax, 14.7 mg/liter) (29), DAP at 12 mg/kg q 24 h plus FOF at 40 mg/kg q8h, and FOF at 40 mg/kg q8h. A growth control was also run for each isolate to verify isolate fitness. Models were performed in duplicate to ensure reproducibility. Supplemental DAP was added at an appropriate rate to FOF combination models to compensate for the higher flow rate required to simulate FOF clearance (31).
Pharmacodynamic analysis.
Samples from each model were collected at 0, 4, 8, 24, 32, 48, 56, and 72 h and diluted in 0.9% saline. Colony counts were determined by spiral plating appropriate dilutions using an automatic spiral plater (WASP; DW Scientific, West Yorkshire, England) to enumerate CFU/ml and avoid antibiotic carryover. Colonies were counted using an automated colony counter (ProtoCOL; Synoptics Limited, Frederick, MD). If the anticipated dilution was near the MIC, then vacuum filtration was also used to avoid antibiotic carryover. When vacuum filtration was used, samples were washed through a 0.45-μm filter with normal saline to remove the antimicrobial agent. For both methods, plates were incubated at 37°C for 18 to 24 h, at which time colony counts were performed. These methods have a lower limit of reliable detection of 1 log10 CFU/ml.
The total reduction in log10 CFU/ml over 72 h was determined by plotting time-kill curves based on the number of remaining organisms over the time period. Bactericidal activity (99.9% kill) and bacteriostatic activity were defined as a ≥3-log10 CFU/ml or a <3-log10 CFU/ml reduction, respectively, in colony count from the initial inoculum. Inactivity was defined as no observed reductions in initial inocula. The time to achieve a 99.9% bacterial load reduction was determined by linear regression (if r2 ≥ 0.95) or visual inspection. The effects of the antimicrobial combinations were interpreted as follows. Enhancement of activity was defined as an increase in kill of ≥2-log10 CFU/ml by the combination of antimicrobials versus the most active single agent of that combination. Improvement was defined as a 1- to 2-log10 CFU/ml increase in kill compared to the most active single agent, while combinations that result in a ≥1-log10 CFU/ml bacterial growth compared to the least active single agent were considered antagonistic (32, 33).
Pharmacokinetic analysis.
Pharmacokinetic samples were obtained through the injection port of each model at 0, 1, 2, 4, 8, 24, 32, 48, 56, and 72 h for verification of target antibiotic concentrations. All samples were stored at −70°C until ready for analysis. FOF concentrations were determined by bioassay using Escherichia coli (ATCC 25922). Blank 1/4-inch disks were spotted with 10 μl of standard concentrations or samples. Each standard was tested in duplicate by placing the disk on agar plates (MHA) inoculated with a 0.5 McFarland suspension of the test organism. This assay demonstrated an intraday coefficient of variance of less than 10% for all standards. Concentrations of daptomycin were determined using a validated high-performance liquid chromatography assay (34–36). This assay has demonstrated an interday coefficient of variation between 0.6 and 7.3% for all standards. The free antimicrobial peak concentrations, half-lives, and free 24-h area under the concentration-time curve (fAUC0–24 h) were determined using PK Analyst software (version 1.10; MicroMath Scientific Software, Salt Lake City, UT). The trapezoidal method was utilized to calculate fAUC0–24 h.
Zeta potential analysis.
Changes in cell surface charge in the presence and absence of subinhibitory concentrations of FOF were measured using a zeta potential analyzer. Briefly, cells were grown overnight in 5 ml of MHB supplemented with 25 mg/liter of G-6-P and 0.5× the MIC of FOF. Cell suspensions were adjusted to an optical density at 600 nm (OD600) of 0.5 and then harvested by centrifugation. Cell pellets were washed 3 times and resuspended in sterile water. Zeta potential was measured using a Brookhaven Instruments ZetaPlus zeta potential analyzer. Results are averages from 10 measurements per organism per exposure. Experiments were conducted on 3 different days.
Daptomycin-bodipy binding studies.
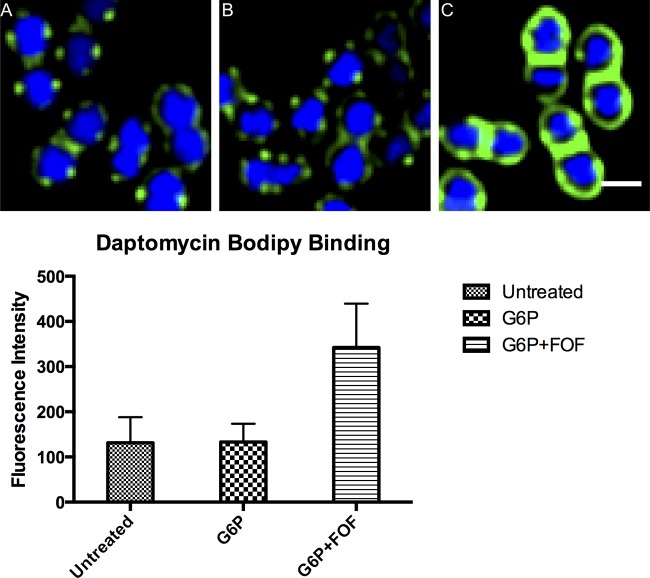
Daptomycin binding was assessed by fluorescence microscopy in the presence and absence of fosfomycin and G-6-P as previously described (24). Briefly, cells were grown to an optical density at 600 nm (OD600) of 0.6, grown for an additional 1 h with or without G-6-P (25 mg/liter) and G-6-P plus FOF (260 mg/liter), and then incubated with 16 mg/liter daptomycin-boron-dipyrromethene (bodipy) for 10 min. Cells were then washed three times in medium to remove unincorporated label, stained with 1 mg/liter 4′,6-diamidino-2-phenylindole (DAPI), and imaged as previously described prior to fluorescence quantification.
Statistical analysis.
Changes in CFU/ml at 24, 48, and 72 h were compared by one-way analysis of variance with Tukey's post hoc test. Zeta potential values and fluorescence intensity of daptomycin-bodipy binding were compared by student's t test. A P value of ≤0.05 was considered significant. All statistical analyses were performed using SPSS Statistical Software (release 20.0; SPSS, Inc., Chicago, IL).
RESULTS
Susceptibility testing and resistance.
Organism MICs to DAP and FOF were 2 and 64, 32 and 128, 1 and 64, and 1 and 32 mg/liter for 8019, 5938, V583, and R7302, respectively. Elevated MICs were observed in 8019 with the DAP 12 and DAP 12 plus FOF simulated regimens (DAP MIC of 16 and 8 mg/liter, respectively). V583 developed nonsusceptibility to DAP (DAP MIC, 16 mg/liter) in DAP 8 and 12 monotherapy regimens. No change in MIC to DAP was observed in R7302. High-level resistance to FOF (MIC of 1,028 mg/liter) developed against all 3 susceptible isolates when utilized as monotherapy.
In vitro PK/PD model.
The average pharmacokinetic parameters observed for FOF were fCmax of 257.9 ± 13.2 mg/liter and half-life of 1.9 ± 0.12 h (targeted, 260 mg/liter and 2.3 h). The average pharmacokinetic parameters observed for DAP 8 were fCmax of 10.22 ± 0.16 mg/liter and half-life of 8.13 ± 0.04 h (targeted, 9.86 mg/liter and 8 h), and for DAP 12 they were fCmax of 14.1 ± 0.12 mg/liter and half-life of 8.98 ± 0.43 h (targeted, 14.7 mg/liter and 8 h).
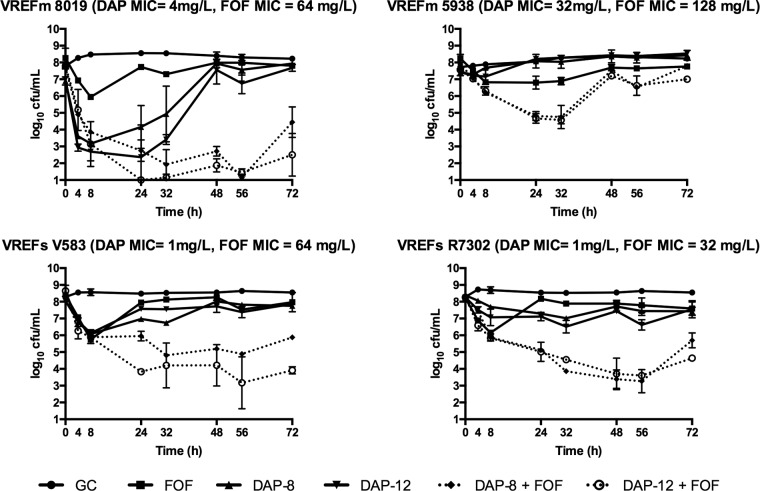
The in vitro killing activity for simulated antimicrobial regimens is summarized in Table 1. The novel combination of FOF and DAP 12 was significantly more active by 72 h (P ≤ 0.05) than either regimen alone for all isolates examined (Fig. 1). This combination also displayed significantly more activity than monotherapy at 24 h against 5938, V583, and R7302 (P ≤ 0.05). DAP 12 plus FOF demonstrated rapid bactericidal activity sustained to 72 h against 8019, V583, and R7302. Enhancement was observed with the addition of FOF to DAP 12 against all isolates at 24 h and against 8019, V583, and R7302 at 72 h. Improvement was displayed against all isolates with DAP 8 and FOF over either agent alone at 24 h, and this was sustained to 72 h with the exception of 5938. Killing was most impressive against 8019, and the addition of FOF prevented the regrowth seen with DAP 8 and DAP 12 alone. The reduction in log10 CFU/ml demonstrated by DAP 8 in combination with FOF demonstrated an effect similar to that of the combination with DAP 12, but the DAP 8 combination was unable to sustain bactericidal activity against the two E. faecalis strains (V583 and R7302). FOF alone was less active than DAP monotherapy in this model, with the exception of the DAP-nonsusceptible mutant 5938. No regimen achieved bactericidal activity against 5938; however, DAP 12 with FOF resulted in significantly lower colony counts than monotherapy.
TABLE 1.
In vitro activity of DAP alone or in combination with FOF in the one-compartment PK/PD model
| Regimena | Activity (log10 CFU/ml) by strain and time pointb |
|||||||
|---|---|---|---|---|---|---|---|---|
| 8019 |
5938 |
V583 |
R7302 |
|||||
| T24 | T72 | T24 | T72 | T24 | T72 | T24 | T72 | |
| FOF | 7.73 ± 0.06 | 7.81 ± 0.01 | 6.81 ± 0.39 | 7.76 ± 0.06 | 7.95 ± 0.24 | 7.97 ± 0.57 | 8.17 ± 0.07 | 7.60 ± 0.39 |
| D8 | 4.17 ± 1.28 | 7.94 ± 0.06 | 8.08 ± 0.41 | 8.43 ± 0.30 | 6.98 ± 0.12 | 7.75 ± 0.00 | 7.29 ± 0.04 | 7.43 ± 0.24 |
| D12 | 2.36 ± 1.92 | 7.68 ± 0.21 | 8.21 ± 0.08 | 8.54 ± 0.05 | 7.57 ± 0.01 | 7.87 ± 0.02 | 7.11 ± 0.24 | 7.55 ± 0.51 |
| D8+FOF | 2.75 ± 0.64 | 4.45 ± 0.91* | 4.81 ± 0.26* | 7.79 ± 0.04 | 3.51 ± 0.35* | 5.88 ± 0.04* | 5.13 ± 0.02* | 5.69 ± 0.44* |
| D12+FOF | 1 ± 0.00 | 2.5 ± 1.27* | 4.67 ± 0.28* | 7 ± 0.17* | 3.58 ± 0.06* | 3.91 ± 0.21* | 5.01 ± 0.57* | 4.64 ± 0.02* |
D8, DAP 8; D12; DAP 12; D8+FOF, DAP 8 with FOF; D12+FOF, DAP 12 with FOF.
T24, 24-h time point; T72, 72-h time point. An asterisk indicates significantly greater log10 CFU/ml reduction over monotherapy (P ≤ 0.05).
FIG 1.
Activity of simulated antimicrobial regimens containing daptomycin at 8 mg/kg (DAP-8), daptomycin at 12 mg/kg (DAP-12), fosfomycin (FOF), or growth control (GC) against each isolate in the in vitro one-compartment PK/PD model.
Zeta potential analysis.
Mean cell surface charge of cells in the presence and absence of subinhibitory concentrations of FOF is depicted in Fig. 2. FOF exposure caused significant reductions in surface charge (4.1 to 5.6 mV) for all 4 strains (P < 0.01). Zeta potential of unexposed cells was positively correlated with the DAP MICs (R2 = 0.97), indicating that the more susceptible strains had a more negatively charged cell surface than less susceptible ones.
FIG 2.
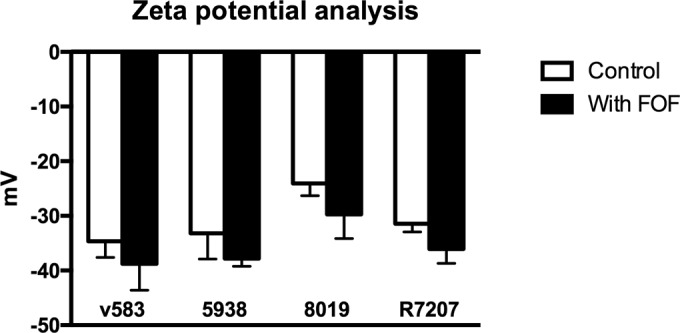
Zeta potential of tested isolates in the presence or absence of fosfomycin.
Daptomycin-bodipy binding studies.
Daptomycin-bodipy binding studies are illustrated in Fig. 3. Daptomycin-bodipy binding increased by 2.6 times in the presence of fosfomycin and G-6-P (P < 0.0001). There was no change in daptomycin binding observed when cells were exposed to G-6-P.
FIG 3.
Daptomycin-bodipy binding to Enterococcus faecium 8019 alone (A) and in the presence of 25 μg/ml of glucose-6-phosphate (G6P) (B) or G6P and fosfomycin (FOF) (C). Cells were stained with DNA-staining dye DAPI (blue) and bodipy-daptomycin (green). Scale bar, 1 μm.
DISCUSSION
Vancomycin-resistant enterococci cause deep-seated, difficult-to-treat infections that have been associated with increased mortality compared to infections caused by vancomycin-susceptible enterococci (37). These therapeutically challenging infections are frequently complicated by resistance to multiple antimicrobials. Daptomycin is one of the only agents to offer bactericidal activity against VRE, but dose optimization remains a challenge (38). The general consensus among experts is that high doses of daptomycin (greater than 8 mg/kg/day) are necessary to achieve clinical success in the setting of serious enterococcal infections, and emerging data indicate that doses of ≥10 mg/kg/day are necessary for resistance prevention (20, 38–42). Resistance to daptomycin in VRE is an emerging threat that requires immediate attention to derive new therapeutic strategies, such as dose optimization and combination therapy, in order to preserve the clinical utility of this drug.
Our study evaluated the activity of daptomycin in combination with fosfomycin against clinical vancomycin-resistant E. faecium and E. faecalis isolates. The combination of daptomycin and fosfomycin may provide improved killing against daptomycin-nonsusceptible vancomycin-resistant E. faecium and E. faecalis; however, this activity was not sustained in our model. It is of interest that all simulated regimens were less effective against vancomycin-resistant E. faecalis strains than against vancomycin-resistant E. faecium relative to the daptomycin and fosfomycin MIC. This may be related to other adaptations, such as enhanced biofilm production in the E. faecalis strains. Of interest, the fosfomycin-containing regimens prevented the emergence of daptomycin nonsusceptibility in 2/3 daptomycin-susceptible strains, whereas an increase in MIC was seen with daptomycin alone. Similar effects have been seen in methicillin-resistant S. aureus, where the addition of fosfomycin to daptomycin delayed the emergence of resistance to daptomycin in vitro (43). Fosfomycin exposure appeared to reduce cell surface charge, which suggests that the mechanism of synergy is similar to that seen with beta-lactams and daptomycin against enterococci and Staphylococcus aureus (27, 44, 45). Support for this hypothesis comes from recent data, which demonstrate that fosfomycin appears to reduce PBP1 expression in S. aureus (10). PBP1 expression is increased with daptomycin exposure and is believed to be important in the bacterial compensatory response in response to peptide-induced injury (46). Thus, either pharmacologic antagonism of PBP1, as seen with beta-lactam antibiotics, or compromising expression of key compensatory proteins like PBP1 by fosfomycin may be a foundation for increasing daptomycin-mediate bacterial killing efficiency.
Daptomycin susceptibility changes have been found to be associated with changes in cell surface charge in E. faecium and E. faecalis (47, 48). Consistent with a previous study which demonstrated 8019 to be more negatively charged than 5938 through cytochrome c binding and increased susceptibility to human cathelicidin LL37 killing (48), we observed a lower zeta potential in 8019, indicating a more negatively charged surface of 8019 than of 5938. The addition of fosfomycin resulted in a further reduction of cell surface charge, which is a plausible explanation for the enhanced killing demonstrated when combined with daptomycin. VRE resistance to daptomycin has previously been linked to genetic changes in genes that regulate cell envelope homeostasis and membrane lipid metabolism (liaFSR, yycFG, cls, and cfa); however, there appears to be a variety of genetic pathways to daptomycin nonsusceptibility (49–55). In addition to altered cell surface charge, an alternative mechanism that has been proposed is related to redistribution of membrane lipids that drive daptomycin binding away from division septa, where it is believed to be more devastating to the organism (53, 55, 56). Mutations of the phosphotransferase system (PTS), previously associated with bacteriocin resistance in E. faecium and E. faecalis, may also contribute to daptomycin nonsusceptibility in E. faecium (48, 57). This finding is especially interesting in the context of the synergy between fosfomycin and daptomycin since the phosphotransferase system plays a role in G-6-P transport, which is an important cofactor for fosfomycin transport. The nature of the relationship between the mutations in this enzyme system observed in daptomycin nonsusceptible strains and the activity of fosfomycin is unclear but warrants further exploration.
As with other published studies with a similar design, this study has some limitations. Due to the practical limitations of in vitro modeling, we simulated one-compartment pharmacokinetics of the study drugs, which may have slightly overestimated their average clinical exposures. The simplification of multicompartmental clinical pharmacokinetics is common in in vitro modeling, even when the investigators use a two-compartment model, such as a simulated endocardial vegetation model or a hollow-fiber model (58–60). While these strategies may modestly overestimate the drug exposures in the model, due to the short distribution phase of these drugs, which was not simulated, we do not believe this had a significant impact on the results. Other studies have successfully utilized a similar model to describe the pharmacokinetic-pharmacodynamic effect of fosfomycin (61). The primary objective of this study was to evaluate the pharmacodynamic interactions between daptomycin and fosfomycin, and we believe that the subtle differences in actual versus simulated pharmacokinetics should have little impact on the overall pharmacodynamics of this combination or the interpretation of the results of this study.
Conclusions.
Daptomycin plus fosfomycin demonstrated sustained bactericidal activity against daptomycin-susceptible vancomycin-resistant E. faecium in vitro and may provide improved pharmacodynamics against daptomycin-nonsusceptible, vancomycin-resistant E. faecium and E. faecalis. Further research is warranted to evaluate the activity of this combination against other strains of VRE, further elucidate the mechanism(s) of synergy, and determine the potential role for this combination in a clinical setting.
ACKNOWLEDGMENTS
M.J.R. has received research support, consulted or participated in speaking for Allergan, Bayer, Cempra, Merck, The Medicine Company, Sunovian, and Theravance, and is supported in part through funding by the National Institutes of Health (R21 AI109266 and R01 AI121400). A.D.S. is an employee of Merck & Co., Inc., and was a postdoctoral fellow at Wayne State during the time this research was conducted. B.J.W. has received research funding from Merck and Allergan. G.S. has received research grant support from Allergan Pharmaceuticals, speaking honoraria from Merck, Allergan, Sunovion, and The Medicines Company, and consulting fees from The Medicines Company. J.P. has an equity interest in Linnaeus Bioscience, Inc., and receives consulting income from the company. The terms of this arrangement have been reviewed and approved by the University of California, San Diego, in accordance with its conflict of interest policies.
P.N., J.P.M., and K.G. have no potential conflicts of interest to disclose.
This work was partially supported by NIH grant AI113295 to J.P.
REFERENCES
- 1.Sader HS, Moet GJ, Farrell DJ, Jones RN. 2011. Antimicrobial susceptibility of daptomycin and comparator agents tested against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: trend analysis of a 6-year period in US medical centers (2005-2010). Diagn Microbiol Infect Dis 70:412–416. doi: 10.1016/j.diagmicrobio.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Lu CL, Liu CY, Huang YT, Liao CH, Teng LJ, Turnidge JD, Hsueh PR. 2011. Antimicrobial susceptibilities of commonly encountered bacterial isolates to fosfomycin determined by agar dilution and disk diffusion methods. Antimicrob Agents Chemother 55:4295–4301. doi: 10.1128/AAC.00349-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perri MB, Hershberger E, Ionescu M, Lauter C, Zervos MJ. 2002. In vitro susceptibility of vancomycin-resistant enterococci (VRE) to fosfomycin. Diagn Microbiol Infect Dis 42:269–271. doi: 10.1016/S0732-8893(02)00370-X. [DOI] [PubMed] [Google Scholar]
- 4.Roussos N, Karageorgopoulos DE, Samonis G, Falagas ME. 2009. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int J Antimicrob Agents 34:506–515. doi: 10.1016/j.ijantimicag.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing; 26th informational supplement. CLSI document M100-S26. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 6.Michalopoulos AS, Livaditis IG, Gougoutas V. 2011. The revival of fosfomycin. Int J Infect Dis 15:e732–e739. doi: 10.1016/j.ijid.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Rosso-Fernandez C, Sojo-Dorado J, Barriga A, Lavin-Alconero L, Palacios Z, Lopez-Hernandez I, Merino V, Camean M, Pascual A, Rodriguez-Bano J, Forest Study Group. 2015. Fosfomycin versus meropenem in bacteraemic urinary tract infections caused by extended-spectrum beta-lactamase-producing Escherichia coli (FOREST): study protocol for an investigator-driven randomised controlled trial. BMJ Open 5:e007363. doi: 10.1136/bmjopen-2014-007363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portier H, Tremeaux JC, Chavanet P, Gouyon JB, Duez JM, Kazmierczak A. 1984. Treatment of severe staphylococcal infections with cefotaxime and fosfomycin in combination. J Antimicrob Chemother 14(Suppl B):277–284. doi: 10.1093/jac/14.suppl_B.277. [DOI] [PubMed] [Google Scholar]
- 9.Neu HC. 1990. Fosfomycin trometamol versus amoxycillin–single-dose multicenter study of urinary tract infections. Chemotherapy 36(Suppl 1):S19–S23. [DOI] [PubMed] [Google Scholar]
- 10.del Rio A, Garcia-de-la-Maria C, Entenza JM, Gasch O, Armero Y, Soy D, Mestres CA, Pericas JM, Falces C, Ninot S, Almela M, Cervera C, Gatell JM, Moreno A, Moreillon P, Marco F, Miro JM, Hospital Clinic Experimental Endocarditis Study Group. 2016. Fosfomycin plus beta-lactams as synergistic bactericidal combinations for experimental endocarditis due to methicillin-resistant and glycopeptide-intermediate Staphylococcus aureus. Antimicrob Agents Chemother 60:478–486. doi: 10.1128/AAC.02139-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw E, Miro JM, Puig-Asensio M, Pigrau C, Barcenilla F, Murillas J, Garcia-Pardo G, Espejo E, Padilla B, Garcia-Reyne A, Pasquau J, Rodriguez-Bano J, Lopez-Contreras J, Montero M, de la Calle C, Pintado V, Calbo E, Gasch O, Montejo M, Salavert M, Garcia-Pais MJ, Carratala J, Pujol M, Spanish Network for Research in Infectious Diseases, GEIH. 2015. Daptomycin plus fosfomycin versus daptomycin monotherapy in treating MRSA: protocol of a multicentre, randomised, phase III trial. BMJ Open 5:e006723. doi: 10.1136/bmjopen-2014-006723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linasmita P. 2012. Successful management of methicillin-resistant Staphylococcus aureus bacteremia unresponsive to vancomycin by adding fosfomycin: a case report. J Med Assoc Thailand 95:960–963. [PubMed] [Google Scholar]
- 13.Miro JM, Entenza JM, Del Rio A, Velasco M, Castaneda X, Garcia de la Maria C, Giddey M, Armero Y, Pericas JM, Cervera C, Mestres CA, Almela M, Falces C, Marco F, Moreillon P, Moreno A, Hospital Clinic Experimental Endocarditis Study Group. 2012. High-dose daptomycin plus fosfomycin is safe and effective in treating methicillin-susceptible and methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother 56:4511–4515. doi: 10.1128/AAC.06449-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munoz-Price LS, Lolans K, Quinn JP. 2005. Emergence of resistance to daptomycin during treatment of vancomycin-resistant Enterococcus faecalis infection. Clin Infect Dis 41:565–566. doi: 10.1086/432121. [DOI] [PubMed] [Google Scholar]
- 15.Arias CA, Torres HA, Singh KV, Panesso D, Moore J, Wanger A, Murray BE. 2007. Failure of daptomycin monotherapy for endocarditis caused by an Enterococcus faecium strain with vancomycin-resistant and vancomycin-susceptible subpopulations and evidence of in vivo loss of the vanA gene cluster. Clin Infect Dis 45:1343–1346. doi: 10.1086/522656. [DOI] [PubMed] [Google Scholar]
- 16.Lewis JS II, Owens A, Cadena J, Sabol K, Patterson JE, Jorgensen JH. 2005. Emergence of daptomycin resistance in Enterococcus faecium during daptomycin therapy. Antimicrob Agents Chemother 49:1664–1665. doi: 10.1128/AAC.49.4.1664-1665.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long JK, Choueiri TK, Hall GS, Avery RK, Sekeres MA. 2005. Daptomycin-resistant Enterococcus faecium in a patient with acute myeloid leukemia. Mayo Clin Proc 80:1215–1216. doi: 10.4065/80.9.1215. [DOI] [PubMed] [Google Scholar]
- 18.King ST, Usery JB, Holloway K, Koeth L, Cleveland KO, Gelfand MS. 2011. Successful therapy of treatment-emergent, non-clonal daptomycin-non-susceptible Enterococcus faecium infections. J Antimicrob Chemother 66:2673–2675. doi: 10.1093/jac/dkr343. [DOI] [PubMed] [Google Scholar]
- 19.Kelesidis T, Chow AL, Humphries R, Uslan DZ, Pegues D. 2012. Case-control study comparing de novo and daptomycin-exposed daptomycin-nonsusceptible Enterococcus infections. Antimicrob Agents Chemother 56:2150–2152. doi: 10.1128/AAC.05918-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casapao AM, Kullar R, Davis SL, Levine DP, Zhao JJ, Potoski BA, Goff DA, Crank CW, Segreti J, Sakoulas G, Cosgrove SE, Rybak MJ. 2013. High-dose daptomycin for treatment of enterococcal infections: a multicenter study. Antimicrob Agents Chemother 57:4190–4196. doi: 10.1128/AAC.00526-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Debbia E, Pesce A, Schito GC. 1988. In vitro activity of LY146032 alone and in combination with other antibiotics against gram-positive bacteria. Antimicrob Agents Chemother 32:279–281. doi: 10.1128/AAC.32.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Descourouez JL, Jorgenson MR, Wergin JE, Rose WE. 2013. Fosfomycin synergy in vitro with amoxicillin, daptomycin, and linezolid against vancomycin-resistant Enterococcus faecium from renal transplant patients with infected urinary stents. Antimicrob Agents Chemother 57:1518–1520. doi: 10.1128/AAC.02099-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakoulas G, Rose W, Nonejuie P, Olson J, Pogliano J, Humphries R, Nizet V. 2014. Ceftaroline restores daptomycin activity against daptomycin-nonsusceptible vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 58:1494–1500. doi: 10.1128/AAC.02274-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Werth BJ, Sakoulas G, Rose WE, Pogliano J, Tewhey R, Rybak MJ. 2013. Ceftaroline increases membrane binding and enhances the activity of daptomycin against daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus in a pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 57:66–73. doi: 10.1128/AAC.01586-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta S, Singh C, Plata KB, Chanda PK, Paul A, Riosa S, Rosato RR, Rosato AE. 2012. Beta-lactams increase the antibacterial activity of daptomycin against clinical methicillin-resistant Staphylococcus aureus strains and prevent selection of daptomycin-resistant derivatives. Antimicrob Agents Chemother 56:6192–6200. doi: 10.1128/AAC.01525-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhand A, Bayer AS, Pogliano J, Yang SJ, Bolaris M, Nizet V, Wang G, Sakoulas G. 2011. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin Infect Dis 53:158–163. doi: 10.1093/cid/cir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakoulas G, Bayer AS, Pogliano J, Tsuji BT, Yang SJ, Mishra NN, Nizet V, Yeaman MR, Moise PA. 2012. Ampicillin enhances daptomycin- and cationic host defense peptide-mediated killing of ampicillin- and vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 56:838–844. doi: 10.1128/AAC.05551-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall Snyder A, Werth BJ, Barber KE, Sakoulas G, Rybak MJ. 2014. Evaluation of the novel combination of daptomycin plus ceftriaxone against vancomycin-resistant enterococci in an in vitro pharmacokinetic/pharmacodynamic simulated endocardial vegetation model. J Antimicrob Chemother 69:2148–2154. doi: 10.1093/jac/dku113. [DOI] [PubMed] [Google Scholar]
- 29.Benvenuto M, Benziger DP, Yankelev S, Vigliani G. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob Agents Chemother 50:3245–3249. doi: 10.1128/AAC.00247-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goto M, Sugiyama M, Nakajima S, Yamashina H. 1981. Fosfomycin kinetics after intravenous and oral administration to human volunteers. Antimicrob Agents Chemother 20:393–397. doi: 10.1128/AAC.20.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blaser J. 1985. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J Antimicrob Chemother 15(Suppl A):125–130. doi: 10.1093/jac/15.suppl_A.125. [DOI] [PubMed] [Google Scholar]
- 32.Vidaillac C, Leonard SN, Sader HS, Jones RN, Rybak MJ. 2009. In vitro activity of ceftaroline alone and in combination against clinical isolates of resistant gram-negative pathogens, including beta-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 53:2360–2366. doi: 10.1128/AAC.01452-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuji BT, Rybak MJ. 2005. Short-course gentamicin in combination with daptomycin or vancomycin against Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother 49:2735–2745. doi: 10.1128/AAC.49.7.2735-2745.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dvorchik BH, Brazier D, DeBruin MF, Arbeit RD. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob Agents Chemother 47:1318–1323. doi: 10.1128/AAC.47.4.1318-1323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kullar R, Chin JN, Edwards DJ, Parker D, Coplin WM, Rybak MJ. 2011. Pharmacokinetics of single-dose daptomycin in patients with suspected or confirmed neurological infections. Antimicrob Agents Chemother 55:3505–3509. doi: 10.1128/AAC.01741-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martens-Lobenhoffer J, Kielstein JT, Oye C, Bode-Boger SM. 2008. Validated high performance liquid chromatography-UV detection method for the determination of daptomycin in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 875:546–550. doi: 10.1016/j.jchromb.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Carmeli Y, Eliopoulos G, Mozaffari E, Samore M. 2002. Health and economic outcomes of vancomycin-resistant enterococci. Arch Intern Med 162:2223–2228. doi: 10.1001/archinte.162.19.2223. [DOI] [PubMed] [Google Scholar]
- 38.Rivera AM, Boucher HW. 2011. Current concepts in antimicrobial therapy against select gram-positive organisms: methicillin-resistant Staphylococcus aureus, penicillin-resistant pneumococci, and vancomycin-resistant enterococci. Mayo Clin Proc 86:1230–1243. doi: 10.4065/mcp.2011.0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werth BJ, Steed ME, Ireland CE, Tran TT, Nonejuie P, Murray BE, Rose WE, Sakoulas G, Pogliano J, Arias CA, Rybak MJ. 2014. Defining daptomycin resistance prevention exposures in vancomycin-resistant Enterococcus faecium and E. faecalis. Antimicrob Agents Chemother 58:5253–5261. doi: 10.1128/AAC.00098-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall AD, Steed ME, Arias CA, Murray BE, Rybak MJ. 2012. Evaluation of standard- and high-dose daptomycin versus linezolid against vancomycin-resistant Enterococcus isolates in an in vitro pharmacokinetic/pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother 56:3174–3180. doi: 10.1128/AAC.06439-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carugati M, Bayer AS, Miro JM, Park LP, Guimaraes AC, Skoutelis A, Fortes CQ, Durante-Mangoni E, Hannan MM, Nacinovich F, Fernandez-Hidalgo N, Grossi P, Tan RS, Holland T, Fowler VG Jr, Corey RG, Chu VH, International Collaboration on Endocarditis. 2013. High-dose daptomycin therapy for left-sided infective endocarditis: a prospective study from the international collaboration on endocarditis. Antimicrob Agents Chemother 57:6213–6222. doi: 10.1128/AAC.01563-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kullar R, Casapao AM, Davis SL, Levine DP, Zhao JJ, Crank CW, Segreti J, Sakoulas G, Cosgrove SE, Rybak MJ. 2013. A multicentre evaluation of the effectiveness and safety of high-dose daptomycin for the treatment of infective endocarditis. J Antimicrob Chemother 68:2921–2926. doi: 10.1093/jac/dkt294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berti AD, Wergin JE, Girdaukas GG, Hetzel SJ, Sakoulas G, Rose WE. 2012. Altering the proclivity towards daptomycin resistance in methicillin-resistant Staphylococcus aureus using combinations with other antibiotics. Antimicrob Agents Chemother 56:5046–5053. doi: 10.1128/AAC.00502-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakoulas G, Nonejuie P, Nizet V, Pogliano J, Crum-Cianflone N, Haddad F. 2013. Treatment of high-level gentamicin-resistant Enterococcus faecalis endocarditis with daptomycin plus ceftaroline. Antimicrob Agents Chemother 57:4042–4045. doi: 10.1128/AAC.02481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Werth BJ, Steed ME, Kaatz GW, Rybak MJ. 2013. Evaluation of ceftaroline activity against heteroresistant vancomycin-intermediate Staphylococcus aureus and vancomycin-intermediate methicillin-resistant S. aureus strains in an in vitro pharmacokinetic/pharmacodynamic model: exploring the “seesaw effect”. Antimicrob Agents Chemother 57:2664–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berti AD, Theisen E, Sauer JD, Nonejuie P, Olson J, Pogliano J, Sakoulas G, Nizet V, Proctor RA, Rose WE. 2016. Penicillin binding protein 1 is important in the compensatory response of Staphylococcus aureus to daptomycin-induced membrane damage and is a potential target for beta-lactam-daptomycin synergy. Antimicrob Agents Chemother 60:451–458. doi: 10.1128/AAC.02071-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arias CA, Panesso D, McGrath DM, Qin X, Mojica MF, Miller C, Diaz L, Tran TT, Rincon S, Barbu EM, Reyes J, Roh JH, Lobos E, Sodergren E, Pasqualini R, Arap W, Quinn JP, Shamoo Y, Murray BE, Weinstock GM. 2011. Genetic basis for in vivo daptomycin resistance in enterococci. N Engl J Med 365:892–900. doi: 10.1056/NEJMoa1011138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Humphries RM, Kelesidis T, Tewhey R, Rose WE, Schork N, Nizet V, Sakoulas G. 2012. Genotypic and phenotypic evaluation of the evolution of high-level daptomycin nonsusceptibility in vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 56:6051–6053. doi: 10.1128/AAC.01318-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reyes J, Panesso D, Tran TT, Mishra NN, Cruz MR, Munita JM, Singh KV, Yeaman MR, Murray BE, Shamoo Y, Garsin D, Bayer AS, Arias CA. 2015. A liaR deletion restores susceptibility to daptomycin and antimicrobial peptides in multidrug-resistant Enterococcus faecalis. J Infect Dis 211:1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munita JM, Mishra NN, Alvarez D, Tran TT, Diaz L, Panesso D, Reyes J, Murray BE, Adachi JA, Bayer AS, Arias CA. 2014. Failure of high-dose daptomycin for bacteremia caused by daptomycin-susceptible Enterococcus faecium harboring LiaSR substitutions. Clin Infect Dis 59:1277–1280. doi: 10.1093/cid/ciu642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diaz L, Tran TT, Munita JM, Miller WR, Rincon S, Carvajal LP, Wollam A, Reyes J, Panesso D, Rojas NL, Shamoo Y, Murray BE, Weinstock GM, Arias CA. 2014. Whole-genome analyses of Enterococcus faecium isolates with diverse daptomycin MICs. Antimicrob Agents Chemother 58:4527–4534. doi: 10.1128/AAC.02686-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mishra NN, Bayer AS, Tran TT, Shamoo Y, Mileykovskaya E, Dowhan W, Guan Z, Arias CA. 2012. Daptomycin resistance in enterococci is associated with distinct alterations of cell membrane phospholipid content. PLoS One 7:e43958. doi: 10.1371/journal.pone.0043958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller C, Kong J, Tran TT, Arias CA, Saxer G, Shamoo Y. 2013. Adaptation of Enterococcus faecalis to daptomycin reveals an ordered progression to resistance. Antimicrob Agents Chemother 57:5373–5383. doi: 10.1128/AAC.01473-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Munita JM, Tran TT, Diaz L, Panesso D, Reyes J, Murray BE, Arias CA. 2013. A liaF codon deletion abolishes daptomycin bactericidal activity against vancomycin-resistant Enterococcus faecalis. Antimicrob Agents Chemother 57:2831–2833. doi: 10.1128/AAC.00021-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tran TT, Panesso D, Gao H, Roh JH, Munita JM, Reyes J, Diaz L, Lobos EA, Shamoo Y, Mishra NN, Bayer AS, Murray BE, Weinstock GM, Arias CA. 2013. Whole-genome analysis of a daptomycin-susceptible enterococcus faecium strain and its daptomycin-resistant variant arising during therapy. Antimicrob Agents Chemother 57:261–268. doi: 10.1128/AAC.01454-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tran TT, Panesso D, Mishra NN, Mileykovskaya E, Guan Z, Munita JM, Reyes J, Diaz L, Weinstock GM, Murray BE, Shamoo Y, Dowhan W, Bayer AS, Arias CA. 2013. Daptomycin-resistant Enterococcus faecalis diverts the antibiotic molecule from the division septum and remodels cell membrane phospholipids. mBio 4:e00281-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hechard Y, Pelletier C, Cenatiempo Y, Frere J. 2001. Analysis of sigma(54)-dependent genes in Enterococcus faecalis: a mannose PTS permease (EII(Man)) is involved in sensitivity to a bacteriocin, mesentericin Y105. Microbiology 147:1575–1580. doi: 10.1099/00221287-147-6-1575. [DOI] [PubMed] [Google Scholar]
- 58.Belley A, Arhin FF, Sarmiento I, Deng H, Rose W, Moeck G. 2013. Pharmacodynamics of a simulated single 1,200-milligram dose of oritavancin in an in vitro pharmacokinetic/pharmacodynamic model of methicillin-resistant staphylococcus aureus infection. Antimicrob Agents Chemother 57:205–211. doi: 10.1128/AAC.01428-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parra-Ruiz J, Bravo-Molina A, Pena-Monje A, Hernandez-Quero J. 2012. Activity of linezolid and high-dose daptomycin, alone or in combination, in an in vitro model of Staphylococcus aureus biofilm. J Antimicrob Chemother 67:2682–2685. doi: 10.1093/jac/dks272. [DOI] [PubMed] [Google Scholar]
- 60.Bhalodi AA, Hagihara M, Nicolau DP, Kuti JL. 2014. In vitro pharmacodynamics of human simulated exposures of ceftaroline and daptomycin against MRSA, hVISA, and VISA with and without prior vancomycin exposure. Antimicrob Agents Chemother 58:672–677. doi: 10.1128/AAC.01516-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.VanScoy BD, McCauley J, Ellis-Grosse EJ, Okusanya OO, Bhavnani SM, Forrest A, Ambrose PG. 2015. Exploration of the pharmacokinetic-pharmacodynamic relationships for fosfomycin efficacy using an in vitro infection model. Antimicrob Agents Chemother 59:7170–7177. doi: 10.1128/AAC.04955-14. [DOI] [PMC free article] [PubMed] [Google Scholar]