Abstract
Vancomycin has been associated with acute kidney injury (AKI). However, the pharmacokinetic/toxicodynamic relationship for AKI is not well defined. Allometrically scaled vancomycin exposures were used to assess the relationship between vancomycin exposure and AKI. Male Sprague-Dawley rats received clinical-grade vancomycin in normal saline (NS) as intraperitoneal (i.p.) injections for 24- to 72-h durations with doses ranging 0 to 200 mg/kg of body weight divided once or twice daily. Urine was collected over the protocol's final 24 h. Renal histopathology was qualitatively scored. Urinary biomarkers (e.g., cystatin C, clusterin, kidney injury molecule 1 [KIM-1], osteopontin, lipocalin 2/neutrophil gelatinase-associated lipocalin 2) were assayed using a Luminex xMAP system. Plasma vancomycin concentrations were assayed by high-performance liquid chromatography with UV detection. A three-compartment vancomycin pharmacokinetic model was fit to the data with the Pmetrics package for R. The exposure-response in the first 24 h was evaluated using Spearman's nonparametric correlation coefficient (rs) values for the area under the concentration-time curve during the first 24 h (AUC0–24), the maximum concentration in plasma during the first 24 h (Cmax0–24), and the lowest (minimum) concentration in plasma after the dose closest to 24 h (Cmin0–24). A total of 52 rats received vancomycin (n = 42) or NS (n = 10). The strongest exposure-response correlations were observed between AUC0–24 and Cmax0–24 and urinary AKI biomarkers. Exposure-response correlations (rs values) for AUC0–24, Cmax0–24, and Cmin0–24 were 0.37, 0.39, and 0.22, respectively, for clusterin; 0.42, 0.45, and 0.26, respectively, for KIM-1; and 0.52, 0.55, and 0.42, respectively, for osteopontin. However, no differences in histopathological scores were observed. Optimal sampling times after administration of the i.p. dose were 0.25, 0.75, 2.75, and 8 h for the once-daily dosing schemes and 0.25, 1.25, 14.5, and 17.25 h for the twice-daily dosing schemes. Our observations suggest that AUC0–24 or Cmax0–24 correlates with increases in urinary AKI biomarkers.
INTRODUCTION
Acute kidney injury (AKI) is a major contributor to patient morbidity and mortality in the hospital setting (1, 2). While the etiology of AKI is multifactorial, many cases among hospital patients are related to medication exposure (2). Not surprisingly, the risk of drug-induced AKI is highest among critically ill, hospitalized patients (3), who carry multiple risk factors for the development and progression of AKI. Vancomycin, the antibiotic most frequently administered in the hospital setting (4), has been implicated as a cause of AKI in a number of clinical (5–7) and animal (8–10) studies. Among the clinical studies, the incidence of vancomycin-induced AKI has been associated with higher doses of vancomycin (11, 12), increasing numbers of vancomycin doses (12), and elevated trough concentrations (7, 13).
While a number of clinical studies have shown that more intensive vancomycin dosing regimens are associated with an increased risk of AKI, these studies could only suggest an association with kidney injury. As these studies were largely observational in nature, it is difficult to discern if the association was reflective of a true effect or was biased due to confounders. For example, patient confounder factors, such as severity of illness, residence in an intensive care unit, and concurrent receipt of nephrotoxins, may influence the vancomycin exposure-response profile that best predicts clinical AKI.
Animal systems are ideally suited to define exposure-response relationships, as they provide flexibility to titrate dosing groups and minimize the influence of external covariates on the observed results. To date, animal models of AKI have confirmed that vancomycin is a nephrotoxin. Dose-ranging studies have revealed that an increase in the vancomycin dose and an increase in the duration of treatment in rats are associated with increases in histopathological damage and elevations in novel urinary biomarkers of AKI (8–10). However, a prospectively derived exposure-AKI (i.e., a pharmacokinetic [PK]/toxicodynamic [TD]) threshold for vancomycin has not been defined. Being cognizant of this knowledge gap, we sought to evaluate vancomycin exposure-response relationships for elevations in several sensitive urinary AKI biomarkers in a paired PK/TD animal study of vancomycin-induced AKI. Secondarily, we sought to define the optimal sampling times for determination of the PKs of vancomycin. These data are important for future animal work, as they will reduce the number of blood draws needed to estimate the vancomycin exposure profile with low bias and high precision.
MATERIALS AND METHODS
This PK/TD study was conducted at Midwestern University, in Downers Grove, IL. The study methods were reviewed and approved by the Midwestern University Institutional Animal Care and Use Committee (IACUC; protocol number 2295).
Experimental design and animals.
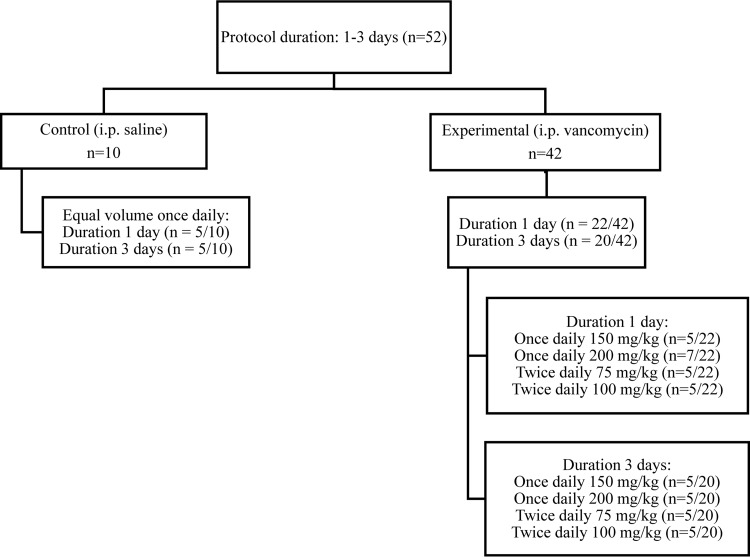
The PK/TD experimental design is summarized in Fig. 1. Animals were divided between experimental (i.e., vancomycin-treated) and control protocol arms. Experimental animals received intraperitoneal (i.p.) injections of clinical-grade vancomycin (Hospira, Lake Forest, IL) in normal saline (NS), and control animals received equivalent volumes of NS as i.p. injections. Experimental and control animals were further enrolled into protocols with 1- and 3-day durations. Finally, experimental animals were divided into allometrically scaled daily doses (i.e., 150 and 200 mg/kg of body weight) and dose fractionation (i.e., once or twice daily) groups. All together there were 2 control groups and 8 treatment groups. The rationale for the choice of these doses was partially based on previous studies that found that these doses produce some renal dysfunction while avoiding overt kidney failure or death in animals (8, 9, 14–16). Likewise, when the principle of allometry is applied, the human equivalent daily dose of vancomycin for rat doses of 150 and 200 mg/kg would approximate to 24.2 and 32.3 mg/kg, respectively, which are doses that are routinely administered according to current clinical practice guidelines (17, 18).
FIG 1.
Allocation of experimental animals across experimental protocols. The experimental flow diagram of animal allocation is according to the duration of exposure and the dose administered.
Male Sprague-Dawley rats (weight, 250 to 350 g; Harlan, Indianapolis, IN, USA) were maintained in plastic cages on a 12-h light/12-h dark cycle. For all experiments, vancomycin-treated animals (n = 5 to 7 per protocol) received i.p. injections of clinical-grade vancomycin at doses of either 150 or 200 mg/kg/day as a 100-mg/ml solution in NS. On the final day of each experimental or control protocol condition, urine samples were collected over 24 h. All animals were allowed free access to water at all times. Food was also available ad libitum, except during the period in which the urine samples were being collected. Data were analyzed for all animals enrolled in each treatment protocol. When animals contributed some predictor data (e.g., vancomycin concentrations) but died prior to urine collection, urinary biomarker outcomes were treated as missing data.
Blood and urine sampling.
Blood samples were withdrawn from internal jugular vein (i.j.) catheters, which were locked with heparin saline solution (100 IU/ml) when not in use. Catheters were surgically implanted while the animals were under ketamine (100 mg/kg) and xylazine (10 mg/kg) anesthesia. Animals were allowed to recover for 24 h prior to withdrawal of blood samples. Blood samples (0.25-ml aliquots) were collected after an experimental dose 24 h before the protocol end (i.e., on day 1 or day 3, depending on the protocol). A maximum of 8 samples were taken within a 24-h period from any animal. Blood samples were taken 0, 5, 15, and 30 min and 1, 2, 4, and 24 h postdose. Time zero corresponded to the time immediately prior to administration of the given dose. For animals who received twice-daily dosing, to maintain total blood sampling limits, the sample collected 5 min after the first dose on the sampling day was omitted to obtain a sample 30 min after the second dose (12.5 h after collection of the sample at time zero). Equivalent volumes of NS were administered via the i.j. catheter after withdrawal of each blood sample. Urine was collected during the 24-h period prior to terminal blood sampling and euthanasia. Animals were placed in metabolic cages for fasting urine collection and quantitation, as previously described (19–21). Urine was collected via metabolic cage (catalogue number 650-0350; Nalgene, Rochester, NY). Animals were placed in the metabolic cage after collection of the sample at 120 min and momentarily removed for collection of other blood samples up until collection of the terminal sample at 24 h. Urine was collected under ambient conditions, and the urine volume was measured at the end of the 22-h residence. Notably, kidney injury molecule 1 (KIM-1), cystatin C, osteopontin (OPN), and neutrophil gelatinase-associated lipocalin 2 (NGAL) levels are stable throughout this time period under ambient conditions. Urine was collected from each animal and centrifuged at 400 × g for 5 min. After the centrifugation step, the entire amount of urine (i.e., the urine pooled over 22 h) from each animal was individually stored at −80°C for batch analysis. Animal urine was not pooled by dose group: each animal's urine was matched with their own PK exposure data.
Chemicals and reagents.
Vancomycin hydrochloride for injection (lot number 343748E03) was obtained commercially (Hospira, Lake Forest, IL). As previously described (22), vancomycin hydrochloride USP with a purity of 99.3% was obtained commercially (Enzo Life Science, CA) for the generation of standard curves using high-performance liquid chromatography (HPLC) with UV detection. Caffeine (purity, 99.7%; Alfa Aesar, Ward Hill, MA, USA), acetonitrile, and methanol were from purchased from VWR International (Radnor, PA). Formic acid was obtained from Fisher Scientific (Waltham, MA). All solvents used were of HPLC or liquid chromatography-tandem mass spectrometry grade. Frozen, nonmedicated, nonimmunized, pooled plasma (anticoagulated with disodium EDTA) from Sprague-Dawley rats was procured (BioreclamationIVT, Westbury, NY) for calibration of standard curves.
Determination of vancomycin concentrations in plasma.
Blood from vancomycin-treated animals was immediately transferred to disodium EDTA-treated Eppendorf microcentrifuge tubes and centrifuged (Eppendorf, Hauppauge, NY) at 3,000 rpm for 10 min. Plasma was separated as a supernatant and was then stored at −80°C for further analysis. Plasma samples underwent phospholipid removal and filtration using Phree columns (Phenomenex, Torrance, CA, USA). Vancomycin was eluted using methanol. The eluted samples were analyzed using a previously reported HPLC method with UV detection (22). Samples were analyzed using a gradient HPLC technique using a Kinetex biphenyl column (particle size, 2.6 μm; 50 by 3 mm; Phenomenex, Torrance, CA, USA). The mobile phase consisted of acetonitrile and water containing 0.1% formic acid, while the UV detector was set at 198 nm. Caffeine was used as the internal standard. The linear range of the assay was 3.0 to 74.9 μg/ml. The coefficient of variation (CV) for intra-assay precision ranged from 2.45 to 15.16%, and the CV for interday assay precision ranged from 2.17 to 15.82%; all of these values met the assay standards (23). The interday accuracy and precision ranges of the assay were 97.4 to 118.5% and 2.18 to 15.81%, respectively.
Determination of urinary biomarkers of AKI.
Urine samples were analyzed for biomarker and creatinine content. Sample aliquots were processed and stored as described above. Urine aliquots were analyzed in batches to determine the concentrations of clusterin, cystatin C, KIM-1, NGAL, and OPN. Biomarkers were assayed using the microsphere-based Luminex xMAP technology (Austin, TX) as described previously (19–21). Urine was aliquoted into 96-well plates supplied with Milliplex MAP rat kidney toxicity magnetic bead panels 1 and 2 (EMD Millipore Corporation, Charles, MO). Samples were prepared and analyzed according to the manufacturer's recommended protocol. The urine creatinine concentration was determined by a colorimetric assay (Cayman Chemical, Ann Arbor, MI). Urine was diluted 1:50; aliquoted into 96-well plates supplied with the kit, which were analyzed according to the manufacturer's instructions; and read at 485 nm on a Beckman Coulter DTX 880 multimode detector (Beckman Coulter, Inc., CA).
Evaluation of histopathological evidence of renal cell damage.
After terminal urine and blood sampling, rats were humanely euthanized by exsanguination from the right atrium while they were under anesthesia (induced by the intraperitoneal injection of 100 mg/kg ketamine and 10 mg/kg xylazine). Necropsies were performed on all rats, and kidneys were harvested for postmortem examination. Each animal's kidneys were removed, briefly washed in cold isotonic saline, and preserved in 10% formalin solution for histologic examination. Histopathological analysis was conducted by Charles River Pathology Associates (Wilmington, MA), using light microscopy on hematoxylin and eosin-stained, paraffin-embedded renal specimens. Pathologists did not have access to any of the TD data concerning surrogate measures of kidney function (i.e., urinary biomarkers) or vancomycin PK exposure data (i.e., the area under the concentration-time curve during the first 24 h [AUC0–24], the maximum concentration in plasma during the first 24 h [Cmax0–24], or the the lowest [minimum] concentration in plasma after the dose closest to 24 h [Cmin0–24]); pathologists were informed only of nominal treatment group status (i.e., the dose [in milligrams per kilogram]). Categorical scoring was according to the histopathological lexicon from the Critical Path Institute's Predictive Safety Testing Consortium Nephrotoxicity Working Group (8, 24–27). Evidence of histopathological damage was rated on an ordinal scale ranging from 0 to 5, where the grades for pathological lesions were 0 for no observable pathology, 1 for minimal pathology, 2 for mild pathology, 3 for moderate pathology, 4 for marked pathology, and 5 for severe pathology; this scale has been validated elsewhere (8, 28). The composite score for an individual animal was calculated as the highest ordinal score for any histopathological process at any kidney site (28).
Vancomycin pharmacokinetic model and exposure determination.
A three-compartment structural model of vancomycin disposition accounting for the rate of absorption from the peritoneal space to the central compartment (Ka) was fit to the PK data. The PK model was parameterized with Ka, the volume of distribution (V), the intercompartmental transfer rates between the central and peripheral compartments (K12 and K21), and the total elimination rate (kel), which were estimated as apparent parameters (i.e., V/F and kel/F, where F is bioavailability) and solved algebraically.
The nonparametric adaptive grid (NPAG) algorithm (29–31) with adaptive gamma implemented within the Pmetrics package (version 1.4.0; Laboratory of Applied Pharmacokinetics and Bioinformatics, Los Angeles, CA) for R (32) was used for all PK model-fitting procedures, as previously described (33–36). There were 80,021 initial grid points. The inverse of the estimated assay variance was used as the first estimate for parameter weighting. Final parameter weighting was accomplished using gamma, a multiplicative observation error model to capture process noise (i.e., error = standard deviation [SD] · gamma), with the initial gamma value being 3. Assay error (SD) was accounted for using an error polynomial as a function of the measured concentration, Y (i.e., SD = C0 + C1 · Y), with inputs of 1.5 and 0.08, respectively. Comparative performance evaluation was completed using Akaike's information criterion, a regression of observed versus predicted concentrations, visual plots of PK parameter-covariate regressions, and the rule of parsimony. The best-fit model from the iterative model-fitting process was utilized to obtain median MAP Bayesian vancomycin exposure estimates. Population predictions were utilized only in sampling time simulations performed by use of the MMopt command within Pmetrics, as described below (see “Calculation of optimal sampling time points for analysis of vancomycin PKs in the rat” below). All exposure-response indices were calculated from individual Bayesian posterior predicted concentrations, as described below (see “Estimation of PK exposure profiles and statistical analysis” below).
Estimation of PK exposure profiles and statistical analysis.
Individual vancomycin exposures for the first 24 h (i.e., AUC0–24, Cmax0–24, and Cmin0–24) were determined for each rat using individual median Bayesian posterior parameter values to calculate plasma vancomycin concentrations every 5 min. Bayesian posterior parameter value distributions were calculated in Pmetrics from the final population model (see Table 2) and each animal's measured vancomycin concentrations, dosing history, and weight. The highest concentration values within the first 24 h were used to determine Cmax0–24, and the lowest concentration observed between 11 and 24 h postdose was used to determine Cmin0–24 across all animals in order to capture the lowest concentration within the first 24 h after i.p. administration. The trapezoidal rule was used to determine AUC0–24. The variability of each PK exposure measure was calculated as the CV by dividing the standard deviation by the mean of the given exposure measure.
TABLE 2.
Final three-compartment population PK model estimatesa
| Parameter | Mean | Median | SD | CV (%) |
|---|---|---|---|---|
| Ka (h−1) | 1.5 | 1.0 | 1.3 | 84.8 |
| V0/F (liter/0.3 kg) | 0.3 | 0.3 | 0.2 | 54.0 |
| kel/F (h−1) | 1.3 | 0.9 | 0.9 | 73.8 |
| K12 (h−1) | 4.8 | 1.9 | 5.3 | 110.3 |
| K21 (h−1) | 4.2 | 1.6 | 5.3 | 124.8 |
Mean and median population pharmacokinetic parameters along with their attendant measures of dispersion are presented. The PK model was derived from 276 observations in 42 animals. Abbreviations: CV, coefficient of variation; Ka, intercompartmental absorption rate from the peritoneal space to the central compartment; kel, central compartment elimination rate; K12 and K21, intercompartmental mass transfer rates between the central and peripheral compartments; V0, volume of distribution standardized to a 0.3-kg body weight; F, apparent bioavailability of clearance and volume, which was not estimated [i.e., CL/F = (V0 · kel)/F].
Association of PK measures with urinary AKI biomarkers and renal histopathology.
All statistical analyses, except where indicated, were completed using Intercooled Stata (version 14.0; StataCorp, College Station, TX). PK/TD exposure-response relationships were assessed for vancomycin exposure profiles (i.e., PKs) and (i) urinary biomarkers of AKI and (ii) composite histopathological damage (i.e., TD). Relationships between exposure and renal endpoints were assessed using Spearman's rank coefficient.
Statistical analyses for between-treatment-group comparisons.
Renal histopathological score, urine output, pharmacokinetic exposure measures (e.g., AUC0–24, Cmax0–24, and Cmin0–24), and body weight loss were analyzed according to vancomycin dose, frequency, and duration. Differences were evaluated using either the Student t test or the Wilcoxon rank sum test, as appropriate. All statistical tests were two-tailed, with an a priori level of alpha set at 0.05 for statistical significance.
Calculation of optimal sampling time points for analysis of vancomycin PKs in the rat.
Once a finalized population PK model was fitted using the NPAG algorithm, the population model consisting of discrete support points (up to one point per animal was included in the model) was leveraged to identify optimal sampling times using the MMopt command within Pmetrics (37). A support point is a collection of values for each parameter in the model and an associated probability of those values, based on how well they predict the observed vancomycin concentrations in the study population. Each support point in the population model can generate a time-concentration profile for vancomycin for a given dosage regimen or regimens for which optimal sampling times are desired. The MMopt algorithm finds the desired number of optimal sampling times (typically, 1 to 4) where the profiles from each support point are maximally separated. In this way, the Bayesian risk of misclassifying a new animal as the wrong support point or combination of support points is minimized. We evaluated 1 to 4 samples for each dose fractionation scheme.
RESULTS
Demographics of animal cohort.
Figure 1 displays the disposition of the 52 rats according to treatment group, fractionation scheme, and duration of exposure. In total, four vancomycin-treated animals (n = 1 from the 150-mg/kg group and n = 3 from the 200-mg/kg group) were euthanized early according to IACUC protocols, and thus, they contributed only partially complete PK and TD data. Of the 52 animals evaluated, 38 vancomycin-treated animals and 10 control animals contributed complete PK and TD data. Differences in animal weight loss postcatheterization according to vancomycin or control status and protocol duration are shown in Table 1. Mean weight loss was not significantly different between vancomycin-treated and control animals overall (23.3 g versus 26.1 g; P = 0.44). Likewise, animal weight loss did not differ between vancomycin-treated and control animals according to protocol duration (P = 0.35 and P = 0.82 for the 1- and 3-day-protocol groups, respectively).
TABLE 1.
Summary of animal weight loss, urine output, histopathology scores, and vancomycin exposure estimatesa
| Characteristic | One-day protocol |
Three-day protocol |
||||
|---|---|---|---|---|---|---|
| Control | Vancomycin | P value | Control | Vancomycin | P value | |
| No. of animals in group/total no. of animals | 5/52 | 22/52 | 5/52 | 20/52 | ||
| Mean ± SD wt loss (g) | 25.6 ± 4.0 | 21.5 ± 9.4 | 0.35 | 26.6 ± 3.6 | 25.3 ± 12.7 | 0.82 |
| Mean ± SD urine output (ml) | NR | 10.6 ± 5.3b | —c | 13.6 ± 2.3 | 12.8 ± 6.7d | 0.79 |
| Median (IQR) worst composite score | 2 (2–2) | 2 (1–2) | 0.68 | 1 (1–2) | 2 (1–3) | 0.41 |
| Median (IQR) AUC0–24 (μg · h/ml) | 0 | 112 (62.2–292) | — | 0 | 202 (83.1–248) | — |
| Median (IQR) Cmax0–24 (μg/ml) | 0 | 17.5 (8.7–57.6) | — | 0 | 26.4 (13.9–51.8) | — |
| Median (IQR) Cmin0–24 (μg/ml) | 0 | 0.04 (0.0–2.5) | — | 0 | 1.8 (0.0–3.2) | — |
Urinary, histopathological, and PK exposure endpoints were stratified by experimental condition (i.e., vancomycin or saline control) and protocol duration (1 or 3 days). Abbreviations: AUC0–24, area under the concentration time curve during the first 24 h; Cmax0–24, maximal concentration during the first 24 h; Cmin0–24, lowest (minimum) concentration after the dose closest to 24 h; IQR, interquartile range. NR, data were not recorded.
Data are for 21 rats.
—, statistical testing against a zero value was not performed.
Data are for 17 rats.
Urine output, concentrations of urinary AKI biomarkers, and renal histopathology.
Urinary output measurements are summarized in Table 1 and were stratified according to vancomycin treatment or control status and protocol duration. On the final protocol day, the overall mean ± SD urine output was 11.8 ± 5.7 ml (n = 43). Mean total urine output did not differ between vancomycin-treated and control animals overall (11.6 ml versus 13.6 ml; P = 0.46). Likewise, mean urine output was not different between vancomycin-treated and control animals in the 3-day-protocol group (12.8 ml versus 13.6 ml; P = 0.79). Urine creatinine concentrations did not differ significantly between vancomycin-treated and control animals (P = 0.28). The range of concentrations of nearly all sensitive urinary AKI biomarkers evaluated (with the exception of the osteopontin concentration) were higher among vancomycin-treated animals in the 3-day-protocol group than control animals and vancomycin-treated animals in the 1-day-protocol group (see Table S1 in the supplemental material). The worst ordinal histopathology score data are also summarized in Table 1 and were stratified according to vancomycin treatment or control status and protocol duration. The highest observed composite histopathology scores did not differ according to treatment group (P = 0.69), nor did they differ according to protocol duration (Table 1).
Vancomycin PK model and exposure measure determination.
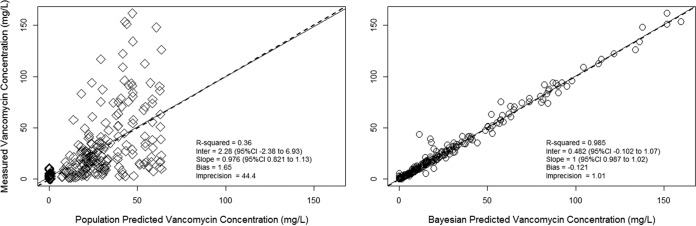
The final population PK model had 34 support points defining a 3-compartment model fitted to 276 vancomycin concentrations in 42 animals. Bias, imprecision, and the coefficient of determination for observations versus Bayesian posterior predictions were −0.12 μg/ml, 1.01 μg2/ml2, and 98.5%, respectively (Fig. 2). For the purposes of determining optimal sampling times, the population mean, median, and dispersion metrics are shown in Table 2, while the interparameter covariances (i.e., the covariance matrix in lower triangular form) for each of the PK model parameters are shown in Table 3. Figure 2 displays the population and individual posterior Bayesian observed versus predicted plots.
FIG 2.
Population and individual goodness-of-fit plots for the three-compartment PK model. The final three-compartment population model parameters are provided in Table 2. Vancomycin observations (n = 276) were obtained from 42 animals (male Sprague-Dawley rats). (Left) Population model goodness-of-fit plot with diamonds; (right) individual Bayesian posterior model goodness-of-fit plot with circles.
TABLE 3.
Covariance matrix in lower triangular form of the final pharmacokinetic modela
| Parameter | Covariance |
||||
|---|---|---|---|---|---|
| kel (h−1) | V0/F (liter/0.3 kg) | Ka (h−1) | K12 (h−1) | K21 (h−1) | |
| kel (h−1) | 0.866 | ||||
| V0/F (liter/0.3 kg) | −0.052 | 0.0228 | |||
| Ka (h−1) | −0.344 | 0.006 | 1.566 | ||
| K12 (h−1) | 0.971 | 0.043 | −1.473 | 28.248 | |
| K21 (h−1) | 1.418 | 0.207 | 1.102 | −4.086 | 27.62759 |
Abbreviations: Ka, intercompartmental absorption rate from the peritoneal space to the central compartment; kel, central compartment elimination rate; K12 and K21, intercompartmental mass transfer rates between the central and peripheral compartments; V0, volume of distribution standardized to s 0.3-kg body weight; F, apparent bioavailability of clearance and volume, which was not estimated [i.e., CL/F = (V0 · kel)/F].
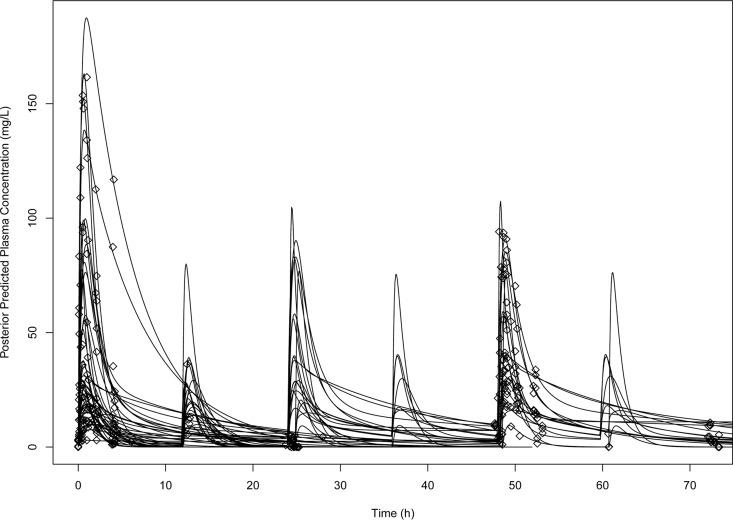
The calculated Bayesian posterior vancomycin exposure estimates are summarized in Table 1 according to treatment status and protocol duration. Among the vancomycin-treated animals, the mean AUC0–24, Cmax0–24, and Cmin0–24 were 202 μg · h/ml, 38.7 μg/ml, and 1.64 μg/ml, respectively. Overall, significant interanimal exposure variability was observed. The CVs among vancomycin-treated animals for AUC0–24, Cmax0–24, and Cmin0–24 were 114%, 118%, and 131%, respectively. The estimated population mean absorption rate constant (Ka) was prolonged at 1.5 h−1 (Table 2). The Bayesian posterior predicted concentrations captured the majority of the observed concentrations with high accuracy and low bias (Fig. 2 and 3).
FIG 3.
Concentration-time profile of vancomycin in rats according to individual Bayesian posterior predictions. Maximum a posteriori Bayesian estimated concentration-time profiles (lines) were fitted to the observed data (diamonds).
PK/TD correlations.
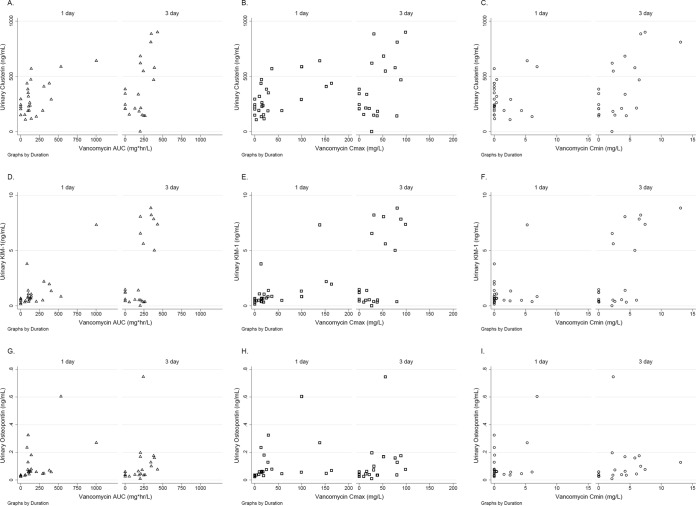
Since vancomycin exposure metrics were not uniformly distributed, nonparametric exposure-response correlations were generated. Urine creatinine concentrations were low for all study animals and did not significantly correlate with vancomycin exposure metrics (P = 0.17, 0.27, and 0.10 for the AUC0–24, Cmax0–24, and Cmin0–24 correlations, respectively; see Fig. S1 in the supplemental material). Sensitive urinary biomarkers of AKI correlated with vancomycin exposure metrics (i.e., AUC0–24, Cmax0–24, and Cmin0–24), as shown in Table 4. Of the urinary AKI biomarkers tested, osteopontin, KIM-1, and clusterin exhibited the highest correlations with vancomycin AUC0–24 and Cmax0–24 (P ≤ 0.05 for all comparisons). Vancomycin exposure-response plots are shown in Fig. 4 for clusterin, KIM-1, and osteopontin. The highest correlations were observed between AUC0–24 and Cmin0–24 and each of these respective biomarkers. None of the vancomycin exposure measures (AUC0–24, Cmax0–24, or Cmin0–24) were significantly correlated with the worst ordinal renal histopathology scores measured (Spearman's nonparametric correlation coefficient [rs] = 0.18, 0.11, and 0.09, respectively). Of note, vancomycin exposure measures were highly intercorrelated across all doses and dosing frequencies, with AUC0–24 and Cmax0–24 being the most highly intercorrelated (rs = 0.898; P < 0.0001), followed by AUC0–24 and Cmin0–24 (rs = 0.783; P < 0.0001) and Cmax0–24 and Cmin0–24 (rs = 0.579; P < 0.0001).
TABLE 4.
Correlations between urinary biomarkers of renal injury and vancomycin PK exposurea
| Urinary biomarker | Association measure | AUC0–24 | Cmax0–24 | Cmin0–24 |
|---|---|---|---|---|
| Clusterin (ng/ml) | rs | 0.372 | 0.393 | 0.225 |
| P value for rs | 0.009 | 0.006 | 0.125 | |
| Cystatin C (ng/ml) | rs | 0.033 | 0.076 | 0.126 |
| P value for rs | 0.822 | 0.610 | 0.393 | |
| KIM-1 (ng/ml) | rs | 0.422 | 0.453 | 0.259 |
| P value for rs | 0.003 | 0.001 | 0.076 | |
| Osteopontin (ng/ml) | rs | 0.517 | 0.546 | 0.419 |
| P value for rs | 0.0002 | 0.0001 | 0.003 | |
| Lipocalin-2/NGAL (ng/ml) | rs | 0.263 | 0.271 | 0.306 |
| P value for rs | 0.071 | 0.062 | 0.035 |
Measures of association between log exposure (i.e., AUC0–24, Cmax0–24, Cmin0–24) and the level of expression of urinary biomarkers. Bold, correlations indicate significance at the level with a P value of <0.05. Abbreviations: AUC0–24, area under the concentration-time curve during the first 24 h; Cmax0–24, maximal concentration during the first 24 h; Cmin0–24, lowest concentration after the dose closest to 24 h; KIM-1, kidney injury molecule 1; rs, Spearman's nonparametric correlation coefficient.
FIG 4.
Exposure-response for vancomycin and early urinary biomarkers of acute kidney injury. Sensitive urinary biomarkers of AKI compared to vancomycin exposure measures were stratified by protocol duration (in days). (A to C) Clusterin expression according to vancomycin exposure measures; (D to F) KIM-1 expression according to vancomycin exposure measures; (G to I) osteopontin expression according to vancomycin exposure measures.
Optimal sampling times for PK analysis in the rat.
For animals receiving vancomycin once every 24 h, the four most informative time points to obtain samples for PK analysis were 0.25, 0.75, 2.75, and 8 h postdose. The Bayes risk of misclassification increased marginally (11.2%) when the number of samples was reduced to 3 at 0.25, 2.75, and 8 h postdose. Alternatively, for animals receiving vancomycin every 12 h, the four most informative time points to obtain samples for PK analysis were 0.25 and 1.25 after the first dose and 2.5 and 5.25 h after the second dose. The Bayes risk of misclassification increased very slightly (9.8%) when the number of samples was reduced to 3 at 0.25, 13, and 16.25 h postdose. Reducing the number of sampling times to 1 or 2 time points increased the Bayes risk of misclassification by >25% compared to that for the four-sample design, irrespective of the dosing schedule. Thus, in the future, sampling times for PK analysis in the rat after i.p. dosing may reasonably be reduced to 3 optimal time points according to the dose fractionation scheme employed.
DISCUSSION
Our study attempted to link PK exposures to vancomycin with elevations in sensitive renal biomarkers in a rat model of nephrotoxicity. While no notable relationships between vancomycin exposure over 24 or 72 h and renal histopathology were observed, our study demonstrated that urinary biomarkers increased as a function of vancomycin duration and intensity. Increasing vancomycin exposures in the form of AUC0–24, Cmax0–24, and Cmin0–24 were all positively correlated with increased concentrations of several urinary biomarkers of AKI. While there was a positive correlation between all PK parameters and urinary biomarkers, the visual exposure-urinary biomarker relationships (Fig. 4) clearly demonstrated that the relationship between increasing urinary biomarkers and exposure was most pronounced for AUC0–24 and Cmax0–24. Our observations support an exposure-response relationship for vancomycin and AKI, a relationship that is critical to elucidate, as vancomycin is the single most common antibiotic administered to hospitalized patients in the United States (4). These data lay the foundation for future experiments that will further clarify the exact PK/TD exposure profile within a larger population treated for longer durations with higher doses. To facilitate the execution of these future studies, this study also provided critical information on the optimal times of sample collection for PK analysis to minimize bias in exposure estimation with the fewest rat blood draws when combined with a population modeling approach.
Our results are generally consistent with those of other preclinical studies that have assessed the relationship between vancomycin and urinary AKI biomarkers. Similar to our findings, Fuchs et al. noted increased concentrations of KIM-1, clusterin, and osteopontin among Wistar rats treated with increasing doses of vancomycin, especially when the daily dose was in excess of 200 mg/kg/day (9). Vaidya et al. also observed that vancomycin produced dose-dependent increases in KIM-1 concentrations over time (8). The authors observed male Han Wistar rats receiving 3, 7, and 14 days of treatment and noted increased expression of KIM-1 with total doses of 140 mg/kg as early as 3 days (8). We observed a similar increase in KIM-1 concentrations among male Sprague-Dawley rats even after only 1 day of treatment with doses of up to 200 mg/kg/day. In light of these findings, we believe that our results reflect the low end of the exposure-response curve. Although we administered allometrically scaled doses (i.e., equivalent doses in humans of approximately 25 to 30 mg/kg/day), the actual amount of vancomycin reaching the rat plasma was likely limited by less than 100% bioavailability after intraperitoneal injection. Furthermore, the durations were relatively short compared to those of typical regimens prescribed to human patients.
Our findings and the findings of others are biologically plausible. In our study, we observed a relationship between the intensity and duration of vancomycin exposure and the elevation in the concentrations of urinary AKI biomarkers, such as KIM-1, which are localized to the proximal tubule. Increases in KIM-1 urinary expression greater than 1.87-fold compared with that for the controls has over 95% specificity for AKI (8). Ample preclinical (8, 9, 38) and clinical (39, 40) data demonstrate that vancomycin can induce proximal tubular damage. Although the exact mechanism by which vancomycin causes proximal tubule damage remains unclear, data suggest that this damage may be due to oxidative stress and mitochondrial dysfunction. In their study of the genomic and transcriptomic response within renal cells among vancomycin-treated BALB/c mice, Dieterich et al. observed a significant downregulation of antioxidant gene expression and an upregulation in oxidative stress and organelle dysfunction markers (41). Similarly, King and Smith observed increased ATP and oxygen consumption concurrently with the proliferation of proximal tubule cells in their study of vancomycin-treated porcine (LLC-PK1) cells (42). Further, multiple groups have demonstrated that antioxidants mitigate the damage done by vancomycin (43–47). As vancomycin is thought to be an oxidative stressor within the proximal tubule, increases in local vancomycin exposures within proximal tubule cells should precede elevations in sensitive biomarkers of AKI. Our observation that increasing exposures are correlated with the rise of novel AKI biomarkers is thus highly biologically plausible.
While we noted elevations in several novel urinary AKI biomarkers, we did not observe a relationship between exposure and histopathological changes. Of note, we did not observe frank histopathological damage among the experimental animals, and the urinary biomarker elevations that we observed did not reach the levels seen in other investigations (8, 9, 15, 16). However, it is important to note that the study protocols used in studies producing frank histopathological damage were longer and the doses were higher than those in the present study. Thus, the doses used in our study likely led only to the low end of the toxic range (i.e., the first bend in a sigmoidal curve). As such, we did not fit an exposure-response model to the data, as more robust sampling of the upper bend in the curve is needed for proper fitting of sigmoidal exposure-response relationships.
Limitations to our study should be considered. First, we observed high degrees of variability in vancomycin exposures among vancomycin-treated animals. The variability seen in our study, as with the variabilities seen in other studies (41), was thought to be inherent because of the i.p. administration route. We utilized vancomycin concentrations of 100 mg/ml, but we did not evaluate any pH-dependent solubility issues that may have occurred with i.p. dosing. Certainly, precipitation of the drug in the peritoneal cavity is possible (48, 49), which may have contributed to the intra-animal variability in absorption. However, the increased variability in vancomycin exposures seen within our study allowed a wide range of drug exposures. Even with high correlations between the pharmacokinetic parameters, some separation was attained, which suggests that the vancomycin AUC0–24 or Cmax0–24 is likely the important target for avoiding kidney injury. Additionally, i.p. dosing provides a depot effect and allows vancomycin concentration-time profiles to more closely mimic those in humans in comparison to the profiles obtained if the doses were simply administered as an intravenous bolus. Previous investigations of the influence of the vancomycin dose on the concentrations of urinary AKI biomarkers in animal models did not evaluate the variability in exposure, as vancomycin PKs either were not evaluated (9) or were not evaluated within the same animals in which TD was measured (41). The variability in absorption and bioavailability due to i.p. administration of vancomycin (an increase in the 50% lethal dose of up to 7-fold has been observed when vancomycin was given i.p. [14, 48, 49]) presents a significant challenge when attempting to make comparisons on a by-dose basis. Thus, our analysis is unique in that paired PKs and TD were measured in the same animals. Accordingly, our analysis was based on exposure as opposed to a strict evaluation by dose category.
We have demonstrated that higher vancomycin exposures are correlated with increasing concentrations of urinary AKI biomarkers. Our preliminary PK/TD correlations support the suggestion that AUC0–24 and Cmax0–24 are most closely associated with the rise of urinary markers of renal insult. Further work is required to further delineate the exact PK/TD profile that produces AKI in a rat model. Clarification of the most predictive profile will have implications for the design of future experiments designed to minimize AKI after administration of humanized exposures.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the following individual who supported the development of the manuscript and provided reviews of the data presented herein: Cristina Miglis. We thank Seema Briyal and Mary Leonard for their assistance with the animal experiments.
We have no conflicts of interest.
The research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases and the National Institute of General Medical Sciences of the National Institutes of Health under award numbers R15-AI105742 and R01-GM068968.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding Statement
Research reported in this publication was supported by National Institute of Allergy and Infectious Diseases and National Institute of General Medical Sciences of the National Institutes of Health under award numbers R15-AI105742 (principal investigator: Marc H. Scheetz) and R01-GM068968. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00591-16.
REFERENCES
- 1.Young MP, Birkmeyer JD. 2000. Potential reduction in mortality rates using an intensivist model to manage intensive care units. Eff Clin Pract 3:284–289. [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C. 2005. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 3.Bagshaw SM, George C, Dinu I, Bellomo R. 2008. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant 23:1203–1210. [DOI] [PubMed] [Google Scholar]
- 4.Kelesidis T, Braykov N, Uslan DZ, Morgan DJ, Gandra S, Johannsson B, Schweizer ML, Weisenberg SA, Young H, Cantey J, Perencevich E, Septimus E, Srinivasan A, Laxminarayan R. 2016. Indications and types of antibiotic agents used in 6 acute care hospitals, 2009-2010: a pragmatic retrospective observational study. Infect Control Hosp Epidemiol 37:70–79. doi: 10.1017/ice.2015.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosso JA, Nappi J, Rudisill C, Wellein M, Bookstaver PB, Swindler J, Mauldin PD. 2011. Relationship between vancomycin trough concentrations and nephrotoxicity: a prospective multicenter trial. Antimicrob Agents Chemother 55:5475–5479. doi: 10.1128/AAC.00168-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minejima E, Choi J, Beringer P, Lou M, Tse E, Wong-Beringer A. 2011. Applying new diagnostic criteria for acute kidney injury to facilitate early identification of nephrotoxicity in vancomycin-treated patients. Antimicrob Agents Chemother 55:3278–3283. doi: 10.1128/AAC.00173-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. 2009. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 49:507–514. doi: 10.1086/600884. [DOI] [PubMed] [Google Scholar]
- 8.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV. 2010. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs TC, Frick K, Emde B, Czasch S, von Landenberg F, Hewitt P. 2012. Evaluation of novel acute urinary rat kidney toxicity biomarker for subacute toxicity studies in preclinical trials. Toxicol Pathol 40:1031–1048. doi: 10.1177/0192623312444618. [DOI] [PubMed] [Google Scholar]
- 10.Konishi H, Morita Y, Mizumura M, Iga I, Nagai K. 2013. Difference in nephrotoxicity of vancomycin administered once daily and twice daily in rats. J Chemother 25:273–278. doi: 10.1179/1973947812Y.0000000067. [DOI] [PubMed] [Google Scholar]
- 11.Lodise TP, Lomaestro B, Graves J, Drusano GL. 2008. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 52:1330–1336. doi: 10.1128/AAC.01602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosch K, McLaughlin MM, Esterly JS, Rhodes NJ, Postelnick MJ, Scheetz MH. 2014. Impact of vancomycin treatment duration and dose on kidney injury. Int J Antimicrob Agents 43:297–298. doi: 10.1016/j.ijantimicag.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J. 2012. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 54:621–629. doi: 10.1093/cid/cir895. [DOI] [PubMed] [Google Scholar]
- 14.Wold JS, Turnipseed SA. 1981. Toxicology of vancomycin in laboratory animals. Rev Infect Dis 3(Suppl):S224–S229. [PubMed] [Google Scholar]
- 15.Endre ZH, Pickering JW, Walker RJ, Devarajan P, Edelstein CL, Bonventre JV, Frampton CM, Bennett MR, Ma Q, Sabbisetti VS, Vaidya VS, Walcher AM, Shaw GM, Henderson SJ, Nejat M, Schollum JB, George PM. 2011. Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int 79:1119–1130. doi: 10.1038/ki.2010.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs TC, Emde B, Czasch S, Landenberg FV, Hewitt P. 2011. Detection of vancomycin induced nephrotoxicity in rats by urinary protein biomarkers. European Renal Association-European Dialysis and Transplant Association, Prague, Czech Republic. [Google Scholar]
- 17.Rybak MJ, Lomaestro BM, Rotscahfer JC, Moellering RC, Craig WA, Billeter M, Dalovisio JR, Levine DP. 2009. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis 49:325–327. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 19.Prozialeck WC, Vaidya VS, Liu J, Waalkes MP, Edwards JR, Lamar PC, Bernard AM, Dumont X, Bonventre JV. 2007. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int 72:985–993. doi: 10.1038/sj.ki.5002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prozialeck WC, Edwards JR, Lamar PC, Liu J, Vaidya VS, Bonventre JV. 2009. Expression of kidney injury molecule-1 (Kim-1) in relation to necrosis and apoptosis during the early stages of Cd-induced proximal tubule injury. Toxicol Appl Pharmacol 238:306–314. doi: 10.1016/j.taap.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prozialeck WC, Edwards JR, Vaidya VS, Bonventre JV. 2009. Preclinical evaluation of novel urinary biomarkers of cadmium nephrotoxicity. Toxicol Appl Pharmacol 238:301–305. doi: 10.1016/j.taap.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhodes NJ, Prozialeck WC, Lodise T, Venkatesan N, O’Donnell JN, Pais G, Lamar P, Gulati A, Kamilar JM, Scheetz M. 2015. Vancomycin exposures associated with elevations in novel urinary biomarkers of acute kidney injury, abstr A-977. Abstr 55th Intersci Conf Antimicrob Agents Chemother, San Diego, CA. [Google Scholar]
- 23.Food and Drug Administration. 2001. Bioanalytical method validation. Guidance for industry. Center for Veterinary Medicine, Center for Drug Evaluation and Research, Food and Drug Administration, U.S. Department of Health and Human Services, Rockville, MD. [Google Scholar]
- 24.Mattes WB, Walker EG. 2009. Translational toxicology and the work of the predictive safety testing consortium. Clin Pharmacol Ther 85:327–330. doi: 10.1038/clpt.2008.270. [DOI] [PubMed] [Google Scholar]
- 25.Dieterle F, Perentes E, Cordier A, Roth DR, Verdes P, Grenet O, Pantano S, Moulin P, Wahl D, Mahl A, End P, Staedtler F, Legay F, Carl K, Laurie D, Chibout SD, Vonderscher J, Maurer G. 2010. Urinary clusterin, cystatin C, beta2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat Biotechnol 28:463–469. doi: 10.1038/nbt.1622. [DOI] [PubMed] [Google Scholar]
- 26.Ozer JS, Dieterle F, Troth S, Perentes E, Cordier A, Verdes P, Staedtler F, Mahl A, Grenet O, Roth DR, Wahl D, Legay F, Holder D, Erdos Z, Vlasakova K, Jin H, Yu Y, Muniappa N, Forest T, Clouse HK, Reynolds S, Bailey WJ, Thudium DT, Topper MJ, Skopek TR, Sina JF, Glaab WE, Vonderscher J, Maurer G, Chibout SD, Sistare FD, Gerhold DL. 2010. A panel of urinary biomarkers to monitor reversibility of renal injury and a serum marker with improved potential to assess renal function. Nat Biotechnol 28:486–494. doi: 10.1038/nbt.1627. [DOI] [PubMed] [Google Scholar]
- 27.Yu Y, Jin H, Holder D, Ozer JS, Villarreal S, Shughrue P, Shi S, Figueroa DJ, Clouse H, Su M, Muniappa N, Troth SP, Bailey W, Seng J, Aslamkhan AG, Thudium D, Sistare FD, Gerhold DL. 2010. Urinary biomarkers trefoil factor 3 and albumin enable early detection of kidney tubular injury. Nat Biotechnol 28:470–477. doi: 10.1038/nbt.1624. [DOI] [PubMed] [Google Scholar]
- 28.Sistare FD, Dieterle F, Troth S, Holder DJ, Gerhold D, Andrews-Cleavenger D, Baer W, Betton G, Bounous D, Carl K, Collins N, Goering P, Goodsaid F, Gu YZ, Guilpin V, Harpur E, Hassan A, Jacobson-Kram D, Kasper P, Laurie D, Lima BS, Maciulaitis R, Mattes W, Maurer G, Obert LA, Ozer J, Papaluca-Amati M, Phillips JA, Pinches M, Schipper MJ, Thompson KL, Vamvakas S, Vidal JM, Vonderscher J, Walker E, Webb C, Yu Y. 2010. Towards consensus practices to qualify safety biomarkers for use in early drug development. Nat Biotechnol 28:446–454. doi: 10.1038/nbt.1634. [DOI] [PubMed] [Google Scholar]
- 29.Leary R, Jelliffe R, Schumitzky A, Van Guilder M. 2001. An adaptive grid nonparametric approach to pharmacokinetic and dynamic (PK/PD) population models, p 389–394. In Proceedings of the 14th IEEE Symposium on Computer-Based Medical Systems, Bethesda, MD. [Google Scholar]
- 30.Tatarinova T, Neely M, Bartroff J, van Guilder M, Yamada W, Bayard D, Jelliffe R, Leary R, Chubatiuk A, Schumitzky A. 2013. Two general methods for population pharmacokinetic modeling: non-parametric adaptive grid and non-parametric Bayesian. J Pharmacokinet Pharmacodyn 40:189–199. doi: 10.1007/s10928-013-9302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Core Team R. 2015. R: a language and environment for statistical computing, 3rd ed R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 33.Loo AS, Neely M, Anderson EJ, Ghossein C, McLaughlin MM, Scheetz MH. 2013. Pharmacodynamic target attainment for various ceftazidime dosing schemes in high-flux hemodialysis. Antimicrob Agents Chemother 57:5854–5859. doi: 10.1128/AAC.00474-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhodes NJ, Gardiner BJ, Neely MN, Grayson ML, Ellis AG, Lawrentschuk N, Frauman AG, Maxwell KM, Zembower TR, Scheetz MH. 2015. Optimal timing of oral fosfomycin administration for pre-prostate biopsy prophylaxis. J Antimicrob Chemother 70:2068–2073. doi: 10.1093/jac/dkv067. [DOI] [PubMed] [Google Scholar]
- 35.Rhodes NJ, Kuti JL, Nicolau DP, Van Wart S, Nicasio AM, Liu J, Lee BJ, Neely MN, Scheetz MH. 2015. Defining clinical exposures of cefepime for Gram-negative bloodstream infections that are associated with improved survival. Antimicrob Agents Chemother 60:1401–1410. doi: 10.1128/AAC.01956-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Donnell JN, Gulati A, Lavhale MS, Sharma SS, Patel AJ, Rhodes NJ, Scheetz MH. 2016. Pharmacokinetics of centhaquin citrate in a rat model. J Pharm Pharmacol 68:56–62. doi: 10.1111/jphp.12498. [DOI] [PubMed] [Google Scholar]
- 37.Neely MN, Bayard DS, Hope WW. 2013. Multiple model optimal (MMopt) sampling for first dose oral voriconazole TDM in children. Abstr 13th Int Congr Ther Drug Monit Clin Toxicol, Salt Lake City, UT. [Google Scholar]
- 38.Marre R, Schulz E, Anders T, Sack K. 1984. Renal tolerance and pharmacokinetics of vancomycin in rats. J Antimicrob Chemother 14:253–260. doi: 10.1093/jac/14.3.253. [DOI] [PubMed] [Google Scholar]
- 39.Le Moyec L, Racine S, Le Toumelin P, Adnet F, Larue V, Cohen Y, Leroux Y, Cupa M, Hantz E. 2002. Aminoglycoside and glycopeptide renal toxicity in intensive care patients studied by proton magnetic resonance spectroscopy of urine. Crit Care Med 30:1242–1245. doi: 10.1097/00003246-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Shah-Khan F, Scheetz MH, Ghossein C. 2011. Biopsy-proven acute tubular necrosis due to vancomycin toxicity. Int J Nephrol 2011:436856. doi: 10.4061/2011/436856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dieterich C, Puey A, Lin S, Swezey R, Furimsky A, Fairchild D, Mirsalis JC, Ng HH. 2009. Gene expression analysis reveals new possible mechanisms of vancomycin-induced nephrotoxicity and identifies gene markers candidates. Toxicol Sci 107:258–269. doi: 10.1093/toxsci/kfn203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.King DW, Smith MA. 2004. Proliferative responses observed following vancomycin treatment in renal proximal tubule epithelial cells. Toxicol In Vitro 18:797–803. doi: 10.1016/j.tiv.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Nishino Y, Takemura S, Minamiyama Y, Hirohashi K, Tanaka H, Inoue M, Okada S, Kinoshita H. 2002. Inhibition of vancomycin-induced nephrotoxicity by targeting superoxide dismutase to renal proximal tubule cells in the rat. Redox Rep 7:317–319. doi: 10.1179/135100002125000884. [DOI] [PubMed] [Google Scholar]
- 44.Nishino Y, Takemura S, Minamiyama Y, Hirohashi K, Ogino T, Inoue M, Okada S, Kinoshita H. 2003. Targeting superoxide dismutase to renal proximal tubule cells attenuates vancomycin-induced nephrotoxicity in rats. Free Radic Res 37:373–379. doi: 10.1080/1071576031000061002. [DOI] [PubMed] [Google Scholar]
- 45.Oktem F, Arslan MK, Ozguner F, Candir O, Yilmaz HR, Ciris M, Uz E. 2005. In vivo evidences suggesting the role of oxidative stress in pathogenesis of vancomycin-induced nephrotoxicity: protection by erdosteine. Toxicology 215:227–233. doi: 10.1016/j.tox.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 46.Cetin H, Olgar S, Oktem F, Ciris M, Uz E, Aslan C, Ozguner F. 2007. Novel evidence suggesting an anti-oxidant property for erythropoietin on vancomycin-induced nephrotoxicity in a rat model. Clin Exp Pharmacol Physiol 34:1181–1185. [DOI] [PubMed] [Google Scholar]
- 47.Basarslan F, Yilmaz N, Ates S, Ozgur T, Tutanc M, Motor VK, Arica V, Yilmaz C, Inci M, Buyukbas S. 2012. Protective effects of thymoquinone on vancomycin-induced nephrotoxicity in rats. Hum Exp Toxicol 31:726–733. doi: 10.1177/0960327111433185. [DOI] [PubMed] [Google Scholar]
- 48.Aronoff GR, Sloan RS, Dinwiddie CB Jr, Glant MD, Fineberg NS, Luft FC. 1981. Effects of vancomycin on renal function in rats. Antimicrob Agents Chemother 19:306–308. doi: 10.1128/AAC.19.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Food and Drug Administration. 2008. Vancomycin solubility study. Report to Office of Generic Drugs. Division of Product Quality Research, Office of Testing and Research, Center for Drug Evaluation and Research, Food and Drug Administration, U.S. Department of Health and Human Services, Rockville, MD: http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm082291.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.