Abstract
We used in vitro and in vivo models of catheter-associated biofilm formation to compare the relative activity of antibiotics effective against methicillin-resistant Staphylococcus aureus (MRSA) in the specific context of an established biofilm. The results demonstrated that, under in vitro conditions, daptomycin and ceftaroline exhibited comparable activity relative to each other and greater activity than vancomycin, telavancin, oritavancin, dalbavancin, or tigecycline. This was true when assessed using established biofilms formed by the USA300 methicillin-resistant strain LAC and the USA200 methicillin-sensitive strain UAMS-1. Oxacillin exhibited greater activity against UAMS-1 than LAC, as would be expected, since LAC is an MRSA strain. However, the activity of oxacillin was less than that of daptomycin and ceftaroline even against UAMS-1. Among the lipoglycopeptides, telavancin exhibited the greatest overall activity. Specifically, telavancin exhibited greater activity than oritavancin or dalbavancin when tested against biofilms formed by LAC and was the only lipoglycopeptide capable of reducing the number of viable bacteria below the limit of detection. With biofilms formed by UAMS-1, telavancin and dalbavancin exhibited comparable activity relative to each other and greater activity than oritavancin. Importantly, ceftaroline was the only antibiotic that exhibited greater activity than vancomycin when tested in vivo in a murine model of catheter-associated biofilm formation. These results emphasize the need to consider antibiotics other than vancomycin, most notably, ceftaroline, for the treatment of biofilm-associated S. aureus infections, including by the matrix-based antibiotic delivery methods often employed for local antibiotic delivery in the treatment of these infections.
INTRODUCTION
Staphylococcus aureus is a leading cause of both hospital and community-acquired infections (1). S. aureus causes many different types of infections, but among the most common are infections associated with indwelling medical devices (2, 3). In fact, with the possible exception of S. epidermidis, S. aureus is easily the single most prominent cause of all types of implant-associated infection, and irrespective of overall prevalence, the virulence of S. aureus makes it by far the most clinically problematic pathogen (4, 5). This is particularly true in the context of implant-associated infections caused by methicillin-resistant S. aureus (MRSA) strains (6). Thus, the increasing prevalence of MRSA strains even among isolates causing community-associated infections (7) makes antibiotics effective against MRSA of particular clinical importance.
The prevalence of S. aureus as a cause of implant-associated infections is due in part to its ability to form a biofilm (5, 8–10). Biofilm formation not only contributes to the establishment and persistence of infection but also greatly complicates treatment owing to intrinsic antibiotic resistance, thus leading to infections that fail to respond to conventional antibiotic therapy even when acquired antibiotic resistance is not an issue (11). This often necessitates surgical intervention to remove the implant and any infected surrounding tissues, often accompanied by some form of local matrix-based antibiotic delivery (4). This is especially true in cases involving indwelling orthopedic devices (8, 12), which are particularly noteworthy in that they are increasing dramatically, with the number of both primary and revision total knee and hip replacement procedures continuing to increase without a decline in the overall infection rate (13).
Despite the increasing prevalence of biofilm-associated infections, antibiotics continue to be developed on the basis of their activity against planktonic bacterial cultures (14). This highlights the need to consider antibiotic activity in the context of a biofilm, and in the case of S. aureus, it has become increasingly important to do so in the context of methicillin resistance. To this end, we previously used an in vitro model of catheter-associated biofilm formation to evaluate the relative activity of daptomycin, linezolid, and vancomycin (11). These studies led to the conclusion that the membrane-active antibiotic daptomycin exhibits greater activity in the context of a biofilm than either vancomycin or linezolid. Subsequent studies using a murine model of catheter-associated biofilm infection confirmed the activity of daptomycin under in vivo conditions (15). These studies support the hypothesis that daptomycin is a viable alternative and perhaps even a preferred alternative to vancomycin in the context of biofilm-associated infections. However, since these studies were done, a number of other antibiotics with activity against MRSA have been introduced into clinical practice. Thus, the purpose of the studies that we report on here was to use our established in vitro and in vivo models of catheter-associated biofilm formation (11, 15) as a first step toward evaluating the relative activity of these additional antibiotics.
MATERIALS AND METHODS
Bacterial strains and antibiotics tested.
The strains included were the USA300 MRSA strain LAC and the USA200 methicillin-sensitive S. aureus (MSSA) strain UAMS-1. The primary antibiotics tested were daptomycin, vancomycin, telavancin, ceftaroline, and tigecycline, all of which are active against MRSA, as assessed under standard in vitro conditions (16). Oxacillin, which is not active against MSRA, was included as a control in these comparisons because the experiments included both the MRSA strain LAC and the MSSA strain UAMS-1. In separate experiments, we also directly compared the relative activity of the lipoglycopeptide antibiotics telavancin, oritavancin, and dalbavancin. All antibiotics were purchased from our hospital pharmacy, except for telavancin, which was kindly provided both in its pharmaceutical formulation (Vibativ) and as telavancin powder by Theravance Biopharma Antibiotics, Inc. (George Town, Cayman Islands).
Assessment of antibiotic susceptibility in vitro.
Antibiotics were tested and compared using our established in vitro catheter model of biofilm formation (11). Briefly, 1-cm segments of fluorinated ethylene propylene catheters (14 gauge; Introcan safety catheter; B. Braun, Bethlehem, PA) were coated with human plasma before being placed into the wells of a 12-well microtiter plate containing 2 ml of tryptic soy broth supplemented with glucose and sodium chloride (biofilm medium [BM]). Each well was then inoculated with LAC or UAMS-1 at an optical density at 600 nm of 0.05. The plate was then incubated at 37°C for 24 h before the catheters were removed and transferred to fresh BM with and without the appropriate antibiotic. For in vitro experiments done with daptomycin, the BM was supplemented with calcium chloride, as previously described (11). In all cases, the medium used for in vitro assays was prepared fresh daily. Comparisons were done using multiple concentrations of each antibiotic. Specifically, the concentrations used corresponded to 5×, 10×, and 20× the breakpoint MIC for a susceptible strain of S. aureus, as defined by the United States Food and Drug Administration (FDA) (Table 1).
TABLE 1.
Relationship between breakpoint MIC and MIC for each test strain
| Antibiotic | BkPta (μg/ml) | LAC |
UAMS-1 |
||
|---|---|---|---|---|---|
| MIC (μg/ml) | Ratio (MIC/BkPt) | MIC (μg/ml) | Ratio (MIC/BkPt) | ||
| Vancomycin | 2.0 | 2.0 | 1.0 | 1.5 | 0.75 |
| Daptomycin | 1.0 | 0.5 | 0.5 | 0.5 | 0.5 |
| Ceftaroline | 1.0 | 0.5 | 0.5 | 0.5 | 0.5 |
| Tigecycline | 0.5 | 0.125 | 0.25 | 0.19 | 0.38 |
| Telavancin | 0.12 | 0.047 | 0.39 | 0.047 | 0.39 |
| Oxacillin | 2.0 | 128 | 64 | 1.5 | 0.75 |
BkPt, breakpoint MIC. The breakpoint MICs cited are those defined by the United States Food and Drug Administration (FDA) for a susceptible strain of S. aureus.
To compensate for the fact that telavancin has a much lower breakpoint MIC than the other antibiotics tested (Table 1), we also evaluated telavancin at 40×, 80×, and 160× its breakpoint MIC, thus allowing us to draw comparisons between approximately equal physical concentrations of telavancin and daptomycin. To make direct comparisons between the lipoglycopeptides, which have been reported to exhibit good penetration and relatively high efficacy in the context of a biofilm (17–19), we also evaluated oritavancin and dalbavancin at concentrations corresponding to 160× the telavancin breakpoint MIC, thus allowing us to draw comparisons between approximately equal physical concentrations of these antibiotics and daptomycin. In addition, a subset of antibiotics chosen for reasons discussed below was also tested at concentrations corresponding to multiples of the actual MICs for the test strains.
Antibacterial effects were assessed after 24 and 72 h of antibiotic exposure. For catheters exposed for 72 h, catheters were removed after 24 and 48 h, rinsed in sterile phosphate-buffered saline (PBS), and transferred to wells with fresh medium with and without antibiotics. After exposure for 24 or 72 h, catheters were removed, rinsed in sterile PBS to remove nonadherent bacteria, and sonicated in sterile PBS to remove adherent bacteria. After sonication, samples were serially diluted and 100-μl aliquots were plated on tryptic soy agar to quantify the number of viable CFU per catheter. Using this experimental method, the limit of detection was 50 CFU per catheter.
Assessment of antibiotic susceptibility in vivo.
To test activity in vivo, we used a murine model of catheter-associated biofilm infection as previously described (15). Briefly, 1-cm catheter sections were implanted subcutaneously into the flanks of NIH Swiss mice. LAC (105 CFU) in a total volume of 100 μl was then injected into the lumen of each catheter. Beginning 24 h later, 100 μl of the test antibiotic at the concentrations indicated below was injected into the lumen daily for 5 days. Control mice were injected daily with 100 μl of sterile PBS. At the completion of each experiment, catheters were processed as previously described (15). The animal studies were approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee.
Statistical methods.
Statistical comparisons were made using the Mann-Whitney test. Using the same experimental model employed in these studies, we previously demonstrated that daptomycin has greater activity than vancomycin in the context of a biofilm formed by UAMS-1 (11). On the basis of this finding, statistical comparisons were made on the basis of activity relative to the activities of these two antibiotics. However, additional comparisons were made to assess the activities of lipoglycopeptide antibiotics relative to each other.
RESULTS AND DISCUSSION
After 24 h in the absence of antibiotic exposure, no significant difference with respect to the average colony counts per catheter was observed between UAMS-1 and LAC (Fig. 1). However, a significant difference was observed after 72 h, with the biofilms formed by UAMS-1 containing, on average, 2.5 times more viable bacterial cells than those formed by LAC (1.16 × 108 ± 1.48 × 108 versus 4.58 × 107 ± 5.71 × 107; n = 54). The finding that there was no significant difference between the number of bacteria within the biofilms formed by these two strains at 24 h suggests that biofilms formed by LAC become relatively static over time, while those formed by UAMS-1 continue to develop. This difference accounts in part for our inclusion of these two time points in our studies, with the other relevant consideration being the likelihood that the results would be impacted in an antibiotic-dependent manner by the time of exposure to the test antibiotic.
FIG 1.
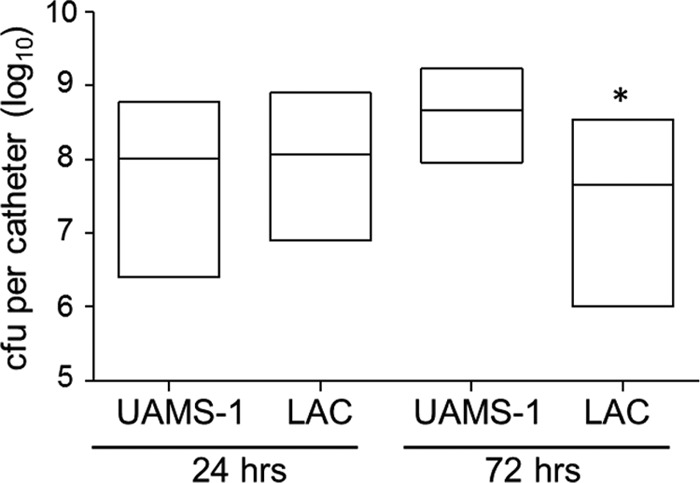
Relative capacity of UAMS-1 and LAC to form a biofilm in vitro. The relative capacity of each strain to form a biofilm was evaluated using our catheter model after 24 and 72 h of colonization without antibiotic exposure. Results are shown as the number of CFU per catheter, with each box illustrating the maximum and minimum values observed within each experimental group and the horizontal line indicating the mean for that group. *, statistically significant difference (P < 0.05) between the number of viable biofilm-associated bacteria formed by LAC relative to the number of viable biofilm-associated bacteria formed by UAMS-1 at 72 h.
The number of potential comparisons in the experiments that we report is very large. Thus, we focused on the fact that vancomycin is the primary antibiotic used for the treatment of MRSA infections and our previous results demonstrating that daptomycin exhibits significantly greater activity than vancomycin in the context of a biofilm (11). Specifically, we based our statistical analysis on whether each antibiotic exhibited increased activity relative to that of each of these two antibiotics when tested at equivalent multiples of the breakpoint MIC for each antibiotic.
After 24 h of exposure of biofilms formed by UAMS-1, daptomycin was found to exhibit significantly greater activity than vancomycin at all concentrations tested (Fig. 2). This is consistent with the results observed in our previous study (11). A significant difference between vancomycin and both oxacillin and tigecycline at all concentrations tested and between ceftaroline and vancomycin at 20× the breakpoint MIC was also observed, with all of these antibiotics exhibiting significantly greater activity than vancomycin when the activities at corresponding concentrations were compared. However, no antibiotic was found to have activity greater than that of daptomycin at any concentration tested at the 24-h time point (Fig. 2).
FIG 2.
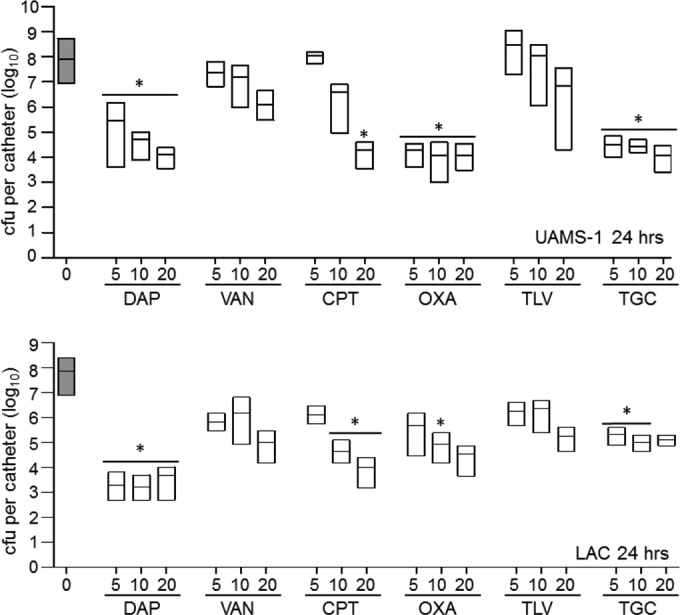
Relative activity of different antibiotics against LAC and UAMS-1 at 24 h in the context of a biofilm in vitro. Activity was evaluated using daptomycin (DAP), vancomycin (VAN), ceftaroline (CPT), oxacillin (OXA), telavancin (TLV), and tigecycline (TGC) at concentrations corresponding to 5×, 10×, and 20× the breakpoint MIC for each antibiotic. Results are shown as the number of CFU per catheter, with each box illustrating the maximum and minimum values observed within each experimental group and the horizontal line indicating the mean for that group. Gray bars, results observed with catheters that were not exposed to any antibiotic; white bars, results observed after exposure of UAMS-1 (top) or LAC (bottom) biofilms to the indicated antibiotics; *, significant reduction in the number of viable bacteria (P ≤ 0.05) relative to that achieved with vancomycin at the equivalent concentration.
After 24 h, the increased activity of daptomycin relative to that of vancomycin was also evident at all concentrations tested when these experiments were repeated using biofilms formed by LAC (Fig. 2). Additionally, while ceftaroline, tigecycline, and even oxacillin exhibited greater activity than vancomycin, depending on the antibiotic concentration, none exhibited greater activity than daptomycin at any of the concentrations tested (Fig. 2). The activity observed with oxacillin was surprising, given that LAC is an MRSA strain. However, when it was tested using UAMS-1, oxacillin exhibited greater activity than vancomycin at every concentration tested, and this was not the case with LAC.
When UAMS-1 biofilms were exposed to the same antibiotics for 72 h, daptomycin, ceftaroline, oxacillin, and tigecycline all exhibited greater activity than vancomycin at one or more of the concentrations tested (Fig. 3). No antibiotic tested exhibited greater activity than daptomycin at an equivalent concentration, with the exception of that activity of oxacillin at 5× the breakpoint MIC compared to that of daptomycin at 5× the breakpoint MIC. Additionally, ceftaroline, oxacillin, and tigecycline all exhibited activity comparable to that observed with daptomycin at one or more concentrations (Fig. 3). Most importantly, only daptomycin at 10× and 20× the breakpoint MIC and ceftaroline at 20× the breakpoint MIC were capable of clearing the catheters of viable biofilm-associated bacteria, as defined by the detection limit of our assay.
FIG 3.
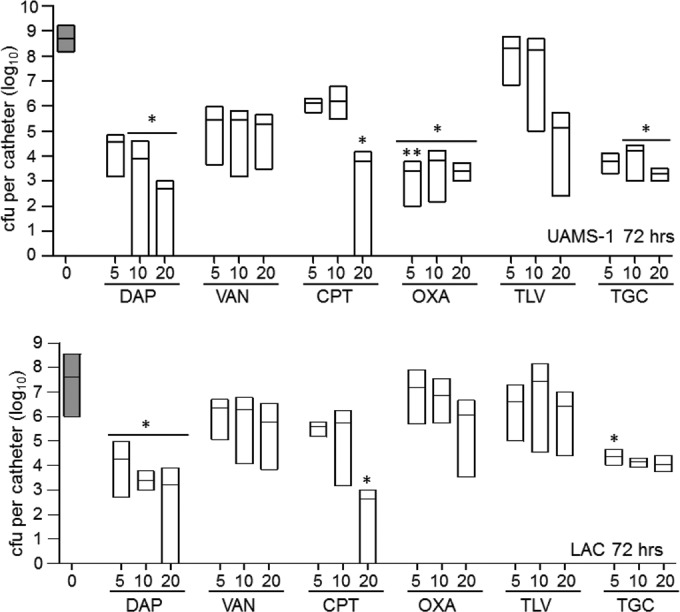
Relative activity of different antibiotics against LAC and UAMS-1 at 72 h in the context of a biofilm in vitro. Activity was evaluated using daptomycin (DAP), vancomycin (VAN), ceftaroline (CPT), oxacillin (OXA), telavancin (TLV), and tigecycline (TGC) at concentrations corresponding to 5×, 10×, and 20× the breakpoint MIC for each antibiotic. Results are shown as the number of CFU per catheter, with each box illustrating the maximum and minimum values observed within each experimental group and the horizontal line indicating the mean for that group. Gray bars, results observed with catheters that were not exposed to any antibiotic; white bars, results observed after exposure of UAMS-1 (top) or LAC (bottom) biofilms to the indicated antibiotics. *, significant reduction in the number of viable bacteria (P ≤ 0.05) relative to that achieved with vancomycin at the equivalent concentration; **, significant reduction relative to that achieved with daptomycin at the equivalent concentration.
With the exception of oxacillin, the same general trends were observed when the 72-h experiments were repeated using biofilms formed by LAC. Specifically, daptomycin was shown to be superior to vancomycin at all concentrations tested. Ceftaroline at 20× the breakpoint MIC and tigecycline at 5× the breakpoint MIC were also found to exhibit greater activity than equivalent concentrations of vancomycin (Fig. 3). As with UAMS-1, daptomycin and ceftaroline were the only antibiotics found to clear any catheters of viable bacteria. In fact, 20× the breakpoint MICs of both daptomycin and ceftaroline were shown to reduce the number of viable bacteria to below the limit of detection in 50% of the catheters tested after 72 h of antibiotic exposure (data not shown). The increased clearance observed with LAC relative to that observed with UAMS-1 is likely a reflection of the fact that UAMS-1 formed a more robust biofilm than LAC at the 72-h time point (Fig. 1).
The lipoglycopeptide antibiotic telavancin includes a membrane-active component, in addition to its cell wall-inhibitory activity, and has shown promise in multiple animal models of infection (20, 21), but it exhibited relatively little activity by comparison to that of daptomycin and ceftaroline against both UAMS-1 and LAC biofilms at the concentrations initially tested in vitro. It is important to note in this respect that neither of these antibiotics was included for comparison in the previous studies focusing on telavancin (20, 21). However, telavancin also has a very low breakpoint MIC by comparison to the breakpoint MICs all of the other antibiotics that we tested (Table 1), thus leaving open the possibility that the results were skewed in favor of these other antibiotics by using multiples of the breakpoint MIC. To address this, we repeated the experiments using telavancin at 40×, 80×, and 160× its breakpoint MIC, with the last concentration (19.2 μg per ml) being comparable to 20× the breakpoint MIC of daptomycin (20 μg per ml). Under these circumstances, telavancin at 160× the breakpoint MIC did, in fact, exhibit activity comparable to that of daptomycin at 20× the breakpoint MIC at 72 h against LAC but not against UAMS-1 (Fig. 4). In fact, telavancin at 160× the breakpoint MIC was capable of reducing the number of viable bacteria to below the limit of detection. After 24 h of exposure, telavancin at 40×, 80×, and 160× the breakpoint MIC exhibited activity comparable to that of daptomycin at 20× the breakpoint MIC when tested using biofilms formed by UAMS-1, but after 72 h, daptomycin at 20× the breakpoint MIC exhibited activity greater than that of telavancin at all of these concentrations (Fig. 4).
FIG 4.
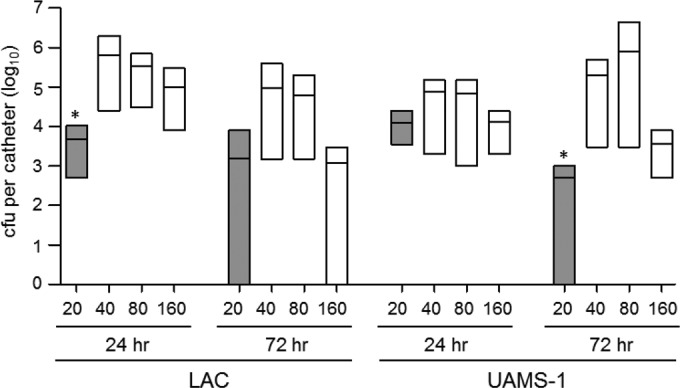
Evaluation of telavancin activity in vitro. Activity was evaluated by comparison of the activity of daptomycin at a concentration corresponding to 20× the breakpoint MIC (gray bars) to that of telavancin at 40×, 80×, and 160× the breakpoint MIC (white bars). Results are shown as the number of CFU per catheter, with each box illustrating the maximum and minimum values observed within each experimental group and the horizontal line indicating the mean for that group. *, significant reduction in the number of viable bacteria (P ≤ 0.05) achieved with daptomycin at 20× the breakpoint MIC relative to that achieved with telavancin at 160× the breakpoint MIC.
To determine whether similar results were observed with other lipoglycopeptides, we also carried out experiments comparing oritavancin and dalbavancin at physical concentrations equivalent to 160× the breakpoint MIC of telavancin (Table 1). In LAC biofilms, telavancin exhibited significantly greater activity than either of these other antibiotics (Fig. 5). Dalbavancin exhibited a level of activity that was greater than that observed with oritavancin but less than that observed with telavancin. In UAMS-1 biofilms, telavancin exhibited significantly greater activity than oritavancin and activity comparable to that of dalbavancin.
FIG 5.
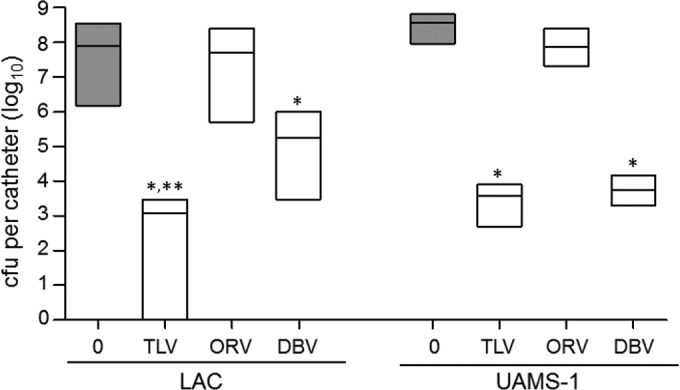
Comparison of the activity of lipoglycopeptide antibiotics in vitro. The activities of telavancin (TLV), oritavancin (ORV), and dalbavancin (DBV) at an equal concentration corresponding to 160× the breakpoint MIC of telavancin (19.2 μg per ml) were compared. The results obtained with catheters that were exposed to antibiotic (white bars) for 72 h relative to those obtained with catheters that were not exposed to antibiotic (gray bars) are shown. Results are shown as the number of CFU per catheter, with each box illustrating the maximum and minimum values observed within each experimental group and the horizontal line indicating the mean for that group. *, significant reduction in the number of viable bacteria (P ≤ 0.05) relative to that achieved with oritavancin; **, significant reduction relative to that achieved with dalbavancin.
These experiments were done using the commercially available formulation of telavancin (Vibativ), which includes a number of inactive ingredients, including hydroxypropyl-beta-cyclodextrin and mannitol (Theravance Biopharma Antibiotics, Inc.), and a recent report demonstrated the need to reconsider the in vitro methods used for determining the MIC of telavancin in multiple bacterial species, including staphylococci (22). To address this, we reassessed the activity of telavancin in the context of a biofilm using the revised protocol recommended by the earlier report (22). The key element of this alternative approach is the use of telavancin powder rather than the commercial preparation and the use of dimethyl sulfoxide as the diluent in the presence of polysorbate 80 (22). The motivation for this was not only the observation that testing methods have a significant impact on the determination of MIC values for all lipoglycopeptides but also the need to consider the activity of antibiotics in alternative clinical contexts, including localized, matrix-based delivery, particularly in postdebridement and/or trauma-associated orthopedic procedures. However, we observed no differences in the results as a function of the drug formulation utilized (data not shown).
Although comparison of the activities of antibiotics on the basis of their respective breakpoint MICs allows the broad applicability of the results, it is possible that this could skew the data in favor of those antibiotics for which a particular strain has a relatively low MIC compared to the breakpoint MIC. More directly, comparisons based on multiples of the breakpoint MIC result in lower multiples of the MIC for any antibiotic for which the ratio of the MIC to the breakpoint MIC is higher than that of another antibiotic. To assess this possibility, the MIC of each antibiotic for each test strain was determined by the Clinical Microbiology Laboratory at Arkansas Children's Hospital. This information was then used to calculate a ratio of the MIC relative to the breakpoint MIC for each strain with each antibiotic (Table 1). We then focused our assessment on a comparison of daptomycin and ceftaroline, which were shown to have greater activity than the other antibiotics tested but also had relatively low ratios (0.5) that could have skewed the results for the reasons discussed above, to vancomycin and oxacillin, which were the only antibiotics that exhibited higher ratios. These antibiotics were compared at concentrations corresponding to 20× and 40× the MIC for each strain.
When tested at 20× the MIC, daptomycin was the only antibiotic found to have significantly greater activity than vancomycin against both UAMS-1 and LAC (Fig. 6). Ceftaroline was also found to have significantly greater activity than vancomycin against LAC but not UAMS-1. Against UAMS-1, daptomycin was also the only antibiotic found to exhibit significantly greater activity than vancomycin when they were tested at 40× the MIC, but this was not true against LAC. In fact, against LAC, even the difference observed between ceftaroline and vancomycin did not reach statistical significance when they were examined at 40× the MIC. However, both daptomycin and ceftaroline did demonstrate the ability to reduce the number of viable bacteria to below the limit of detection in some catheters, and this was not true with vancomycin or, with respect to UAMS-1, oxacillin (Fig. 6). These results are consistent with comparisons made on the basis of breakpoint MICs, thus confirming that daptomycin and ceftaroline do in fact exhibit greater activity than vancomycin in the context of a biofilm.
FIG 6.
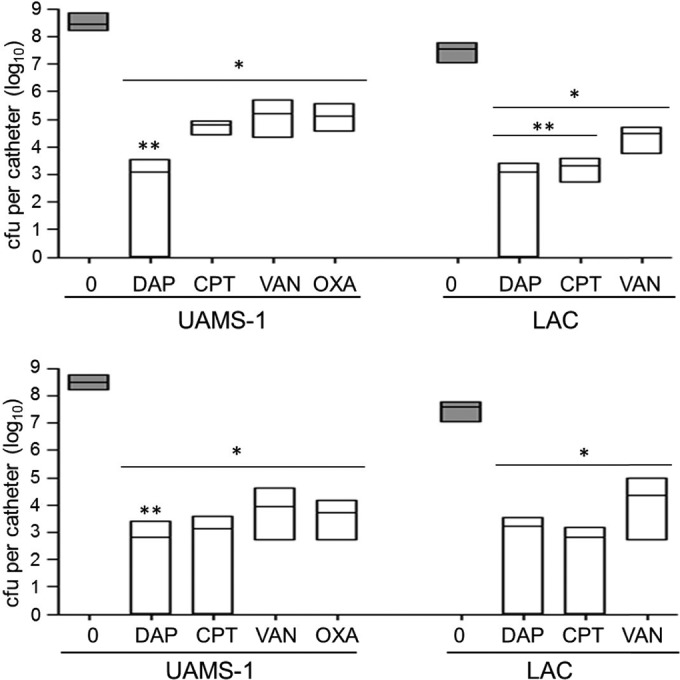
Relative activity of different antibiotics assessed in vitro based on the MIC. Activity was evaluated after 72 h of exposure using daptomycin (DAP), ceftaroline (CPT), vancomycin (VAN), and oxacillin (OXA) at concentrations corresponding to 20× (top) and 40× (bottom) the MIC for each test strain, as determined by Etest. Gray bars, results observed with catheters that were not exposed to any antibiotic; white bars, results observed after exposure to the indicated antibiotics. *, significant reduction in the number of viable bacteria (P ≤ 0.05) relative to the number of untreated control bacteria; **, significant reduction relative to that achieved with vancomycin at the equivalent concentration.
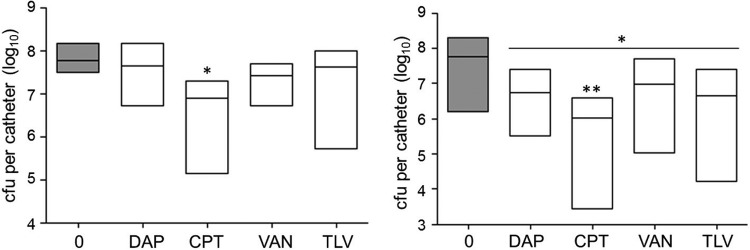
We next tested the efficacy of daptomycin, ceftaroline, and telavancin relative to that of vancomycin under in vivo conditions using concentrations corresponding to 20× and 40× the breakpoint MIC for each antibiotic. These three antibiotics were chosen because they were the only antibiotics capable of reducing the number of viable bacteria to below the limit of detection under any of the conditions tested. Interestingly, at 20× the breakpoint MIC, none of the differences observed between these antibiotics reached statistical significance, and only treatment with ceftaroline was found to result in a significant reduction in the number of bacteria relative to the number of bacteria for the PBS-treated control (Fig. 7). At 40× the breakpoint MIC, all antibiotics were found to have a significant effect relative to that of the PBS-treated control, but only ceftaroline was found to exhibit greater efficacy than vancomycin.
FIG 7.
Relative activity of different antibiotics assessed in vivo. Catheters colonized with LAC were evaluated after 5 days of exposure to daptomycin (DAP), ceftaroline (CPT), vancomycin (VAN), and telavancin (TLV) at a concentration corresponding to 20× (left) or 40× (right) the breakpoint MIC for each antibiotic. Gray bars, results observed with catheters that were not exposed to any antibiotic; white bars, results observed after exposure to the indicated antibiotics. *, significant reduction in the number of viable bacteria (P ≤ 0.05) relative to the number of untreated control bacteria; **, statistical significance by comparison to the results obtained with vancomycin.
In summary, these results illustrate the intrinsic resistance that defines S. aureus biofilms, thus emphasizing the need to evaluate antibiotics in the context of an established biofilm. In fact, it is not clear if the concentrations used in our study can be achieved at the site of infection when antibiotics are administered systemically. However, antibiotics are also often administered locally using some form of matrix-based delivery system, particularly in orthopedic medicine (11, 23–25). We also recently described a novel nanocage-based system for the targeted local delivery of daptomycin (26). Thus, even if sufficient systemic levels cannot be achieved, it remains important to prioritize antibiotics for use in local antibiotic delivery systems.
In this respect, the results that we report confirm the greater activity of the membrane-active agent daptomycin relative to that of vancomycin. They also demonstrate that ceftaroline has activity comparable to that of daptomycin in the context of biofilms formed by both MSSA and MRSA strains, particularly at the higher concentrations tested, and even greater efficacy than daptomycin when tested under in vivo conditions. Indeed, the activity of these antibiotics relative to that of oxacillin suggests that they may offer a therapeutic advantage even in the context of MSSA infections. It is also worth noting that, in the context of biofilms formed by MRSA strain LAC, telavancin exhibited greater activity than any other lipoglycopeptide. This suggests that in at least some patients suffering from biofilm-associated infections caused by MRSA strains, telavancin may also be a viable therapeutic option, particularly since it was one of only three antibiotics shown to clear an established biofilm under any test condition. Most importantly, all of these results must be interpreted relative to those for vancomycin, which was relatively ineffective by comparison to daptomycin and ceftaroline yet remains the primary choice for the treatment of MRSA infections.
ACKNOWLEDGMENT
The content is solely the responsibility of the authors and does not represent the views of the NIH or the U.S. Department of Defense.
Funding Statement
Additional support was provided by core facilities supported by the Center for Microbial Pathogenesis and Host Inflammatory Responses (P20-GM103450) and Translational Research Institute (UL1TR000039).
REFERENCES
- 1.Brady RA, Leid JG, Calhoun JH, Costerton JW, Shirtliff ME. 2013. Evaluation of genetically inactivated alpha toxin for protection in multiple mouse models of Staphylococcus aureus infection. PLoS One 8:e63040. doi: 10.1371/journal.pone.0063040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korol E, Johnston K, Waser N, Sifakis F, Jafri HS, Lo M, Kyaw MH. 2013. A systematic review of risk factors associated with surgical site infections among surgical patients. PLoS One 8:e83743. doi: 10.1371/journal.pone.0083743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mootz JM, Benson MA, Heim CE, Crosby HA, Kavanaugh JS, Dunman PM, Kielian T, Torres VJ, Horswill AR. 2015. Rot is a key regulator of Staphylococcus aureus biofilm formation. Mol Microbiol 96:388–404. doi: 10.1111/mmi.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darouiche RO. 2004. Treatment of infections associated with surgical implants. N Engl J Med 350:1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 5.Jorgensen NP, Meyer R, Dagnaes-Hansen F, Fuursted K, Peterson E. 2014. A modified chronic infection model for testing treatment of Staphylococcus aureus biofilms on implants. PLoS One 9:e103688. doi: 10.1371/journal.pone.0103688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parvizi J, Pawasarat IM, Azzam KA, Joshi A, Hansen EN, Bozic KJ. 2010. Periprosthetic joint infection: the economic impact of methicillin-resistant infections. J Arthroplasty 25(Suppl 6):S103–S107. doi: 10.1016/j.arth.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 7.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. 2010. Community-associated methicillin-resistant Staphylococcus aureus. Lancet 375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brady RA, Leid JG, Calhoun JH, Costerton JW, Shirtliff ME. 2008. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol Med Microbiol 52:13–22. doi: 10.1111/j.1574-695X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- 9.Jennings JA, Carpenter DP, Troxel KS, Beenken KE, Smeltzer MS, Courtney HS, Haggard WO. 2015. Novel antibiotic-loaded point-of-care implant coating inhibits biofilm. Clin Orthop Relat Res 473:2270–2282. doi: 10.1007/s11999-014-4130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Römling U, Balsalobre C. 2012. Biofilm infections, their resilience to therapy and innovative treatment strategies. J Intern Med 272:541–561. doi: 10.1111/joim.12004. [DOI] [PubMed] [Google Scholar]
- 11.Weiss EC, Spencer HJ, Daily SJ, Weiss BD, Smeltzer MS. 2009. Impact of sarA on antibiotic susceptibility of Staphylococcus aureus in a catheter-associated in vitro model of biofilm formation. Antimicrob Agents Chemother 53:2475–2482. doi: 10.1128/AAC.01432-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cierny G. 2011. Surgical treatment of osteomyelitis. Plast Reconstr Surg 127(Suppl 1):190S–204S. doi: 10.1097/PRS.0b013e3182025070. [DOI] [PubMed] [Google Scholar]
- 13.Kurtz SM, Ong KL, Lau E, Bozic KJ. 2014. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am 96:624–630. doi: 10.2106/JBJS.M.00285. [DOI] [PubMed] [Google Scholar]
- 14.Bjarnsholt T, Ciofu O, Molin S, Givskov M, Høiby N. 2013. Applying insights from biofilm biology to drug development—can a new approach be developed? Nat Rev Drug Discov 12:791–808. doi: 10.1038/nrd4000. [DOI] [PubMed] [Google Scholar]
- 15.Weiss EC, Zielinska A, Beenken KE, Spencer HJ, Daily SJ, Smeltzer MS. 2009. Impact of sarA on daptomycin susceptibility of Staphylococcus aureus biofilms in vivo. Antimicrob Agents Chemother 53:4096–4102. doi: 10.1128/AAC.00484-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmes NE, Howden BP. 2014. What's new in the treatment of serious MRSA infection? Curr Opin Infect Dis 27:471–478. doi: 10.1097/QCO.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 17.Baldoni D, Furustrand Tafin U, Aeppli S, Angevaare E, Oliva A, Haschke M, Zimmerli W, Trampuz A. 2013. Activity of dalbavancin, alone and in combination with rifampicin, against methicillin-resistant Staphylococcus aureus in a foreign-body infection model. Int J Antimicrob Agents 42:220–225. doi: 10.1016/j.ijantimicag.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Belley A, Neesham-Grenon E, McKay G, Arhin FF, Harris R, Beveridge T, Parr TR, Moeck G. 2009. Oritavancin kills stationary-phase and biofilm Staphylococcus aureus cells in vitro. Antimicrob Agents Chemother 53:918–925. doi: 10.1128/AAC.00766-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirker KR, Fisher ST, James GA. 2015. Potency and penetration of telavancin in staphylococcal biofilms. Int J Antimicrob Agents 46:451–455. doi: 10.1016/j.ijantimicag.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Hegde SS, Janc JW. 2014. Efficacy of telavancin, a lipoglycopeptide antibiotic, in experimental models of Gram-positive infection. Expert Rev Anti Infect Ther 12:1463–1475. doi: 10.1586/14787210.2014.979789. [DOI] [PubMed] [Google Scholar]
- 21.Chan C, Hardin TC, Smart JI. 2015. A review of telavancin activity in in vitro biofilms and animal models of biofilm-associated infections. Future Microbiol 10:1325–1338. doi: 10.2217/fmb.15.53. [DOI] [PubMed] [Google Scholar]
- 22.Farrell DJ, Mendes RE, Rhomberg PR, Jones RN. 2014. Revised reference broth microdilution method for testing telavancin: effect on MIC results and correlation with other testing methodologies. Antimicrob Agents Chemother 58:5547–5551. doi: 10.1128/AAC.03172-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beenken KE, Smith JK, Skinner RA, McLaren SG, Bellamy W, Gruenwald MJ, Spencer HJ, Haggard WO, Smeltzer MS. 2014. Chitosan coating to enhance the therapeutic efficacy of calcium sulfate-based antibiotic therapy in the treatment of chronic osteomyelitis. J Biomater Appl 29:514–523. doi: 10.1177/0885328214535452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beenken KE, Bradney L, Bellamy W, Skinner RA, McLaren SG, Gruenwald MJ, Spencer HJ, Smith JK, Haggard WO, Smeltzer MS. 2012. Use of xylitol to enhance the therapeutic efficacy of polymethylmethacrylate-based antibiotic therapy in treatment of chronic osteomyelitis. Antimicrob Agents Chemother 56:5839–5844. doi: 10.1128/AAC.01127-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker AC, Beenken KE, Jennings JA, Hittle L, Shirtliff ME, Bumgardner JD, Smeltzer MS, Haggard WO. 2015. Characterization of local delivery with amphotericin B and vancomycin from modified chitosan sponges and functional biofilm prevention evaluation. J Orthop Res 33:439–447. doi: 10.1002/jor.22760. [DOI] [PubMed] [Google Scholar]
- 26.Meeker DG, Jenkins SV, Miller EK, Beenken KE, Loughran AJ, Powless A, Muldoon TJ, Galanzha EI, Zharov VP, Smeltzer MS, Chen J. 2016. Synergistic photothermal and antibiotic killing of biofilm-associated Staphylococcus aureus using targeted antibiotic-loaded gold nanoconstructs. ACS Infect Dis 2:241–250. doi: 10.1021/acsinfecdis.5b00117. [DOI] [PMC free article] [PubMed] [Google Scholar]