Abstract
We report that the tuberculosis drug SQ109 [N-adamantan-2-yl-N′-((E)-3,7-dimethyl-octa-2,6-dienyl)-ethane-1,2-diamine] has potent activity against the intracellular amastigote form of Leishmania mexicana (50% inhibitory concentration [IC50], ∼11 nM), with a good selectivity index (>500). It is also active against promastigotes (IC50, ∼500 nM) and acts as a protonophore uncoupler, in addition to disrupting Ca2+ homeostasis by releasing organelle Ca2+ into the cytoplasm, and as such, it is an interesting new leishmaniasis drug hit candidate.
TEXT
There is a need for new drugs to treat the neglected tropical diseases, in particular, Chagas disease and leishmaniasis. In previous work (1–5), we discovered that the antiarrhythmia drugs amiodarone (Fig. 1, compound 1) and dronedarone (Fig. 1, compound 2) had activity against Trypanosoma cruzi as well as Leishmania mexicana, the causative agents of Chagas disease and one form of cutaneous leishmaniasis, respectively. In addition, amiodarone was found to have partial in vivo activity against T. cruzi in mice, which was considerably increased when added in combination with posaconazole (1), and in initial clinical work in humans, it has been used to treat parasitic infections (6, 7). The mechanism of action of compounds 1 and 2 is thought to involve uncoupling activity, with the release of Ca2+ from intracellular organelles (acidocalcisomes and mitochondria), as well as inhibition of oxidosqualene synthase and hence, ergosterol biosynthesis. Interestingly, another type of uncoupler, the nitrothiazole nitazoxanide (Fig. 1, compound 3), and its active metabolite, tizoxanide (Fig. 1, compound 4), also have activity against T. cruzi and L. mexicana (8), and compound 4 has been shown to act, at least in part, as an uncoupler, in Mycobacterium tuberculosis (9).
FIG 1.
Structures of compounds discussed in the text.
Since we and others recently reported (10–13) that another M. tuberculosis drug/drug lead (13–15), SQ109 (Fig. 1, compound 5) [N-adamantan-2-yl-N′-((E)-3,7-dimethyl-octa-2,6-dienyl)-ethane-1,2-diamine], also acted as an uncoupler in Mycobacterium smegmatis, we tested it against T. cruzi, finding 50% inhibitory concentrations (IC50s) of ∼50 nM against trypomastigotes, ∼5 μM against epimastigotes, and ∼1 μM against amastigotes (13). The amastigote result was disappointing, being less effective than that we found with dronedarone (∼1 nM); however, since both amiodarone (Cordarone) and dronedarone (Multaq) come with “black box” warnings, we elected to test SQ109 against L. mexicana, since it seemed possible that it might have good activity against this parasitic protozoan, much in the same way as it does against M. tuberculosis (and M. smegmatis).
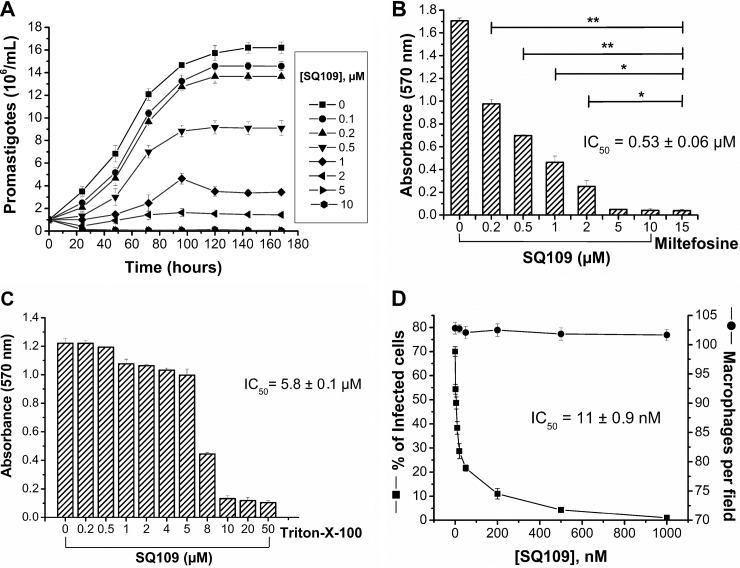
We show in Fig. 2A and B the effects of SQ109 on the viability of L. mexicana promastigotes and intracellular amastigotes (inside J774 macrophages) and on macrophage viability (Fig. 2C and D). As can be seen in Fig. 2A and B, SQ109 inhibits the viability of L. mexicana promastigotes in a dose-dependent manner, and as shown in Fig. 2B, the IC50 (after 72 h of treatment) is 0.53 ± 0.06 μM (plus/minus indicates the standard error of the mean for at least three independent experiments). There is little effect on macrophage viability, since the IC50 (after 72 h of treatment) is 5.8 ± 0.1 μM (Fig. 2C).
FIG 2.
Effects of SQ109 on L. mexicana and J774 macrophages. (A) Promastigote growth as a function of time and SQ109 concentration. (B) Effects of SQ109 on the viability of L. mexicana promastigotes. The IC50 (after 72 h of treatment) is 0.53 ± 0.06 μM. Miltefosine is shown as a positive control. The asterisks represent statistically significant differences, determined using the Student t test, P ≤ 0.05 (*) and P ≤ 0.01 (**). (C) Effects of SQ109 on J774 macrophage viability; IC50 (after 72 h of treatment) is 5.8 ± 0.1 μM. (D) Effects of SQ109 on macrophages infected with L. mexicana amastigotes. SQ109 potently inhibits amastigote proliferation, yielding an IC50 (after 48 h of treatment) of 11 ± 0.9 nM. The protocols used in panels A and D are the same as those described in reference 5 and for panels B and C in reference 16 (the error bars represent standard deviation for least three independent experiments).
More significantly, in the intracellular assay (Fig. 2D), the 50% inhibitory concentration against amastigotes in infected macrophages (after 48 h of treatment) was 11 ± 0.9 nM, with no observable effect on uninfected macrophages. These results are clearly much more impressive than those we reported earlier with SQ109 in T. cruzi and represent a good selectivity index (calculated by IC50 of J774 macrophages/IC50 of L. mexicana amastigotes) of >500. The question then arises, what is the mechanism of action of SQ109 in L. mexicana?
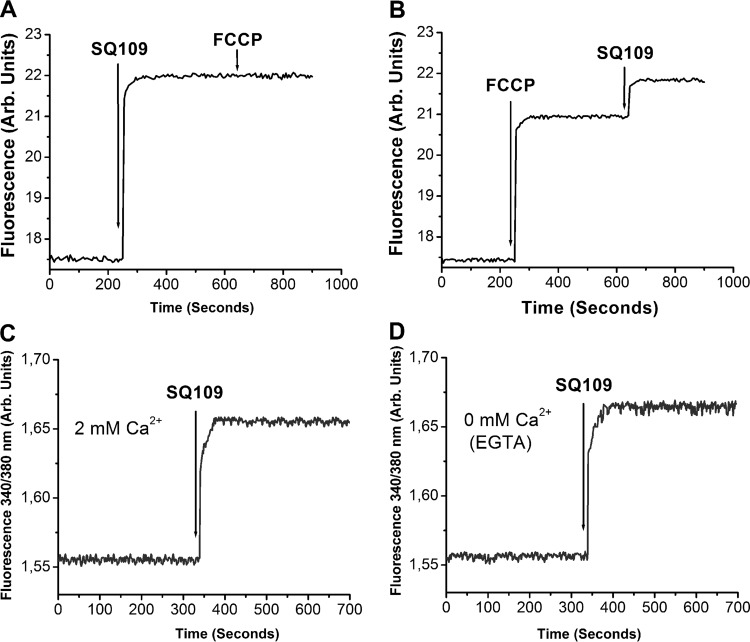
As noted above, in earlier work on M. smegmatis, we and others (10–12) showed that a major mechanism of action of SQ109 (as well as amiodarone) was on the proton motive force (PMF), with SQ109 collapsing pH gradients, as determined by nuclear magnetic resonance spectroscopy, and electrochemical potentials (in both M. smegmatis and Escherichia coli membrane vesicles), as determined by fluorescence spectroscopy. Similar results were obtained with amiodarone and dronedarone in T. cruzi and L. mexicana (1–5). Here, as shown in Fig. 3A, we find that SQ109 collapses the PMF in L. mexicana promastigotes, as determined using rhodamine 123. The addition of the known uncoupler FCCP [carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazine] after the addition of SQ109 (5 μM) has no further effect, since the PMF is already collapsed, but if FCCP (2 μM) is added first, there is a partial collapse in the PMF, which is complete after the addition of SQ109 (5 μM) (Fig. 3B). These results are similar to the effects of dronedarone on L. mexicana (5). Likewise, we find that SQ109 causes a release of Ca2+ from internal Ca2+ (Ca2+i) stored in organelles (Fig. 3C and D). In the presence of 2 mM external Ca2+, the same amount of Ca2+ is released by SQ109 (as determined using fura 2) as in the absence of external Ca2+ (i.e., in the presence of 8 mM ethylene glycol tetraacetic acid [EGTA], a high-affinity Ca2+-specific chelator), which means that it is not the external Ca2+ that is involved in the increase in Ca2+i. Rather, Ca2+ is released from internal stores, mitochondria and acidocalcisomes, again, just as found for T. cruzi and L. mexicana with amiodarone and dronedarone (1–5). This disruption of Ca2+ homeostasis in addition to the effects on the proton motive force (as seen also in mycobacteria [10–12]) are likely to make major contributions to L. mexicana cell killing.
FIG 3.
Effects of SQ109 and FCCP on the mitochondrial electrochemical potential and SQ109 on Ca2+ flux. (A) SQ109 at 5 μM, followed by 2 μM FCCP using rhodamine 123 as a probe of the electrochemical potential. (B) Same as panel A but FCCP added first and then SQ109. (C) Ca2+i as determined using the radiometric fluorescent Ca2+ indicator fura 2 in the presence of 2 mM external Ca2+. (D) Same as panel C but no external Ca2+ (8 mM EGTA). Methods are as described in reference 5.
Overall, the results we described above are of interest, since we find that the tuberculosis drug SQ109, currently in clinical trials, has potent activity (IC50, 11 ± 0.9 nM) against the clinically relevant amastigote form of Leishmania mexicana, inside macrophages, with a selectivity index of >500. SQ109 appears to act, at least in part, as a protonophore uncoupler (as it does in mycobacteria), in addition to releasing Ca2+ from intracellular stores, basically the same mechanism as that found with amiodarone and dronedarone, and as such, it may represent a potential new hit candidate for treating leishmanial diseases.
ACKNOWLEDGMENT
We thank Otto Geoffroy, Alchem Laboratories Corporation, for providing the SQ109.
Funding Statement
This work was supported by the Fondo Nacional de Ciencias, Tecnología e Investigación, Venezuela (FONACIT; grant 2011000884 to G.B.), by the Consejo de Desarrollo Científico y Humanístico-Universidad Central de Venezuela (CDCH-UCV; grant PG-03-8728-2013/2 to G.B.), and in part by the U.S. Public Health Service (NIH grants CA158191 and GM065307 to E.O.), a Harriet A. Harlin Professorship (E.O.), and the University of Illinois Foundation/Oldfield Research Fund.
REFERENCES
- 1.Benaim G, Sanders JM, Garcia-Marchan Y, Colina C, Lira R, Caldera AR, Payares G, Sanoja C, Burgos JM, Leon-Rossell A, Concepcion JL, Schijman AG, Levin M, Oldfield E, Urbina JA. 2006. Amiodarone has intrinsic anti-Trypanosoma cruzi activity and acts synergistically with posaconazole. J Med Chem 49:892–899. doi: 10.1021/jm050691f. [DOI] [PubMed] [Google Scholar]
- 2.Serrano-Martín X, García-Marchan Y, Fernandez A, Rodriguez N, Rojas H, Visbal G, Benaim G. 2009. Amiodarone destabilizes the intracellular Ca2+ homeostasis and the biosynthesis of sterols in Leishmania mexicana. Antimicrob Agents Chemother 53:1403–1410. doi: 10.1128/AAC.01215-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benaim G, Hernandez-Rodriguez V, Mujica-Gonzalez S, Plaza-Rojas L, Silva ML, Parra-Gimenez N, Garcia-Marchan Y, Paniz-Mondolfi A, Uzcanga G. 2012. In vitro anti-Trypanosoma cruzi activity of dronedarone, a novel amiodarone derivative with an improved safety profile. Antimicrob Agents Chemother 56:3720–3725. doi: 10.1128/AAC.00207-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benaim G, Paniz-Mondolfi AE. 2012. The emerging role of amiodarone and dronedarone in Chagas disease. Nat Rev Cardiol 9:605–609. doi: 10.1038/nrcardio.2012.108. [DOI] [PubMed] [Google Scholar]
- 5.Benaim G, Casanova P, Hernandez-Rodriguez V, Mujica-Gonzalez S, Parra-Gimenez N, Plaza-Rojas L, Concepcion JL, Liu Y-L, Oldfield E, Paniz-Mondolfi A, Suarez AI. 2014. Dronedarone, an amiodarone analog with improved anti-Leishmania mexicana efficacy. Antimicrob Agents Chemother 58:2295–2303. doi: 10.1128/AAC.01240-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paniz-Mondolfi AE, Perez-Alvarez AM, Reyes-Jaimes O, Socorro G, Zerpa O, Slova D, Concepcion JL. 2008. Concurrent Chagas' disease and borderline disseminated cutaneous leishmaniasis: the role of amiodarone as an antitrypanosomatidae drug. Ther Clin Risk Manag 4:659–663. doi: 10.2147/TCRM.S2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paniz-Mondolfi AE, Perez-Alvarez AM, Lanza G, Marquez E, Concepcion JL. 2009. Amiodarone and itraconazole: a rational therapeutic approach for the treatment of chronic Chagas' disease. Chemotherapy 55:228–233. doi: 10.1159/000219436. [DOI] [PubMed] [Google Scholar]
- 8.Chan-Bacab MJ, Hernández-Núñez E, Navarrete-Vázquez G. 2009. Nitazoxanide, tizoxanide and a new analogue [4-nitro-N-(5-nitro-1,3-thiazol-2-yl)benzamide; NTB] inhibit the growth of kinetoplastid parasites (Trypanosoma cruzi and Leishmania mexicana) in vitro. J Antimicrob Chemother 63:1292–1293. doi: 10.1093/jac/dkp117. [DOI] [PubMed] [Google Scholar]
- 9.de Carvalho LPS, Darby CM, Rhee KY, Nathan C. 2011. Nitazoxanide disrupts membrane potential and intrabacterial pH homeostasis of Mycobacterium tuberculosis. ACS Med Chem Lett 2:849–854. doi: 10.1021/ml200157f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li K, Schurig-Briccio LA, Feng X, Upadhyay A, Pujari V, Lechartier B, Fontes FL, Yang H, Rao G, Zhu W, Gulati A, No JH, Cintra G, Bogue S, Liu YL, Molohon K, Orlean P, Mitchell DA, Freitas-Junior L, Ren F, Sun H, Jiang T, Li Y, Guo RT, Cole ST, Gennis RB, Crick DC, Oldfield E. 2014. Multitarget drug discovery for tuberculosis and other infectious diseases. J Med Chem 57:3126–3139. doi: 10.1021/jm500131s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Upadhyay A, Fontes F, North EJ, Wang Y, Crans DC, Grzegorzewicz AE, Jones V, Franzblau SG, Lee RE, Crick DC, Jackson M. 2014. Novel insights into the mechanism of inhibition of MmpL3, a target of multiple pharmacophores in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:6413–6423. doi: 10.1128/AAC.03229-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng X, Zhu W, Schurig-Briccio LA, Lindert S, Shoen C, Hitchings R, Li J, Wang Y, Baig N, Zhou T, Kim BK, Crick DC, Cynamon M, McCammon JA, Gennis RB, Oldfield E. 2015. Antiinfectives targeting enzymes and the proton motive force. Proc Natl Acad Sci U S A 112:E7073–E7082. doi: 10.1073/pnas.1521988112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veiga-Santos P, Li K, Lameira L, de Carvalho TMU, Huang G, Galizzi M, Shang N, Li Q, Gonzalez-Pacanowska D, Hernandez-Rodriguez V, Benaim G, Guo RT, Urbina JA, Docampo R, de Souza W, Oldfield E. 2015. SQ109, a new drug lead for Chagas disease. Antimicrob Agents Chemother 59:1950–1961. doi: 10.1128/AAC.03972-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinrich N, Dawson R, du Bois J, Narunsky K, Horwith G, Phipps A, Nacy C, Aarnoutse R, Boeree M, Gillespie S, Venter A, Henne S, Rachow A, Phillips P, Hoelscher M, Diacon AH, Pan African Consortium for the Evaluation of Antituberculosis Antibiotics (PanACEA). 2015. Early phase evaluation of SQ109 alone and in combination with rifampicin in pulmonary TB patients. J Antimicrob Chemother 70:1558–1566. doi: 10.1093/jac/dku553. [DOI] [PubMed] [Google Scholar]
- 15.Sacksteder K, Protopopova M, Barry C, Andries K, Nacy C. 2012. Discovery and development of SQ109: a new antitubercular drug with a novel mechanism of action. Future Microbiol 7:823–837. doi: 10.2217/fmb.12.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muelas-Serrano S, Nogal-Ruiz J, Gómez-Barrio A. 2000. Setting of a colorimetric method to determine the viability of Trypanosoma cruzi epimastigotes. Parasitol Res 86:999–1002. doi: 10.1007/PL00008532. [DOI] [PubMed] [Google Scholar]