Abstract
The direct-acting antiviral regimen of 25 mg ombitasvir–150 mg paritaprevir–100 mg ritonavir once daily (QD) plus 250 mg dasabuvir twice daily (BID) is approved for the treatment of hepatitis C virus genotype 1 infection, including patients coinfected with human immunodeficiency virus. This study was performed to evaluate the pharmacokinetic, safety, and tolerability effects of coadministering the regimen of 3 direct-acting antivirals with two antiretroviral therapies (dolutegravir or abacavir plus lamivudine). Healthy volunteers (n = 24) enrolled in this phase I, single-center, open-label, multiple-dose study received 50 mg dolutegravir QD for 7 days or 300 mg abacavir plus 300 mg lamivudine QD for 4 days, the 3-direct-acting-antiviral regimen for 14 days, followed by the 3-direct-acting-antiviral regimen with dolutegravir or abacavir plus lamivudine for 10 days. Pharmacokinetic parameters were calculated to compare combination therapy with 3-direct-acting-antiviral or antiretroviral therapy alone, and safety/tolerability were assessed throughout the study. Coadministration of the 3-direct-acting-antiviral regimen increased the geometric mean maximum plasma concentration (Cmax) and the area under the curve (AUC) of dolutegravir by 22% (central value ratio [90% confidence intervals], 1.219 [1.153, 1.288]) and 38% (1.380 [1.295, 1.469]), respectively. Abacavir geometric mean Cmax and AUC values decreased by 13% (0.873 [0.777, 0.979]) and 6% (0.943 [0.901, 0.986]), while those for lamivudine decreased by 22% (0.778 [0.719, 0.842]) and 12% (0.876 [0.821, 0.934]). For the 3-direct-acting-antiviral regimen, geometric mean Cmax and AUC during coadministration were within 18% of measurements made during administration of the 3-direct-acting-antiviral regimen alone, although trough concentrations for paritaprevir were 34% (0.664 [0.585, 0.754]) and 27% (0.729 [0.627, 0.847]) lower with dolutegravir and abacavir-lamivudine, respectively. All study treatments were generally well tolerated, with no evidence of increased rates of adverse events during combination administration. These data indicate that the 3-direct-acting-antiviral regimen can be administered with dolutegravir or abacavir plus lamivudine without dose adjustment.
INTRODUCTION
Hepatitis C virus (HCV) and human immunodeficiency virus (HIV) are often found as co-occurring infections. The Centers for Disease Control and Prevention estimate that 25% of individuals with HIV in the United States are coinfected with HCV (1). This proportion increases substantially among individuals with HIV who are injection drug users, a group with an approximately 80% prevalence of comorbid HCV. The co-occurrence of these two infectious diseases markedly enhances the risk for liver-related morbidity and mortality and complicates disease management (1, 2). The multidrug regimens that are typically used to treat HIV and HCV infections can contribute to potential drug-drug interactions (DDI), which may compromise the therapeutic efficacy or safety of these regimens when used in combination (2–4).
The 3-direct-acting-antiviral regimen is a newer treatment option for HCV that does not include interferon; rather, the 3-direct-acting-antiviral regimen is comprised of paritaprevir, ombitasvir, and dasabuvir plus ritonavir, which serves as a pharmacokinetic enhancer for paritaprevir. Paritaprevir is a nonstructural 3/4A (NS3/4A) protease inhibitor identified as a lead compound by AbbVie and Enanta that is coadministered with ritonavir (r) and administered once daily (paritaprevir/r); ombitasvir is an NS5A protease inhibitor administered once daily, and dasabuvir is a nonnucleoside NS5B RNA polymerase inhibitor administered twice daily. The 3-direct-acting-antiviral regimen has demonstrated antiviral efficacy across diverse populations of patients with HCV infection, including those with HIV-1 coinfection (5–12).
Drug-drug interactions with the 3-direct-acting-antiviral regimen may arise through effects on metabolic enzymes or transport proteins. Paritaprevir and ritonavir are metabolized primarily by CYP3A, and dasabuvir is metabolized primarily by CYP2C8 and, to a lesser extent, by CYP3A. Ombitasvir is metabolized by hydrolysis followed by oxidative metabolism. Ritonavir is a known CYP3A inhibitor, whereas paritaprevir, ombitasvir, and dasabuvir do not inhibit CYP enzymes. At clinically relevant concentrations, paritaprevir is an inhibitor of organic anion transporting polypeptide (OATP) 1B1/B3, and paritaprevir, ritonavir, and dasabuvir are potential inhibitors of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP). Paritaprevir, ombitasvir, dasabuvir, and ritonavir are in vitro substrates of P-gp and BCRP, and paritaprevir is also a substrate of OATP1B1/B3 (13). The phase II/III clinical trial that enrolled patients with HCV-HIV-1 coinfection was preceded by formal DDI studies that explored the potential for changes in pharmacokinetic profiles of the components of 3-direct-acting-antiviral regimens and HIV-1 antiretroviral therapy (ART) drugs due to coadministration of the 3-direct-acting-antiviral regimen and atazanavir and raltegravir, as well as HIV-1 nucleos(t)ide ART drugs, such as tenofovir disoproxil fumarate and emtricitabine (14, 15).
The objective of the current study was to evaluate the pharmacokinetics, safety, and tolerability of coadministering the 3-direct-acting-antiviral regimen with dolutegravir or abacavir plus lamivudine. The study was designed to determine potential two-way drug interactions between the 3-direct-acting-antiviral regimen and dolutegravir or abacavir plus lamivudine.
MATERIALS AND METHODS
The study protocol was approved by an institutional review board (Vista Health System, Vista Medical Center East, Waukegan, IL), and study procedures were conducted in accordance with the ethical principles set forth in the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guidelines, and local regulations.
Study population.
Adult male and female volunteers aged 18 to 55 years were eligible to participate in the study. Subjects must have been in general good health with a body mass index between 18 and 30 kg/m2, inclusive. Subjects considered for treatment arm 2, in which abacavir plus lamivudine was to be administered, were required to have a negative HLA-B*5701 allele test to avoid inclusion of individuals who were at increased risk for abacavir hypersensitivity reactions (16). Exclusion criteria included regular use of prescription or over-the-counter medications; use of any known inhibitor or inducer of CYP3A, CYP2C8, or OATP1B1 within 1 month of study drug administration; receipt of any drug by injection within 30 days of study drug administration or within a time frame equivalent to 10 half-lives of the medication; use of any medication within 2 weeks of first study drug dose or within 10 half-lives of the medication; and consumption of grapefruit, Seville oranges, starfruit, and/or quinine/tonic water within 72 h of study drug administration. Subjects with positive test results for hepatitis (A, B, or C) or HIV were excluded from the study. All participants provided written informed consent.
Study design.
Subjects enrolled in this phase I, single-center, open-label, multiple-dose study were divided into two treatment arms (Fig. 1). Subjects in treatment arm 1 received 50 mg dolutegravir (Tivicay; ViiV Healthcare, Research Triangle Park, NC, USA) once daily in the morning for 7 days (i.e., period 1, study days 1 to 7). After a washout period of 7 days, subjects received the 3-direct-acting-antiviral regimen, which consisted of ombitasvir/paritaprevir/ritonavir coformulated tablets (two tablets to provide a total dose of 25, 150, and 100 mg, respectively) administered once daily in the morning and a 250-mg dasabuvir tablet administered twice daily (morning and evening) for 14 days (i.e., period 2, study days 1 to 14). Starting on study day 15 of period 2, 50 mg dolutegravir once daily was coadministered with the 3-direct-acting-antiviral regimen (25 mg ombitasvir–150 mg paritaprevir–100 mg ritonavir administered once daily in the morning and 250 mg dasabuvir administered twice daily) for 10 days. Subjects in treatment arm 2 received 300 mg abacavir (as two tablets of 150 mg each) once daily (Ziagen; ViiV Healthcare, Research Triangle Park, NC, USA) plus a 300-mg lamivudine tablet (Epivir; ViiV Healthcare, Research Triangle Park, NC, USA) once daily in the morning for 4 days (i.e., period 1, study days 1 to 4). After a washout period of 5 days, subjects received the 3-direct-acting-antiviral regimen (as described above) for 14 days (i.e., period 1, study days 1 to 14). Beginning on study day 15 of period 2, subjects received abacavir and lamivudine coadministered with the 3-direct-acting-antiviral regimen (25 mg ombitasvir–150 mg paritaprevir–100 mg ritonavir administered once daily in the morning and 250 mg dasabuvir administered twice daily) for 10 days.
FIG 1.
Study design. The 3-direct-acting-antiviral (3D) regimen consists of two 25 mg ombitasvir–150 mg paritaprevir–100 mg ritonavir coformulated tablets once daily in the morning and one dasabuvir 250-mg tablet twice daily (morning and evening). Dolutegravir at 50 mg, abacavir at 600 mg, and lamivudine at 300 mg were all administered once daily in the morning. All study drugs were taken under nonfasting conditions. PK, pharmacokinetic.
All study drugs were administered under nonfasting conditions. Morning doses were administered approximately 30 min after the start of a standardized breakfast. The evening dose of dasabuvir was administered approximately 30 min after the start of an evening snack.
Pharmacokinetic and safety assessments.
In both treatment arms, blood samples for determination of ombitasvir, paritaprevir, ritonavir, dasabuvir, and the major dasabuvir metabolite (M1) plasma concentrations were collected on study days 14 and 24 of period 2 to characterize the effect of the ARTs on paritaprevir, ombitasvir, dasabuvir, and ritonavir at steady state. Blood samples were collected on day 7 in period 1 and day 24 in period 2 to determine the effect of the 3-direct-acting-antiviral regimen on dolutegravir (treatment arm 1) and on day 4 in period 1 and day 24 in period 2 to determine the effect of the 3-direct-acting-antiviral regimen on abacavir and lamivudine (treatment arm 2) at steady state. Nominal sampling times are provided in Fig. 1.
In arm 1, blood samples were obtained prior to dosing and at 1, 2, 3, 4, 5, 6, 9, 12, 16, 24, 48, and 72 h after dosing on day 7 in period 1 and day 24 in period 2 for dolutegravir. In arm 2, blood samples were obtained prior to dosing and at 1, 1.5, 2, 3, 4, 5, 6, 9, 12, 16, 24, 36, and 48 h after dosing on day 4 in period 1, and samples were obtained prior to dosing and at 1, 1.5, 2, 3, 4, 5, 6, 9, 12, 16, 24, 36, 48, and 72 h on day 24 in period 2 for abacavir and lamivudine. In both arms, blood samples were obtained prior to dosing and at 1, 2, 3, 4, 5, 6, 9, 12, 16, and 24 h after dosing on day 14 in period 2 and prior to dosing and at 1, 2, 3, 4, 5, 6, 9, 12, 16, 24, 48, and 72 h after dosing on day 24 in period 2 for ombitasvir, paritaprevir, ritonavir, dasabuvir, and the major dasabuvir metabolite (M1).
We determined plasma concentrations of paritaprevir, ritonavir, ombitasvir, dasabuvir, and the M1 metabolite of dasabuvir (dasabuvir M1) using validated liquid chromatography methods with tandem mass spectrometric detection as described previously (17). Plasma concentrations of dolutegravir, abacavir, and lamivudine were determined using validated liquid chromatography methods with tandem mass spectrometric detection at commercial laboratories (PPD, Middleton, WI, for dolutegravir and inVentive Health Clinique, Quebec, Canada, for abacavir and lamivudine). The lower limits of quantification (LLOQ) were 0.567 ng/ml (paritaprevir), 4.63 ng/ml (ritonavir), 0.445 ng/ml (ombitasvir), 4.31 ng/ml (dasabuvir), 4.58 ng/ml (dasabuvir M1), 20 ng/ml (dolutegravir), 5 ng/ml (abacavir), and 10 ng/ml (lamivudine). Results below the LLOQ were recorded as a value of 0.
Safety and tolerability were evaluated based on adverse event monitoring, vital sign measurements, physical examinations, electrocardiography, and clinical laboratory assessments. Subjects were also monitored for the appearance of signs and symptoms of abacavir hypersensitivity; study drug treatment was withheld if hypersensitivity was suspected.
Pharmacokinetic variables.
Pharmacokinetic parameters, including maximum plasma concentration (Cmax), predose trough plasma concentration (Ctrough; C24 and C12 for drugs administered once and twice daily, respectively), time to Cmax (Tmax), area under the plasma concentration-time curve (AUC; AUC24 and AUC12 for drugs administered once and twice daily, respectively), and terminal elimination half-life (t1/2) were estimated using noncompartmental methods.
Statistical analysis.
Statistical analyses were performed using SAS, version 9.2 (SAS Institute, Inc., Cary, NC, USA), with SAS procedure PROC MIXED. Effects of the 3-direct-acting-antiviral regimen on dolutegravir or abacavir plus lamivudine and vice versa were estimated by analyzing natural log-transformed Cmax, AUC, and Ctrough (C24 for once-daily and C12 for twice-daily regimens) values under a repeated-measures analysis framework. Central value ratios and 90% confidence intervals (CIs) for Cmax, AUC, and Ctrough were calculated to quantify the magnitude of drug interactions.
A sample size of 12 subjects per treatment arm provides at least 97.3% power for the test on Cmax if the ratio of the central values is 2-fold (complete data were obtained from 11 subjects). The power calculations were performed using logarithmic transformation with an assumed error term variance of 0.1409 for the natural logarithm of Cmax. This value was selected based on results from within-subject comparisons of paritaprevir, which showed higher variability than other drugs used in previous studies.
RESULTS
Subject demographics.
A total of 24 subjects were enrolled in the study: 12 in treatment arm 1 and 12 in treatment arm 2. Twenty-two subjects completed the study as planned. One subject in treatment arm 1 withdrew consent on day 17 of period 2. Another subject discontinued from treatment arm 2 due to allergic dermatitis before receiving the morning study drug dose on day 3 of period 2. Data from these two subjects were not included in the statistical analysis. Data from one additional subject was excluded from the lamivudine Ctrough analysis because the 24-h concentration value for this subject in period 2 was considerably higher than the concentration value at a previous time point in the same period, indicating erroneous data.
The majority (79.2%) of subjects enrolled in the study were male, and the median age of the overall population was 36.5 years (Table 1). White and black subjects were enrolled in approximately equal numbers.
TABLE 1.
Summary of subject demographics
| Characteristic | Value for treatment arm: |
||
|---|---|---|---|
| 1 (n = 12) | 2 (n = 12) | All subjects (n = 24) | |
| Age, yra | 36.5 (21.0, 46.0) | 37.0 (22.0, 51.0) | 36.5 (21.0, 51.0) |
| Weight, kga | 79.7 (64.9, 97.3) | 72.9 (46.0, 87.4) | 74.9 (46.0, 97.3) |
| Height, cma | 180.2 (162.3, 194.8) | 170.7 (144.2, 194.6) | 172.3 (144.2, 194.8) |
| Sex, n (%) | |||
| Male | 11 (91.7) | 8 (66.7) | 19 (79.2) |
| Female | 1 (8.3) | 4 (33.3) | 5 (20.8) |
| Race, n (%) | |||
| White | 3 (25.0) | 8 (66.7) | 11 (45.8) |
| Black | 9 (75.0) | 4 (33.3) | 13 (54.2) |
Data are presented as median (range).
Effect of the 3-direct-acting-antiviral regimen on dolutegravir pharmacokinetics.
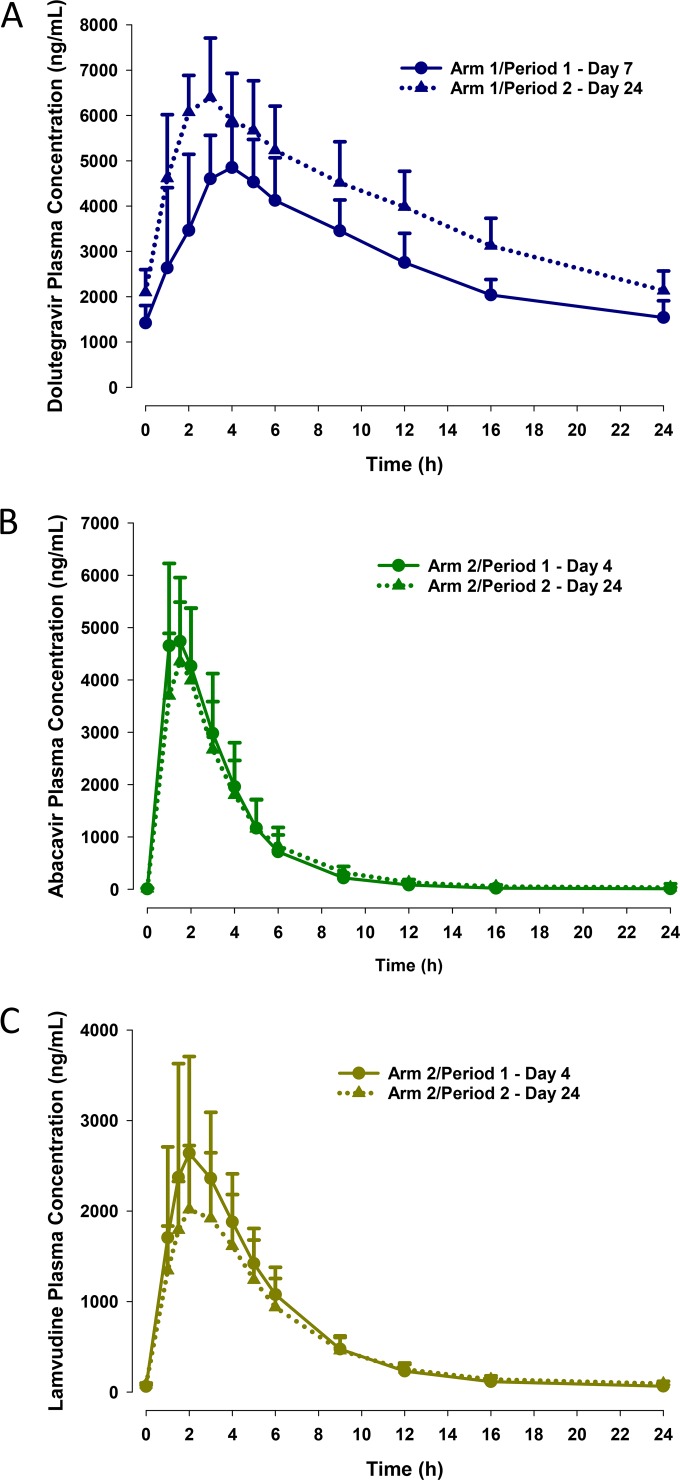
The pharmacokinetic profile of dolutegravir was moderately affected by coadministration of the 3-direct-acting-antiviral regimen (Fig. 2A and Table 2). Comparison of dolutegravir exposures at steady state revealed a 22% increase in Cmax (central value ratio [90% CI], 1.219 [1.153, 1.288]), a 38% increase in AUC (1.380 [1.295, 1.469]), and a 36% increase in Ctrough (1.357 [1.190, 1.548]) when the drug was administered with the 3-direct-acting-antiviral regimen versus when administered alone (Table 2). The Tmax and t1/2 of dolutegravir were not affected by coadministration with the 3-direct-acting-antiviral regimen.
FIG 2.
Concentration-time profiles (means plus standard deviations) for dolutegravir (A), abacavir (B), and lamivudine (C). Period 1, day 7, and period 1, day 4, were the final days of administration of antiretroviral therapy (dolutegravir or abacavir plus lamivudine) alone in treatment arms 1 and 2, respectively. Period 2, day 15, and period 2, day 24, were the initial and final days, respectively, of coadministration of the 3-direct-acting-antiviral regimen with antiretroviral therapy.
TABLE 2.
HIV antiretroviral drug pharmacokinetic properties
| Parameter | Value(s) for: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dolutegravir |
Abacavir |
Lamivudine |
|||||||
| Period 1, day 7 (n = 12) | With 3-direct-acting-antiviral regimen, period 2, day 24 (n = 11) | Ratios of central valuesa (90% CIs) | With lamivudine, period 1, day 4 (n = 12) | With lamivudine and 3-direct-acting-antiviral regimen, period 2, day 24 (n = 11) | Ratios of central valuesa (90% CIs) | With abacavir, period 1, day 4 (n = 12) | With abacavir and 3-direct-acting-antiviral regimen, period 2, day 24 (n = 11) | Ratios of central valuesa (90% CIs) | |
| Cmax, ng/mlb | 5,290 (15.8) | 6,560 (18.5) | 1.219 (1.153, 1.288) | 5,080 (23.5) | 4,460 (26.9) | 0.873 (0.777, 0.979) | 2,720 (33.7) | 2,120 (27.6) | 0.778 (0.719, 0.842) |
| Tmax, hc | 3.0 (1.0, 6.0) | 3.0 (1.0, 4.0) | 1.3 (1.0, 2.0) | 1.5 (1.0, 2.0) | 2.0 (1.0, 4.0) | 2.0 (1.0, 4.0) | |||
| AUC24, ng · h/mlb | 66,600 (17.0) | 93,700 (17.0) | 1.380 (1.295, 1.469) | 17,100 (27.5) | 16,500 (28.4) | 0.943 (0.901, 0.986) | 15,000 (23.7) | 13,200 (23.7) | 0.876 (0.821, 0.934) |
| Ctrough, ng/mlb | 1,500 (24.0) | 2,100 (20.3) | 1.357 (1.190, 1.548) | 12.9e (279) | 15.6f (226) | NDg | 62.7 (25.4) | 88.0 (30.6) | 1.287 (1.050, 1.578)i |
| t1/2, hd | 15.5 (1.87) | 12.6 (1.87) | 2.26 (0.46) | 2.98 (0.94) | 14.1 (10.3) | 13.7 (4.7) | |||
Values shown are ratios of central values (90% CIs) for ART plus the 3-direct-acting-antiviral regimen versus ART alone. Data are point estimates (90% confidence intervals). Point estimates are derived from the antilogarithm of the difference of the least-squares means for logarithms.
Geometric mean (percent coefficient of variation).
Median (range).
Harmonic mean (pseudostandard deviation).
Eight subjects had concentrations below the lower limit of quantitation.
Two subjects had concentrations below the lower limit of quantitation.
ND, not done. The effect of the 3-direct-acting antiviral regimen on abacavir Ctrough could not be estimated, as abacavir Ctrough concentration was below the lower limit of quantification in the majority of subjects in period 1.
For period 2, only data up to 48 h postdose were used for half-life determination.
One subject was excluded from the Ctrough analysis for lamivudine.
Effect of the 3-direct-acting-antiviral regimen on abacavir and lamivudine pharmacokinetics.
Coadministration of the 3-direct-acting-antiviral regimen minimally affected the pharmacokinetic profile of abacavir (Fig. 2B and Table 2). Comparisons of central values for Cmax and AUC showed decreases of 13% (0.873 [0.777, 0.979]) and 6% (0.943 [0.901, 0.986]), respectively, during coadministration compared with administration of abacavir and lamivudine alone (Table 2). The effect of the 3-direct-acting-antiviral regimen on the Ctrough values of abacavir could not be reliably determined because the measurements were below the LLOQ for the majority of subjects (8 of 12) in period 1. The Tmax and t1/2 values for abacavir were similar with or without the 3-direct-acting-antiviral regimen.
Effects of 3-direct-acting-antiviral regimen coadministration on the lamivudine pharmacokinetic profile were modest (Fig. 2C and Table 2). Coadministration with the 3-direct-acting-antiviral regimen resulted in a 22% (0.778 [0.719, 0.842]) lower Cmax and a 12% (0.876 [0.821, 0.934]) lower AUC for lamivudine (Table 2). In contrast, lamivudine Ctrough values increased by 29% (1.287 [1.050, 1.578]) during concomitant administration of the 3-direct-acting-antiviral regimen versus administration of abacavir and lamivudine alone. The Tmax and t1/2 values of lamivudine were not affected by the 3-direct-acting-antiviral regimen.
Effect of HIV-1 antiretroviral drugs on 3-direct-acting-antiviral regimen pharmacokinetics.
Exposure changes for paritaprevir, ritonavir, ombitasvir, and dasabuvir were generally minimal (<17% change) during coadministration with dolutegravir (Table 3). Notable exceptions were the Ctrough values for paritaprevir and ritonavir, which decreased by 34% (0.664 [0.585, 0.754]) and 28% (0.720 [0.678, 0.764]), respectively, during concomitant administration of the 3-direct-acting-antiviral regimen and dolutegravir (Table 3). The dasabuvir M1 Cmax and AUC changed by 7% (1.073 [0.979, 1.177]) or less, and the Ctrough decreased by 12% (0.879 [0.795, 0.972]) during coadministration with dolutegravir (data not shown in the tables).
TABLE 3.
3-Direct-acting-antiviral regimen pharmacokinetic properties with and without dolutegravir
| Drug and parameter | Value(s) for: |
||
|---|---|---|---|
| 3-Direct-acting-antiviral regimen, period 2, day 14 (n = 12) | 3-Direct-acting-antiviral regimen + dolutegravir, period 2, day 24 (n = 11) | Ratios of central valuesa (90% CIs) | |
| Paritaprevir | |||
| Cmax, ng/mlb | 2,050 (61.9) | 1,780 (58.8) | 0.888 (0.694, 1.135) |
| Tmax, hc | 4.0 (2.0, 5.0) | 4.0 (2.0, 4.0) | |
| AUC24, ng · h/mlb | 8,850 (70.6) | 7,310 (58.8) | 0.835 (0.668, 1.042) |
| Ctrough, ng/mlb | 22.5 (98.3) | 15.6 (80.8) | 0.664 (0.585, 0.754) |
| t1/2, hd | NDe | 4.73 (1.18) | |
| Ritonavir | |||
| Cmax, ng/mlb | 1,840 (31.7) | 1,670 (21.3) | 0.932 (0.827, 1.052) |
| Tmax, hc | 4.0 (2.0, 5.0) | 4.0 (2.0, 4.0) | |
| AUC24, ng · h/mlb | 10,600 (26.5) | 8,850 (20.7) | 0.852 (0.794, 0.915) |
| Ctrough, ng/mlb | 30.4 (39.7) | 21.3 (42.3) | 0.720 (0.678, 0.764) |
| t1/2, hd | ND | 3.96 (0.75) | |
| Ombitasvir | |||
| Cmax, ng/mlb | 129 (23.2) | 120 (29.1) | 0.960 (0.894, 1.031) |
| Tmax, hc | 5.0 (2.0, 5.0) | 5.0 (5.0, 5.0) | |
| AUC24, ng · h/mlb | 1,330 (30.0) | 1,250 (34.9) | 0.952 (0.902, 1.004) |
| Ctrough, ng/mlb | 26.5 (39.5) | 24.3 (43.9) | 0.922 (0.867, 0.981) |
| t1/2, hd | ND | 21.8 (6.15) | |
| Dasabuvir | |||
| Cmax, ng/mlb | 1,300 (30.7) | 1,330 (20.3) | 1.014 (0.924, 1.114) |
| Tmax, hc | 3.5 (2.0, 5.0) | 3.0 (2.0, 5.0) | |
| AUC12, ng · h/mlb | 9,280 (37.3) | 9,250 (29.5) | 0.983 (0.921, 1.048) |
| Ctrough, ng/mlb | 419 (56.6) | 394 (43.5) | 0.917 (0.851, 0.987) |
| t1/2, hd | ND | ND | |
Values shown are ratios of central values (90% CIs) for 3-direct-acting-antiviral regimen plus dolutegravir versus 3-direct-acting-antiviral regimen alone. Data are point estimates (90% confidence intervals). Point estimates are derived from the antilogarithm of the difference of the least-squares means for logarithms.
Geometric mean (percent coefficient of variation).
Median (range).
Harmonic mean (pseudostandard deviation).
ND, not done. Half-life was not determined due to insufficient data.
Exposure changes for paritaprevir, ombitasvir, dasabuvir, and ritonavir were also generally modest (≤18% lower) during coadministration of the 3-direct-acting-antiviral regimen with abacavir and lamivudine (Table 4). The greatest difference during concomitant treatment was a 27% (0.729 [0.627, 0.847]) decrease in paritaprevir Ctrough. Dasabuvir M1 exposures decreased by 17% (0.832 [0.757 to 0.916]) or less during coadministration of the 3-direct-acting-antiviral regimen with abacavir and lamivudine.
TABLE 4.
3-Direct-acting-antiviral regimen pharmacokinetic properties with and without abacavir plus lamivudine
| Parameter | 3-Direct-acting-antiviral regimen, period 2, day 14 (n = 11) | 3-Direct-acting-antiviral regimen + abacavir + lamivudine, period 2, day 24 (n = 11) | Ratios of central valuesa (90% CIs) |
|---|---|---|---|
| Paritaprevir | |||
| Cmax, ng/mlb | 3,570 (80.1) | 2,990 (74.1) | 0.836 (0.686, 1.019) |
| Tmax, hc | 4.0 (3.0, 6.0) | 5.0 (3.0, 6.0) | |
| AUC, ng · h/mlb | 16,800 (84.5) | 13,800 (72.5) | 0.822 (0.695, 0.971) |
| Ctrough, ng/mlb | 41.2 (62.9) | 30.0 (68.6) | 0.729 (0.627, 0.847) |
| t1/2, hd | NDe | 4.40 (0.46) | |
| Ritonavir | |||
| Cmax, ng/mlb | 2,100 (35.4) | 1,840 (33.3) | 0.876 (0.770, 0.997) |
| Tmax, hc | 4.0 (3.0, 5.0) | 4.0 (3.0, 6.0) | |
| AUC, ng · h/mlb | 12,000 (34.2) | 10,500 (34.7) | 0.877 (0.816, 0.942) |
| Ctrough, ng/mlb | 32.6 (51.1) | 30.1 (58.8) | 0.925 (0.834, 1.025) |
| t1/2, hd | ND | 3.91 (0.72) | |
| Ombitasvir | |||
| Cmax, ng/mlb | 167 (36.4) | 137 (34.4) | 0.822 (0.758, 0.891) |
| Tmax, hc | 5.0 (5.0, 6.0) | 5.0 (3.0, 6.0) | |
| AUC, ng · h/mlb | 1,830 (30.5) | 1,660 (28.3) | 0.909 (0.866, 0.953) |
| Ctrough, ng/mlb | 41.3 (34.9) | 37.8 (34.4) | 0.916 (0.876, 0.958) |
| t1/2, hd | ND | 26.0 (8.73) | |
| Dasabuvir | |||
| Cmax, ng/mlb | 983 (54.5) | 924 (51.5) | 0.940 (0.862, 1.026) |
| Tmax, hc | 4.0 (3.0, 5.0) | 4.0 (3.0, 6.0) | |
| AUC, ng · h/mlb | 6,930 (47.9) | 6,300 (48.9) | 0.910 (0.864, 0.958) |
| Ctrough, ng/mlb | 322 (43.4) | 304 (42.0) | 0.945 (0.875, 1.020) |
| t1/2, hd | ND | ND |
Values are ratios of central values (90% CIs) of 3-direct-acting-antiviral regimen plus abacavir plus lamivudine versus the 3-direct-acting-antiviral regimen alone. Data are point estimates (90% confidence interval). Point estimates are derived from the antilogarithm of the difference of the least-squares means for logarithms.
Geometric mean (percent coefficient of variation).
Median (range).
Harmonic mean (pseudostandard deviations).
ND, not done. Half-life was not determined due to insufficient data.
Safety and tolerability.
In aggregate, 12 subjects reported treatment-emergent adverse events during the various treatment phases assessed in the study, all of which were mild in severity. One subject, receiving the 3-direct-acting-antiviral regimen alone, experienced allergic dermatitis and discontinued from the study due to this event, which resolved within 3 days of study drug discontinuation. The pattern of adverse events was similar regardless of whether the subject was receiving the 3-direct-acting-antiviral regimen and ARTs alone or in combination.
No serious adverse events occurred during the study, and none of the subjects displayed evidence of an abacavir hypersensitivity reaction. Results for other safety measures (e.g., vital sign measurements, electrocardiography findings, and laboratory assessments) were unremarkable for each treatment group.
DISCUSSION
Dolutegravir is an integrase strand transfer inhibitor that is a preferred agent for treatment-naive HIV-1 patients based on its highly favorable efficacy and safety profiles (18, 19). The combination of abacavir plus lamivudine (both nucleoside analog reverse transcriptase inhibitors) is a recommended adjunct to dolutegravir as well as other HIV-1 ART (20). Through assessment of DDI potential, this study informs the dosing recommendation for these HIV-1 ART drugs and the 3-direct-acting-antiviral regimen when used in combination in patients with HIV-1 and HCV coinfection.
Results from this drug-drug interaction study demonstrate that the 3-direct-acting-antiviral regimen does not have a clinically meaningful effect on the pharmacokinetics of dolutegravir, abacavir, and lamivudine. Changes in drug exposures were <40% during coadministration of antiretroviral drugs with the 3-direct-acting-antiviral regimen compared with antiretroviral administration alone. This study also showed that the antiretroviral agents dolutegravir and abacavir plus lamivudine have minimal effect on the pharmacokinetics of the 3-direct-acting-antiviral regimen. The Cmax and AUC values for 3-direct-acting-antiviral regimen components during coadministration with dolutegravir or abacavir plus lamivudine were within 18% of measurements made during 3-direct-acting-antiviral administration alone. Although paritaprevir and ritonavir Ctrough values were 34% and 28% lower, respectively, during dolutegravir coadministration, and the Ctrough for paritaprevir was 27% lower during coadministration of abacavir plus lamivudine, these differences are not considered to be clinically significant. During phase II studies, considerably higher and lower doses and exposures of the 3-direct-acting-antiviral regimen components were evaluated (21). The resulting exposure variances and associated efficacy and safety findings suggest that exposure increases of 100% or decreases of 50% do not necessitate dose adjustment (21). These findings support that the changes in exposure for paritaprevir, ombitasvir, and dasabuvir with dolutegravir or abacavir plus lamivudine (i.e., <40%) do not require dose adjustment for the 3-direct-acting-antiviral regimen.
Although some increase in dolutegravir exposure was observed during coadministration with the 3-direct-acting-antiviral regimen, the magnitude of the change is not expected to be clinically meaningful. The degree of increase in Cmax, AUC, and Ctrough values during coadministration of dolutegravir with the 3-direct-acting-antiviral regimen is comparable with the reported increases in dolutegravir exposures during DDI studies with 25 mg rilpivirine once daily or 750 mg telaprevir every 8 h (22, 23). Greater increases in dolutegravir exposure (up to 180%) have been observed during coadministration with atazanavir or atazanavir-ritonavir (24). Even with these larger increases in dolutegravir exposure, no dose adjustment is recommended during concomitant use of atazanavir or atazanavir-ritonavir with dolutegravir (24, 25).
The lack of an appreciable interaction between the 3-direct-acting-antiviral regimen and dolutegravir is not unexpected given the low risk for DDIs that has been observed with dolutegravir (19, 25). The nominal interaction potential of dolutegravir has been linked to the characteristics of its metabolism and disposition as well as to its limited impact on metabolic enzymes and transport proteins (19, 26). Dolutegravir is primarily metabolized by UDP glucuronosyltransferase (UGT1A1), with contribution from CYP3A4 and minor roles for UGT1A3 and UGT1A9 (26). The increase in dolutegravir exposures can be attributed to CYP3A inhibition and/or UGT1A1 inhibition by the 3-direct-acting-antiviral regimen. The reason for the decrease in paritaprevir and ritonavir Ctrough in the presence of dolutegravir is not known.
The 6% to 22% decreases in Cmax and AUC for abacavir and lamivudine and the 29% increase in Ctrough for lamivudine when administered with the 3-direct-acting-antiviral regimen are not expected to influence the efficacy or safety profiles of either medication. Although the effects of the 3-direct-acting-antiviral regimen on the Ctrough values of abacavir were not determined because of the large proportion of subjects (8 of 12) with values below the LLOQ in period 1, the lack of 3-direct-acting-antiviral regimen effect on the t1/2 of abacavir and the minimal impact of 3-direct-acting-antiviral treatment on abacavir Cmax and AUC suggest that any influence on Ctrough would be minimal.
This drug interaction study evaluated the two-way interaction between HIV antiretroviral drugs and the 3-direct-acting-antiviral regimen. While a randomized crossover design may be ideal for evaluating a 2-way interaction, the current study design (sequential crossover) was chosen as it allowed for evaluation of 2-way interactions without unduly prolonging the study duration to allow washout of mechanism-based inhibition as well as enzyme induction effects of ritonavir. The sequential crossover study design has been used fairly commonly to evaluate drug interactions, especially for ritonavir-based regimens. In addition, although the intended population for combination therapy with the 3-direct-acting-antiviral regimen and ART includes patients with HCV-HIV coinfection, healthy volunteers who were not receiving any concomitant medications were evaluated in this study to more accurately ascertain the magnitude of drug interactions.
Coadministration of the 3-direct-acting-antiviral regimen with dolutegravir or abacavir plus lamivudine for 10 days was generally well tolerated by the healthy volunteers in this study. All of the reported adverse events were mild in severity, and only one event resulted in study discontinuation. As this study was a short-term study, conclusions regarding a longer duration of coadministration (i.e., during the 12- or 24-week course of 3-direct-acting-antiviral therapy) are limited. Further information on the safety and tolerability of coadministration of the 3-direct-acting-antiviral regimen with various HIV antiretroviral agents will come from the ongoing TURQUOISE-I study in patients with comorbid HCV and HIV-1 infection who are on a stable ART regimen. Recently published results from part 1a of TURQUOISE-I demonstrated high sustained virologic response rates (91% to 94%), with a favorable safety profile and no adverse events leading to study discontinuation, among patients with HCV-HIV-1 coinfection who were treated with the 3-direct-acting-antiviral regimen plus ribavirin for 12 or 24 weeks on a background of atazanavir- or raltegravir-based ART (12). Data from patients receiving darunavir or dolutegravir will be obtained in subsequent parts of this ongoing study.
Results from this DDI study of the 3-direct-acting-antiviral regimen may be extrapolated to the 2-direct-acting-antiviral regimen of ombitasvir, paritaprevir, and ritonavir, which is approved in Japan for the treatment of HCV genotype 1 infection and in the United States and the European Union for the treatment of HCV genotype 4 infection. Based on the lack of a clinically meaningful DDI as indicated by the results for the 3-direct-acting-antiviral regimen in the current study, no dose adjustment is indicated during concomitant use of the 2-direct-acting-antiviral regimen with dolutegravir or abacavir plus lamivudine. While the once-daily regimen of dolutegravir was evaluated in this study, the recommendations also apply to the twice-a-day regimen, where a similar or lower interaction is anticipated.
The lack of clinically significant DDIs between the 3-direct-acting-antiviral regimen and dolutegravir or abacavir plus lamivudine (based on both pharmacokinetic and safety/tolerability evaluations) indicates that 3-direct-acting-antiviral therapy is a viable option for HCV treatment in patients receiving dolutegravir or abacavir plus lamivudine for HIV-1 management. Moreover, the pharmacokinetic assessments indicate that no dose adjustment is needed during concomitant therapy with the 3-direct-acting-antiviral regimen and dolutegravir or abacavir plus lamivudine. Ongoing phase III studies will further elucidate the role of 3-direct-acting-antiviral therapy in patients with HCV-HIV-1 coinfection receiving ART.
ACKNOWLEDGMENTS
We thank Crystal Murcia and Lamara D. Shrode of The JB Ashtin Group, Inc., for assistance (writing, technical editing, and proofreading) in preparing the manuscript for publication on behalf of AbbVie Inc. We also acknowledge AbbVie employees Pamela Watson, Jeffrey Enejosa, Jill Polzin, Peter Probst (who was an employee of PRA Health Sciences working under a contract with AbbVie), Yi-Lin Chiu, Matthew Rosebraugh, and David Carter for their contributions to the study conduct, analyses, and clinical study report.
Authors made the following contributions to this work: original research project conception and design and data interpretation, A.K., R.T., T.P., and R.M.; review and critique of the manuscript throughout the editorial process and approval of the final manuscript draft submitted for publication, A.K., R.T., W.Z., T.P., and R.M.; data acquisition, statistical analysis, and data interpretation, W.Z.
Funding Statement
This work was supported by AbbVie Inc. AbbVie contributed to the study design, research, and interpretation of data and the writing, reviewing, and approving the manuscript for publication. All authors are AbbVie employees and may hold AbbVie stocks or options.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2014. HIV and viral hepatitis fact sheet. March. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 2.Toussaint-Miller KA, Andres J. 2015. Treatment considerations for unique patient populations with HCV genotype 1 infection. Ann Pharmacother 49:1015–1030. doi: 10.1177/1060028015592015. [DOI] [PubMed] [Google Scholar]
- 3.Cope R, Pickering A, Glowa T, Faulds S, Veldkamp P, Prasad R. 2015. Majority of HIV/HCV patients need to switch antiretroviral therapy to accommodate direct acting antivirals. AIDS Patient Care STDS 29:379–383. doi: 10.1089/apc.2015.0004. [DOI] [PubMed] [Google Scholar]
- 4.El-Sherif O, Khoo S, Solas C. 2015. Key drug-drug interactions with direct-acting antiviral in HIV-HCV coinfection. Curr Opin HIV AIDS 10:348–354. doi: 10.1097/COH.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 5.Andreone P, Colombo MG, Enejosa JV, Koksal I, Ferenci P, Maieron A, Mullhaupt B, Horsmans Y, Weiland O, Reesink HW, Rodrigues L Jr, Hu YB, Podsadecki T, Bernstein B. 2014. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology 147:359–365. doi: 10.1053/j.gastro.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 6.Dore GJ, Conway B, Luo Y, Janczewska E, Knysz B, Liu Y, Streinu-Cercel A, Caruntu FA, Curescu M, Skoien R, Ghesquiere W, Mazur W, Soza A, Fuster F, Greenbloom S, Motoc A, Arama V, Shaw D, Tornai I, Sasadeusz J, Dalgard O, Sullivan D, Liu X, Kapoor M, Campbell A, Podsadecki T. 2016. Efficacy and safety of ombitasvir/paritaprevir/r and dasabuvir compared to IFN-containing regimens in genotype 1 HCV patients: the MALACHITE-I/II trials. J Hepatol 64:19–28. doi: 10.1016/j.jhep.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, Aguilar H, Xiong J, Pilot-Matias T, DaSilva-Tillmann B, Larsen L, Podsadecki T, Bernstein B. 2014. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 370:1594–1603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 8.Forns X, Poordad F, Pedrosa M, Berenguer M, Wedemeyer H, Ferenci P, Shiffman ML, Fried MW, Lovell S, Trinh R, Lopez-Talavera JC, Everson G. 2015. Ombitasvir/paritaprevir/r, dasabuvir and ribavirin for cirrhotic HCV patients with thrombocytopaenia and hypoalbuminaemia. Liver Int 35:2358–2362. doi: 10.1111/liv.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lalezari J, Sullivan JG, Varunok P, Galen E, Kowdley KV, Rustgi V, Aguilar H, Felizarta F, McGovern B, King M, Polepally AR, Cohen DE. 2015. Ombitasvir/paritaprevir/r and dasabuvir plus ribavirin in HCV genotype 1-infected patients on methadone or buprenorphine. J Hepatol 63:364–369. doi: 10.1016/j.jhep.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, Shiffman ML, Wedemeyer H, Berg T, Yoshida EM, Forns X, Lovell SS, Da Silva-Tillmann B, Collins CA, Campbell AL, Podsadecki T, Bernstein B. 2014. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 370:1973–1982. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 11.Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, Bourliere M, Sulkowski MS, Wedemeyer H, Tam E, Desmond P, Jensen DM, Di Bisceglie AM, Varunok P, Hassanein T, Xiong J, Pilot-Matias T, DaSilva-Tillmann B, Larsen L, Podsadecki T, Bernstein B. 2014. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 370:1604–1614. doi: 10.1056/NEJMoa1401561. [DOI] [PubMed] [Google Scholar]
- 12.Sulkowski MS, Eron JJ, Wyles D, Trinh R, Lalezari J, Wang C, Slim J, Bhatti L, Gathe J, Ruane PJ, Elion R, Bredeek F, Brennan R, Blick G, Khatri A, Gibbons K, Hu YB, Fredrick L, Schnell G, Pilot-Matias T, Tripathi R, Da Silva-Tillmann B, McGovern B, Campbell AL, Podsadecki T. 2015. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA 313:1223–1231. doi: 10.1001/jama.2015.1328. [DOI] [PubMed] [Google Scholar]
- 13.Badri PS, King JR, Polepally AR, McGovern BH, Dutta S, Menon RM. 2015. Dosing recommendations for concomitant medications during 3D anti-HCV therapy. Clin Pharmacokinet doi: 10.1007/s40262-015-0317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khatri A, Dutta S, Wang H, Podsadecki T, Trinh R, Awni W, Menon R. 2016. Evaluation of drug-drug interactions between hepatitis C antiviral agents ombitasvir, paritaprevir/ritonavir, and dasabuvir and HIV-1 protease inhibitors. Clin Infect Dis 62:972–979. doi: 10.1093/cid/civ1213. [DOI] [PubMed] [Google Scholar]
- 15.Khatri A, Dutta S, Dunbar M, Podsadecki T, Trinh R, Awni W, Menon R. 2016. Evaluation of drug-drug interactions between direct-acting anti-hepatitis C virus combination regimens and the HIV-1 antiretroviral agents raltegravir, tenofovir, emtricitabine, efavirenz, and rilpivirine. Antimicrob Agents Chemother 60:2965–2971. doi: 10.1128/AAC.02605-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma JD, Lee KC, Kuo GM. 2010. HLA-B*5701 testing to predict abacavir hypersensitivity. PLoS Curr 2:RRN1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polepally AR, Dutta S, Hu B, Podsadecki TJ, Awni WM, Menon RM. 24 January 2016. Drug–drug interaction of omeprazole with the HCV direct-acting antiviral agents paritaprevir/ritonavir and ombitasvir with and without dasabuvir. Clin Pharmacol Drug Dev doi: 10.1002/cpdd.246. [DOI] [PubMed] [Google Scholar]
- 18.Department of Health and Human Services. 2015. April panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, Washington, DC. [Google Scholar]
- 19.Max B, Vibhakar S. 2014. Dolutegravir: a new HIV integrase inhibitor for the treatment of HIV infection. Future Virol 9:967–978. doi: 10.2217/fvl.14.80. [DOI] [Google Scholar]
- 20.Dall'Agata M, Gramenzi A, Biselli M, Bernardi M. 2014. Hepatitis C virus reinfection after liver transplantation: is there a role for direct antiviral agents? World J Gastroenterol 20:9253–9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menon RM, Badri PS, Wang T, Polepally AR, Zha J, Khatri A, Wang H, Hu B, Coakley EP, Podsadecki TJ, Awni WM, Dutta S. 2015. Drug-drug interaction profile of the all-oral anti-hepatitis C virus regimen of paritaprevir/ritonavir, ombitasvir, and dasabuvir. J Hepatol 63:20–29. doi: 10.1016/j.jhep.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 22.Ford SL, Gould E, Chen S, Margolis D, Spreen W, Crauwels H, Piscitelli S. 2013. Lack of pharmacokinetic interaction between rilpivirine and integrase inhibitors dolutegravir and GSK1265744. Antimicrob Agents Chemother 57:5472–5477. doi: 10.1128/AAC.01235-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson M, Borland J, Chen S, Savina P, Wynne B, Piscitelli S. 2014. Effects of boceprevir and telaprevir on the pharmacokinetics of dolutegravir. Br J Clin Pharmacol 78:1043–1049. doi: 10.1111/bcp.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song I, Borland J, Chen S, Lou Y, Peppercorn A, Wajima T, Min S, Piscitelli SC. 2011. Effect of atazanavir and atazanavir/ritonavir on the pharmacokinetics of the next-generation HIV integrase inhibitor, S/GSK1349572. Br J Clin Pharmacol 72:103–108. doi: 10.1111/j.1365-2125.2011.03947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitt C, Riek M, Winters K, Schutz M, Grange S. 2009. Unexpected hepatotoxicity of rifampin and saquinavir/ritonavir in healthy male volunteers. Arch Drug Infect 2:8–16. doi: 10.1111/j.1753-5174.2009.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reese MJ, Savina PM, Generaux GT, Tracey H, Humphreys JE, Kanaoka E, Webster LO, Harmon KA, Clarke JD, Polli JW. 2013. In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a HIV integrase inhibitor. Drug Metab Dispos 41:353–361. doi: 10.1124/dmd.112.048918. [DOI] [PubMed] [Google Scholar]