Abstract
Among 230 target-synthesized indole-based compounds, seven 3-triazenoindoles showed MICs of 0.2 to 0.5 μg/ml against Mycobacterium tuberculosis strain H37Rv and isoniazid-resistant human isolate CN-40. The TU112 compound was active also against a dormant form of M. tuberculosis. Some of these triazenoindoles were active against Mycobacterium avium, with MICs of 0.05 to 0.5 μg/ml. The selectivity indices (SI) for M. tuberculosis and M. avium were significantly higher than 10, making these compounds acceptable for the next testing step.
TEXT
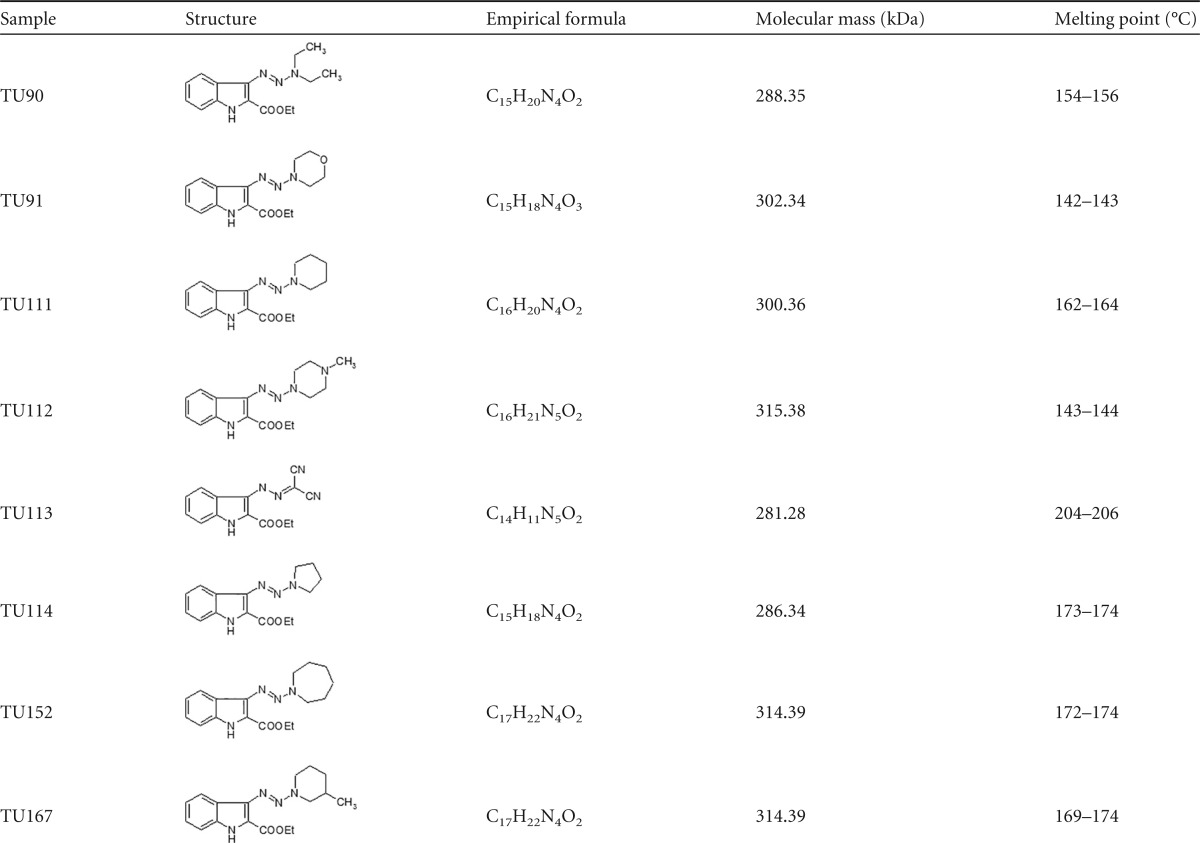
A series of indole-based compounds possessing antimycobacterial activity were reported during last decade (1–3), including those from our panel of Mycobacterium-targeting N-N-containing indoles synthesized according the procedure described previously (4). Here, we present a few indole agents containing three lined-up nitrogen atoms representing a series of 3-triazenoindoles and demonstrating the most prominent antimycobacterial capacities. Synthesis of the target 3-triazenoindoles was performed using 2-ethoxycarbonyl-3H-diazoindole and appropriate secondary amines (or malononitrile for the compound TU113) by the method reported earlier (5). The characteristics of the compounds are displayed in Table 1.
TABLE 1.
Characteristics of 3-triazenoindoles and hydrazone TU113
All reagents and solvents were purchased from commercial sources and were used without further purification. The yields refer to purified products and are not optimized. Melting points were uncorrected. Infrared (IR) spectra were run as KBr disks on an IR Fourier Magna-IR 750 Nicolet spectrometer. 1H nuclear magnetic resonance (NMR) and 13C NMR spectra were performed on a Bruker Avance-300 and Bruker Avance-400 (300 and 400 MHz, respectively), using tetramethylsilane (TMS) as an integral standard and hexadeuterodimethyl sulfoxide (DMSO-d6) and CDC1 as solvents. Splitting patterns are described as singlet (s), doublet (d), triplet (t), quartet (q), multiplet (m), and broad (br); chemical shifts are expressed as δ ppm, and the coupling constant (J) is given in hertz (Hz). Elemental analysis was performed at the laboratory of microanalysis of the A. N. Nesmeyanov Institute of Organoelement Compounds, Moscow, Russia. Mass spectra were recorded on Finnigan PolarisQ mass spectrometer.
Mycobacterium tuberculosis strain H37Rv, a clinical isolate of isoniazid (INH)-resistant M. tuberculosis CN-40, and Mycobacterium avium strain 724R were obtained from the Department of Immunology of Central Institute for Tuberculosis (Moscow, Russia). The origin, storage conditions, properties, and preparation of bacterial cultures were described earlier (6–8). Initially, the compounds were tested for their capacity to inhibit [3H]uracil incorporation (as preliminary screen) into M. tuberculosis H37Rv and CN-40 (4, 6), followed by MIC testing of the most active ones. MICs were determined by a standard microdilution assay using microtubes with Dubos medium containing 0.05% Tween 80. The lowest concentration of a compound resulting in no visible growth of M. tuberculosis for 2 weeks was considered the MIC (4, 9, 10). All samples were tested twice in triplicate. In addition, the samples were plated on Dubos agar for CFU counting and determination of the MIC99. We also used a micro method developed in our laboratory based on measurement under a microscope of the volume of growing compact mycobacterial culture in the wells of round-bottom 96-well plates in the presence or absence of a tested compound (K. Majorov, unpublished data). The MICs for M. avium were determined in an identical manner, except with a 1-week incubation instead of two.
To assess whether or not the compounds under study were toxic for mammalian cells, the level of macrophage lysis was determined by measuring the enzymatic activity of lactate dehydrogenase (LDH) in culture supernatants using the CytoTox 96 kit (Promega). Specific lysis was calculated according to the formula (A490 from experimental well − A490 spontaneous release)/(A490 maximal release − A490 spontaneous release) × 100 (6). The selectivity index (SI) then was calculated by dividing the 50% inhibitory concentration (IC50) by the MIC; an SI of >10 was considered sufficient for a compound's further evaluation (11). The in vitro model of M. tuberculosis dormancy and resuscitation was described previously (12).
All compounds displayed in Table 2, except TU113, demonstrated a high level of in vitro activity against M. tuberculosis H37Rv (MIC, 0.25 to 0.5 μg/ml, comparable to that of INH) and against INH-resistant strain CN-40 (MIC, 0.2 to 0.5 μg/ml, comparable to that of rifampin [RIF]). These compounds demonstrated also a high level of activity against the virulent M. avium strain 724R, comparable to that of clarithromycin (Table 2). The SIs for compounds TU111, TU112, TU114, and TU152 were much higher than 10 regarding all three mycobacterial strains, indicating the necessity of their further testing.
TABLE 2.
Antimycobacterial testing results of 3-triazenoindoles
| Compounda | Activity (MIC99 [μg/ml]) againstb: |
IC50 (μg/ml)c | SI ford: |
||||
|---|---|---|---|---|---|---|---|
| M. tuberculosis H37Rv | INHr CN-40 strain | M. avium | M. tuberculosis H37Rv | INHr CN-40 | M. avium | ||
| TU90 | 0.46 | 0.46 | NT | 18 | 39 | 39 | NT |
| TU91 | 0.36 | 0.45 | NT | 10 | 28 | 22 | NT |
| TU111 | 0.25 | 0.5 | 0.1 | 100 | 400 | 200 | 2,000 |
| TU112 | 0.25 | 0.2 | 0.05 | 18 | 72 | 90 | 360 |
| TU113 | >1 | 2–10 | 1 | 7 | <7 | <3.5–0.7 | 7 |
| TU114 | 0.3 | 0.1–1 | 0.25 | 50 | 166 | 50–500 | 200 |
| TU152 | 0.1–0.5 | ≤0.5 | <0.1 | 50 | 100–500 | 100 | 500 |
| TU167 | <0.5 | <0.5 | 2 | 10 | >20 | >20 | 5 |
| INH | 0.05 | 20 | NT | NT | NT | NT | NT |
| RIF | 0.1 | 0.1 | NT | NT | NT | NT | NT |
| CLA | NT | NT | 0.2 | NT | NT | NT | NT |
INH, isoniazid; RIF, rifampin; CLA, clarithromycin.
INHr, INH resistant; NT, not tested.
IC50, concentration killing 50% of host macrophages.
SI, selectivity index.
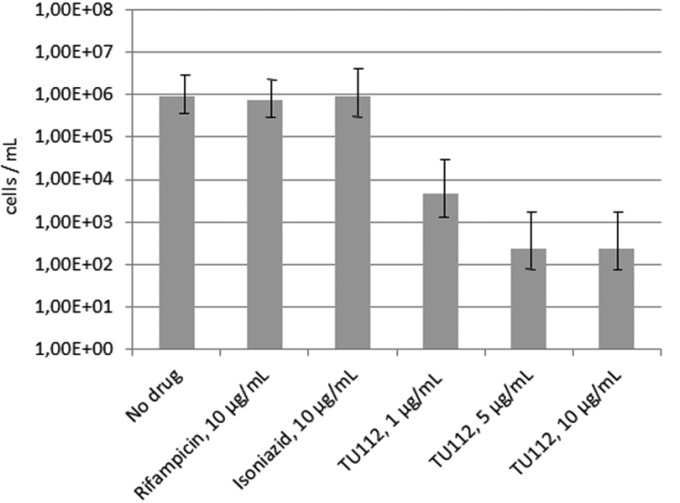
In addition, compounds TU111 and TU112 were tested with respect to their activity against nonculturable dormant cells of M. tuberculosis H37Rv. Since the compound TU111 demonstrated no activity (data not shown), a more detailed assessment was performed with the compound TU112. Remarkably, whereas RIF and INH were ineffective against metabolically inactive mycobacteria, TU112 killed these cells even at the 10-fold-lower concentration (Fig. 1). The privileged indole core of the compounds tested is directly connected to the line assembly of three nitrogen atoms as a side chain substituent. The compound TU113 with two nitrogen atoms at the side chain and 2-ethoxycarbonylindole without any substituent (data not shown) proved to be, respectively, poorly active and inactive against all three mycobacterial strains. Therefore, the substituents present in the above-mentioned species are relevant for antituberculosis activity and differ in their absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties. The study of these properties is now in progress. Regarding the chemical structure of the compounds tested, the presence of three lined-up nitrogen atoms seems to be a key requirement for the antimycobacterial activity (Table 2). Six compounds presented herein should be considered very promising drug candidates for further testing in other models, including in vivo testing. The compound TU167 possesses significant activity against H37Rv and CN-40 strains but is toxic, so we do not consider it a promising candidate. Significant activity of the triazenoindole TU112 with N-methyl piperazine substituent against M. tuberculosis H37Rv, including dormant cells, and against CN-40 suggests that this substituent controls physicochemical properties within an optimal range (neither too lipophilic, nor too hydrophilic).
FIG 1.
Activity of the compound TU112 against dormant nonculturable M. tuberculosis. Mycobacteria were incubated in the presence of different concentrations of the compound TU112 for 7 days at 37°C. Viability of mycobacteria was tested by measuring the numbers of cells recovered from a nonculturable state by most probable number (MPN) assay as described earlier (12). The difference between TU112 groups and the untreated control was significant, with a P value of <0.01. Error bars indicate standard deviation.
ACKNOWLEDGMENTS
This work was financially supported by the Russian Foundation for Basic Research (grant 14-04-01688) and the Russian Scientific Foundation (grant 15-15-30020 for studying drug toxicity against macrophages and IC50).
We thank Igor K. Egorov (The Jackson Laboratory, Bar Harbor, ME) for critically reading the manuscript and Vadim Makarov (Research Center of Biotechnology of the Russian Academy of Sciences, Moscow) for useful remarks.
REFERENCES
- 1.Velezheva VS, Brennan PJ, Marshakov VY, Gusev DV, Lisichkina IN, Peregudov AS, Tchernousova LN, Smirnova TG, Andreevskaya SN, Medvedev AE. 2004. Novel pyridazino[4,3-b]indoles with dual inhibitory activity against Mycobacterium tuberculosis and monoamine oxidase. J Med Chem 47:3455–3461. doi: 10.1021/jm030479g. [DOI] [PubMed] [Google Scholar]
- 2.Matviiuk T, Rodriguez F, Saffon N, Mallet-Ladeira S, Gorichko M, de Jesus Lopes Ribeiro AL, Pasca MR, Lherbet C, Voitenko Z, Baltas M. 2013. Design, chemical synthesis of 3-(9H-fluoren-9-yl)pyrrolidine-2,5-dione derivatives and biological activity against enoyl-ACP reductase (InhA) and Mycobacterium tuberculosis. Eur J Med Chem 70:37–48. doi: 10.1016/j.ejmech.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 3.Onajole OK, Pieroni M, Tipparaju SK, Lun S, Stec J, Chen G, Gunosewoyo H, Guo H, Ammerman NC, Bishai WR, Kozikowski AP. 2013. Preliminary structure-activity relationships and biological evaluation of novel antitubercular indolecarboxamide derivatives against drug-susceptible and drug-resistant Mycobacterium tuberculosis strains. J Med Chem 56:4093–4103. doi: 10.1021/jm4003878. [DOI] [PubMed] [Google Scholar]
- 4.Velezheva V, Brennan P, Ivanov P, Kornienko A, Lyubimov S, Kazarian K, Nikonenko B, Majorov K, Apt A. 2016. Synthesis and antituberculosis activity of indole-pyridine derived hydrazides, hydrazide-hydrazones, and thiosemicarbazones. Bioorg Med Chem Lett 26:978–985. doi: 10.1016/j.bmcl.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 5.Simakov S, Velezheva V, Kozik T, Ershova Y, Chernov V, Suvorov N. 1983. Synthesis and antitumor activity of some 4-oxo-1,2,3-triazino[5,6-b]indoles and 1,1-dialkyl-3-[indol-3-yl]triazenes. Pharm Chem J 17:707–712. doi: 10.1007/BF00765019. [DOI] [Google Scholar]
- 6.Majorov KB, Lyadova IV, Kondratieva TK, Eruslanov EB, Rubakova EI, Orlova MO, Mischenko VV, Apt AS. 2003. Different innate ability of I/St and A/Sn mice to combat virulent Mycobacterium tuberculosis: phenotypes expressed in lung and extrapulmonary macrophages. Infect Immun 71:697–707. doi: 10.1128/IAI.71.2.697-707.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikonenko BV, Samala R, Einck L, Nacy CA. 2004. Rapid, simple in vivo screen for new drugs active against Mycobacterium tuberculosis. Antimicrob Agents Chemother 48:4550–4555. doi: 10.1128/AAC.48.12.4550-4555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondratieva E, Logunova N, Majorov K, Averbakh M, Apt A. 2010. Host genetics in granuloma formation: human-like lung pathology in mice with reciprocal genetic susceptibility to M. tuberculosis and M. avium. PLoS One 5:e10515. doi: 10.1371/journal.pone.0010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubuisson T, Bogatcheva E, Krishnan MY, Collins MT, Einck L, Nacy CA, Reddy VM. 2010. In vitro antimicrobial activities of capuramycin analogues against non-tuberculous mycobacteria. J Antimicrob Chemother 65:2590–2597. doi: 10.1093/jac/dkq372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogatcheva E, Hanrahan C, Nikonenko B, de los Santos G, Reddy V, Chen P, Barbosa F, Einck L, Nacy C, Protopopova M. 2011. Identification of SQ609 as a lead compound from a library of dipiperidines. Bioorg Med Chem Lett 21:5353–5357. doi: 10.1016/j.bmcl.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orme I, Tuberculosis Drug Screening Program. 2001. Search for new drugs for treatment of tuberculosis. Antimicrob Agents Chemother 45:1943–1946. doi: 10.1128/AAC.45.7.1943-1946.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salina E, Ryabova O, Kaprelyants A, Makarov V. 2014. New 2-thiopyridines as potential candidates for killing both actively growing and dormant Mycobacterium tuberculosis cells. Antimicrob Agents Chemother 58:55–60. doi: 10.1128/AAC.01308-13. [DOI] [PMC free article] [PubMed] [Google Scholar]