Abstract
The ability of Pseudomonas aeruginosa to rapidly modulate its response to antibiotic stress and persist in the presence of antibiotics is closely associated with the process of cell-to-cell signaling. The alternative sigma factor RpoN (σ54) is involved in the regulation of quorum sensing (QS) and plays an important role in the survival of stationary-phase cells in the presence of carbapenems. Here, we demonstrate that a ΔrpoN mutant grown in nutrient-rich medium has increased expression of pqsA, pqsH, and pqsR throughout growth, resulting in the increased production of the Pseudomonas quinolone signal (PQS). The link between pqsA and its role in carbapenem tolerance was studied using a ΔrpoN ΔpqsA mutant, in which the carbapenem-tolerant phenotype of the ΔrpoN mutant was abolished. In addition, we demonstrate that another mechanism leading to carbapenem tolerance in the ΔrpoN mutant is mediated through pqsE. Exogenously supplied PQS abolished the biapenem-sensitive phenotype of the ΔrpoN ΔpqsA mutant, and overexpression of pqsE failed to alter the susceptibility of the ΔrpoN ΔpqsA mutant to biapenem. The mutations in the ΔrpoN ΔrhlR mutant and the ΔrpoN ΔpqsH mutant led to susceptibility to biapenem. Comparison of the changes in the expression of the genes involved in QS in wild-type PAO1 with their expression in the ΔrpoN mutant and the ΔrpoN mutant-derived strains demonstrated the regulatory effect of RpoN on the transcript levels of rhlR, vqsR, and rpoS. The findings of this study demonstrate that RpoN negatively regulates the expression of PQS in nutrient-rich medium and provide evidence that RpoN interacts with pqsA, pqsE, pqsH, and rhlR in response to antibiotic stress.
INTRODUCTION
Pseudomonas aeruginosa, an opportunistic Gram-negative bacterium, is the leading cause of infections in immunocompromised patients and those suffering from cystic fibrosis. To cause disease, P. aeruginosa expresses and secretes virulence factors that promote colonization and survival within its host. P. aeruginosa infections are particularly difficult to treat due to their high levels of resistance to antimicrobials. In contrast to antibiotic resistance, which is attributed to a wide repertoire of mechanisms, P. aeruginosa displays another phenotype known as antibiotic tolerance, characterized by the presence of a small population of antibiotic-tolerant cells that persist in the presence of antibiotics (1). The presence of antibiotic-tolerant cells results in relapse of the bacterial infection after cessation of antibiotic treatment; therefore, determining the precise mechanism for antibiotic tolerance is a prerequisite for adequately fighting persistent infections caused by P. aeruginosa.
P. aeruginosa is capable of overcoming the effects of virtually any commercially available antibiotic, including β-lactam antibiotics, such as carbapenems. Despite this, carbapenems are still commonly used to treat infections caused by P. aeruginosa. The main advantage of carbapenems is their resistance to the β-lactamase AmpC and their ability to bind to multiple different penicillin binding proteins (PBPs) and inhibit cell wall synthesis (2, 3). It has been demonstrated, however, that the presence of carbapenems induces cell rounding, a morphotype that promotes β-lactam tolerance (4).
However, the molecular mechanism underlying antibiotic tolerance is largely unknown. Environmental signals, including prolonged nutrient deprivation (5), oxidative stress (6), DNA-damaging agents (7), and activation of the stringent response (5, 8), promote antibiotic tolerance. P. aeruginosa closely regulates its response to environmental conditions through a cell-to-cell communication system or quorum sensing (QS). In P. aeruginosa, three interconnected QS systems control the global gene regulation implicated in the production of virulence factors and biofilm formation (9, 10). Two QS systems, the LasR/I and RhlR/I systems, modulate gene transcription in response to increasing concentrations of the N-acyl-l-homoserine lactones synthesized by LasI [N-(3-oxododecanoyl) homoserine lactone] and RhlI (N-butanoyl homoserine lactone) (11–13). In addition to the las and rhl regulatory systems, P. aeruginosa has a third QS system, known as the Pseudomonas quinolone signal (PQS), which acts as a link between the las and rhl systems and signals via 4-hydroxy-2-alkylquinolines (HAQs) (14, 15).
The quinolone signaling network is encoded by the pqsABCDE operon, which is positively regulated by the transcriptional regulator PqsR (also known as MvfR) (15). The first four genes in the operon are required for the synthesis of 2-heptyl-4-quinolone (HHQ), which is oxidized to PQS through the action of PqsH (15). In addition to HHQ and PQS, the synthesis of the other main HAQ, 2-heptyl-4-hydroxyquinoline N-oxide (HQNO), depends on PqsABCD; however, its synthesis is not dependent on the presence of HHQ (15). Transcription of pqsABCDE leads to the expression of the fifth gene in the operon, PqsE. The precise function of PqsE is not understood; however, structural analysis indicated that PqsE is related to the metallo-β-lactamase superfamily and is not required for HAQ biosynthesis but independently regulates genes that are under PQS control (16–18). PqsE is required for swarming motility and biofilm formation and can activate virulence in the absence of a functional pqs system (19). PqsE is closely tied with the elements of the rhl signaling system (18, 20). PQS positively regulates the rhl system, and rhl can negatively impact the production of PQS (21–23). PQS controls the production of the virulence factors pyocyanin (17) and elastase and influences biofilm development (21). Furthermore, PQS plays a role in antibiotic stress through its dual pro- and antioxidant functions, serving as a bacterial stress regulator (24).
Major factors contributing to antibiotic tolerance are transcriptional regulators, such as sigma factors, which initiate transcription by associating with the core RNA polymerase enzyme and direct promoter-specific transcription, thus affecting the expression of a wide range of genes (25). The RpoS sigma factor (σS) is a master regulator of the stress response and is closely linked with the regulation of QS, production of the virulence factors pyocyanin and exotoxin A (26), and biofilm formation (27). Furthermore, RpoS activity serves as a defense mechanism in the presence of carbapenems and fluoroquinolones (28, 29). Another alternative sigma factor, RpoN (σ54), controls the expression of genes involved in nitrogen metabolism and motility (30–33). Recent studies showed the complex control of expression of the type VI secretion system (T6SS), an important factor of the bacterial pathogenesis, by RpoN (34). RpoN has been linked with the regulation of QS by repressing lasR-lasI and rhlR-rhlI (35). Depending on nutrient availability, RpoN can also influence biofilm formation in P. aeruginosa (36). The goal of this study was to expand on previous work from our laboratories demonstrating the role of RpoN in antibiotic tolerance to carbapenems in P. aeruginosa (37).
To investigate the mechanism by which RpoN contributes to carbapenem tolerance, we initially constructed an rpoN in-frame deletion mutant and demonstrated that the ΔrpoN mutant has increased expression of pqsA, pqsH, and pqsR and higher levels of PQS. This observation prompted us to investigate the link between increased PQS levels and the carbapenem-tolerant phenotype of the ΔrpoN mutant. We constructed an in-frame deletion of pqsA in the ΔrpoN mutant background and confirmed that pqsA positively affects carbapenem tolerance in the ΔrpoN mutant. In addition, we also show that the pqsE, pqsH, and rhlR genes have a positive effect on the survival of the ΔrpoN mutant in the presence of carbapenems, suggesting that PQS and PQS-associated virulence factors mediate tolerance to carbapenems. Deletion of vqsR in the ΔrpoN mutant background did not affect the bacterial response to carbapenems. Using the results of transcriptional analysis of the wild-type PAO1, ΔrpoN mutant, and ΔrpoN mutant-derived strains, we provide further insights into the regulatory network involved in carbapenem tolerance.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains, plasmids, and primers used and generated in this study are shown in Table 1. Bacteria were routinely cultured at 37°C in Luria-Bertani (LB) medium or on L-agar plates supplemented with 1 mM l-glutamine, and when necessary, with 10% sucrose. Vogel-Bonner minimal medium (VBMM) (38) was used in mating experiments. Antibiotics for plasmid selection and propagation were added as required: gentamicin at 20 μg/ml for Escherichia coli or 100 μg/ml for P. aeruginosa.
TABLE 1.
Bacterial strains, plasmids, and primers used in the study
| Bacterial strain, plasmid or primer | Relevant characteristicsa | Reference or source |
|---|---|---|
| Bacterial strains | ||
| E. coil S17-1 | thi pro hsdR recA Tra+ | These laboratories |
| P. aeruginosa | ||
| PAO1 | Wild type | |
| PAO1 ΔrpoN | PAO1 with an in-frame deletion of rpoN | This study |
| PAO1 ΔrpoN ΔvqsR | PAO1 with an in-frame deletion of rpoN and vqsR | This study |
| PAO1 ΔrpoN ΔpqsA | PAO1 with an in-frame deletion of rpoN and pqsA | This study |
| PAO1 ΔrpoN ΔpqsE | PAO1 with an in-frame deletion of rpoN and pqsE | This study |
| PAO1 ΔrpoN ΔpqsH | PAO1 with an in-frame deletion of rpoN and pqsH | This study |
| PAO1 ΔrpoN ΔrhlR | PAO1 with an in-frame deletion of rpoN and rhlR | This study |
| Plasmids | ||
| pEX18Gm | Broad-host-range gene replacement vector; sacB Gmr | 41 |
| pJN105 | Arabinose-inducible gene expression vector; pBRR-1 MCS; araC-PBAD Gmr | 42 |
| pEX18Gm-ΔrpoN | rpoN deletion suicide vector | This study |
| pEX18Gm-ΔvqsR | vqsR deletion suicide vector | This study |
| pEX18Gm-ΔpqsA | pqsA deletion suicide vector | This study |
| pEX18Gm-ΔpqsE | pqsE deletion suicide vector | This study |
| pEX18Gm-ΔpqsH | pqsH deletion suicide vector | This study |
| pEX18Gm-ΔrhlR | rhlR deletion suicide vector | This study |
| Primers | ||
| Primers for construction of mutant strains | ||
| rpoN-upF | ATAGAATTCCGATCTCGGTCGGCGACATC | |
| rpoN-upR | ATAGGATCCCTGGAGGTCCAGGGTGGATAG | |
| rpoN-downF | ATAGGATCCGGCATAGCCCCTTCGAGCGAG | |
| rpoN-downR | ATAAAGCTTCTCCGGCAGCTCCCTGGCTA | |
| vqsR-upF | ATAGAGCTCGCCGGTGACCACCACGGTGG | |
| vqsR-upR | ATAGGATCCGACGCACGCTAGTGCGCCGC | |
| vqsR-downF | ATAGGATCCTGCGATATCCACACAACTACTCCTC | |
| vqsR-downR | ATAGCATGCGGCATAGCGATTGCCGGGAT | |
| pqsA-upF | CTAATGGAGCTCAGGGAAGCCTGCAAATGGCAG | |
| pqsA-upR | GTGATAAAGGGTGTCGCTGGCCTGGGAGAGAATGTA | |
| pqsA-downF | GACACCCTTTATCACGACAACCTTCCGGAGG | |
| pqsA-downR | CTAATGAAGCTTGCAATCCAGGTTGGGCCGGCA | |
| pqsE-upF | ATAGAATTCGCCGGCGAGAGTCTCGAA | |
| pqsE-upR | ATAGGATCCAAGCCTCAACATGGCCGGT | |
| pqsE-downF | ATAGGATCCCTGGACTGAGACGGGACAT | |
| pqsE-downR | ATAAAGCTTAGGCTGGACAGGCCATGC | |
| pqsH-upF | CGACTCTAGAGGATCGTAAGGGGTTGACAGGAG | |
| pqsH-upR | CACCGGCGGCTACTGGGTCATCCGTTGCTCCTT | |
| pqsH-downF | CAGTAGCCGCCGGTGCCA | |
| pqsH-downR | CCATGATTACGAATTGGACGACATCCGGCTTCA | |
| rhlR-upF | CGACTCTAGAGGATCCGCCTACGCGCCACTGGG | |
| rhlR-upR | GATGAGACCCAGCGCCCTCATTGCAGTAAGCCC | |
| rhlR-downF | GCGCTGGGTCTCATCTGAAG | |
| rhlR-downR | CCATGATTACGAATTTAGCGCGAAAGCTCCCAG | |
| pqsESD-F | TTGGGCTAGCGAATTAGGAAACAGCTATGTTGAGGCTTTCGG | |
| pqsES-R | TGGCGGCCGCTCTAGTCAGTCCAGAGGCAGCGCCTGGC | |
| Primers for qRT-PCR | ||
| vqsR-F | GCTGTCGATCGCCACTATCA | |
| vqsR-R | TCGGAGTGGGACTTCACGTT | |
| pqsA-F | CATCTCGCCGAACAGATTCC | |
| pqsA-R | CCAACTTGCCGTTGTCGTT | |
| pqsR-F | GCAGCTGATCGGCGACAT | |
| pqsR-F | CAGCAGCACCCGGAGATT | |
| pqsH-F | GCGCGGATCGAGTTCATC | |
| pqsH-R | CAGGGCGATTCCCACTGA | |
| rhlR-F | TTCGCCGTCCTGGAAAAG | |
| rhlR-R | CGCCATAGGCGTAGTAATCGA | |
| rpoS-F | CACTTCCTTCTCTCCAAACAACA | |
| rpoS-R | AGCTGCGTTGCGTCCAA | |
| omlA-F | CGAACTATCAACCAGCTGGTG | |
| omlA-R | GCTGTGCTCTTGCAGGTTGTG |
Gmr, resistance to gentamicin; MCS, multiple-cloning site. In the oligonucleotide sequences, introduced restriction sites are underlined, and the sequences introduced for overlap extension PCR are in italics.
Reagents.
Biapenem was purchased from Meiji Seika Pharma Co., Ltd. (Yokohama, Japan), and doripenem was purchased from Shionogi & Co., Ltd. (Osaka, Japan). Both were used at a concentration of 32 μg/ml. Synthetic PQS was obtained from Toyama Chemical Co., Ltd. (Tokyo, Japan), was solubilized in methanol, and was used at a concentration of 50 μM for killing assays.
Antibiotic susceptibility testing.
The MIC of each agent was determined using the broth microdilution method as previously described (39), with the following modifications: bacterial suspensions were incubated in LB medium at a density of 1 × 106 CFU/ml. MICs were determined after 24 h of incubation at 37°C. The MIC was defined as the lowest concentration of antimicrobial agent that completely inhibited the growth of the organism, as detected by the unaided eye.
Time-kill assays.
For time-kill studies, approximately 108 CFU/ml of stationary-phase cells grown in 10 ml of LB medium in 50-ml conical tubes at 37°C were centrifuged at 3,000 × g for 15 min. Subsequently, sedimented cells were resuspended in fresh 10-ml aliquots of LB medium and allowed to grow with antimicrobial agents in a shaker (200 rpm) at 37°C for 24 h. Samples were collected at several time points, 10-fold serial dilutions were prepared with 0.85% NaCl, and 100-μl samples were plated onto L-agar plates in duplicate. Microbial killing was assessed at defined time points by counting the colonies and calculating the percent survival relative to the number of untreated cells at time zero. Plates with 30 to 300 colonies were used for CFU counts. Data were collected from at least three independent experiments.
Generation of mutant strains.
Unmarked deletions of rpoN, vqsR, pqsE, pqsA, rhlR, and pqsH were constructed in P. aeruginosa PAO1 using the pEX18Gm suicide vector (40), which uses the sacB-based counterselection method (41). A strain with the single deletion of rpoN was first constructed, and that mutant was then used to generate the ΔrpoN ΔvqsR, ΔrpoN ΔpqsA, and ΔrpoN ΔpqsE double mutants. To delete rpoN, the following procedure was used: a PCR fragment containing 545 nucleotides upstream of the rpoN start site and 150 nucleotides within the open reading frame was digested with EcoRI-BamHI and then inserted into pEX18Gm that had been digested with the same enzymes and linked to a BamHI-HindIII-digested PCR fragment containing 39 nucleotides of the rpoN gene and 516 nucleotides downstream of the rpoN stop codon. The deletion includes the helix-turn-helix motif and the rpoN box. The pEX18Gm-ΔrpoN construct was sequenced to confirm that PCR did not introduce a mutation before the construct was mobilized from E. coli S17-1 into P. aeruginosa. The transconjugants carrying the integrated plasmid on the chromosome were selected on L-agar plates containing 10% (wt/vol) sucrose, and sucrose-resistant colonies were screened using colony PCR to identify mutants. To generate double deletions, the aforementioned procedure was used. The vqsR and pqsE upstream and downstream fragments were generated using the primer pairs listed in Table 1. The two PCR products for each gene were digested with SacI-BamHI (upstream) and BamHI-SphI (downstream) for vqsR and with EcoRI-BamHI (upstream) and BamHI-HindIII (downstream) for pqsE. Inserts were then cloned into pEX18Gm to generate pEX18Gm-ΔvqsR and pEX18Gm-ΔpqsE, respectively. The constructs were transferred from E. coli S17-1 to the P. aeruginosa ΔrpoN mutant. To delete pqsA, flanking upstream and downstream regions of approximately 650 bp were amplified from the chromosome of PAO1. The resulting DNA fragments contained a 15-bp overlap and were joined by overlap extension PCR. This final PCR product was cloned into pEX18Gm using the SacI and HindIII restriction sites to generate pEX18Gm-ΔpqsA. In-frame deletion constructs of rhlR and pqsH were performed using an In-Fusion HD cloning kit (Clontech Laboratories, Inc.). The upstream fragment of each gene contained a 15-bp overlap complementary to a 15-bp region of the downstream fragment. The amplified fragments contained a 5′ end homology to the vector pEX18Gm and were fused together using in-fusion cloning in order to produce the deletion constructs pEX18Gm-ΔrhlR and pEX18Gm-ΔpqsH. The ΔrpoN ΔrhlR mutant and ΔrpoN ΔpqsH mutant were generated by allelic exchange of the rhlR and pqsH genes, respectively, in the ΔrpoN mutant background with pEX18Gm-ΔrhlR and pEX18Gm-ΔpqsH, respectively, using sucrose counterselection.
Construction of inducible overexpression of pqsE.
To construct an expression plasmid for PqsE, the pqsE gene was amplified from P. aeruginosa PAO1 chromosomal DNA using the primers pqsESD-F and pqsESD-R. Using the In-Fusion cloning kit, this PCR product was cloned under the control of an arabinose-inducible promoter in the pJN105 plasmid (42) that had been digested with EcoRI-XbaI, resulting in plasmid pJN105-pqsE, which was further verified by sequence analysis. The pJN105 and pJN105-pqsE plasmids were introduced into wild-type P. aeruginosa PAO1 and the ΔrpoN ΔpqsA mutant via conjugation. For pqsE overexpression experiments, 0.2% arabinose was added to the stationary-phase culture 30 min prior to biapenem treatment.
PQS extraction and quantification.
To analyze PQS production, wild-type PAO1 and the ΔrpoN mutant were used to inoculate 10-ml cultures. Following 16 h of incubation at 37°C, the cultures were washed and used to inoculate 10-ml subcultures to a final optical density at 595 nm (OD595) of 0.01. Cultures were grown for 6 h, 10 h, and 24 h and extracted with acidified ethyl acetate. The phases were separated by centrifugation, and the organic phase was collected and dried. Extracts were resuspended in 20 μl of methanol. Aliquots (10 μl) of extracts were loaded onto silica gel thin-layer chromatography (TLC) plates (60F254; Merck) that had previously been soaked in 5% KH2PO4 for 30 min and activated at 100°C for 1 h. Two microliters of the 10 mM PQS standard was used as a control. Samples were resolved using a solvent mixture consisting of dichloromethane-methanol (95:5). Fluorescent spots were visualized under long-wave UV light and photographed.
RNA isolation and qRT-PCR analysis.
Strains were grown overnight in 10 ml of LB medium at 37°C for 16 h, after which time the cells were washed and used to inoculate 10-ml subcultures in LB medium to a final OD595 of 0.01. The cultures were incubated at 37°C and were sampled at 6 h, 10 h, and 24 h. Total RNA was isolated from cells using the TRIzol reagent (Invitrogen) according to the manufacturer's instructions. To remove traces of contaminating DNA, total RNA was treated with Turbo DNase (Ambion) as suggested by the manufacturer. cDNA was generated from 550 ng of DNase-treated RNA using a Transcriptor first-strand cDNA synthesis kit (Roche Applied Science). Quantitative real-time PCRs (qRT-PCRs) were carried out in a LightCycler apparatus (Roche Molecular Biochemicals) using a LightCycler FastStart DNA MasterPlus SYBR green I kit (Roche Applied Science) according to the specifications of the supplier. Data were analyzed using LightCycler software (version 3.5). To correct for the differences in the amount of starting material, omlA was used as a reference gene. The software calculated the concentrations of the transcripts studied using a standard curve. The oligonucleotide primers used to detect the expression of each gene of interest are listed in Table 1. At least three technical replicates were performed for each cDNA sample analyzed.
Pyocyanin quantification.
The P. aeruginosa PAO1, ΔrpoN mutant, and ΔrpoN mutant-derived strains were grown for 16 h in LB medium; pyocyanin was extracted from 5 ml of bacterial supernatant with chloroform and then reextracted with 0.2 N HCl to yield a pink solution. The absorbance at 520 nm was measured, and the amount of pyocyanin was calculated as previously described (43).
Growth assay.
To assess the growth of the wild-type PAO1, ΔrpoN mutant, and ΔrpoN mutant-derived strains, cultures grown overnight were washed and used to inoculate 10-ml subcultures in LB medium to an OD595 of ~0.01. The absorbance at 595 nm was monitored every 2 h for 12 h, with an additional measurement being taken at 24 h.
RESULTS
RpoN represses the expression of pqsA, pqsR, pqsH, and lasR.
We have previously shown that RpoN downregulates the expression of the QS transcriptional regulator VqsR and that of sigma factor PvdS (37). VqsR has been implicated in the regulation of another transcriptional regulator, QscR (44), as well as in the regulation of siderophore synthesis and genes in the PQS operon (45, 46). The connection between PQS and iron is well established; PQS is known to chelate iron and induce the expression of genes involved in the regulation of pvdS and the biosynthesis of pyoverdine and is essential for pyocyanin production (19, 21, 47, 48).
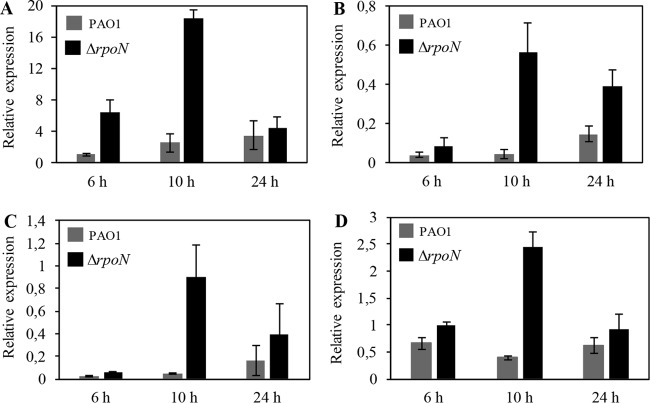
The implication of the involvement of RpoN in the regulation of the las and rhl QS systems prompted us to expand on the potential role of RpoN in regulating PQS. To this end, we isolated RNA from wild-type strain PAO1 and the ΔrpoN mutant that had been grown in LB medium for 6 h (OD595, ∼0.6), 10 h (OD595, ∼0.9), and 24 h (OD595, ∼1.1) and compared the transcript levels of pqsA, the first gene in the pqsABCDE operon, in each strain. The most pronounced difference was observed at the 6-h and 10-h time points, at which time the ΔrpoN mutant expressed 6 and 7 times more pqsA, respectively, than the wild type. By 24 h, the levels of pqsA transcripts in the rpoN mutant were only 1.3 times higher than those in the wild type (Fig. 1A).
FIG 1.
Expression of pqsA (A), pqsR (B), pqsH (C), and lasR (D) in wild-type PAO1 and the ΔrpoN mutant throughout growth. Overnight cultures of PAO1 and the ΔrpoN mutant were diluted to an OD595 of 0.01 in LB medium supplemented with 1 mM glutamine, grown at 37°C, and harvested at an OD595 of ∼0.6 (6 h), 0.9 (10 h), and 1.1 (24 h) for total RNA isolation. The levels of the pqsA, pqsR, pqsH, and lasR transcripts were measured by qRT-PCR and normalized to the level of omlA expression. All results are the averages from at least three independent experiments, and the error bars represent SDs.
The expression of the pqsABCDE operon requires the LysR-type transcriptional activator PqsR, which binds to the pqsA promoter. PqsR binding is increased in the presence of PQS (23, 49); therefore, we also measured pqsR expression. As we suspected, the ΔrpoN mutant had higher levels of pqsR than the wild type at all time points (Fig. 1B).
Having established the role of rpoN in the regulation of pqsA and pqsR, we subsequently sought to confirm that the increased levels of pqsA and pqsR correlate with an increase in the expression of pqsH, which is responsible for the conversion of HHQ into PQS and is directly controlled by LasR (15). We found that pqsH expression was upregulated in the ΔrpoN mutant relative to its expression in the wild type (Fig. 1C).
The close regulatory link between LasR and PQS, with LasR being a dominant regulator of pqsR and pqsH expression (23, 50), prompted us to investigate the transcriptional levels of lasR in wild-type PAO1 and in the ΔrpoN mutant. The level of lasR expression in the ΔrpoN mutant was consistently higher than that in wild-type PAO1 at all tested time points (Fig. 1D). Based on the findings presented above, we conclude that in nutrient-rich medium, RpoN promotes the downregulation of the pqsA, pqsR, and pqsH genes through the regulatory mechanism governed by LasR.
RpoN negatively affects PQS production.
To correlate the transcriptional data with the production of PQS in the wild-type and ΔrpoN mutant, the strains were cultured and sampled as described above for the transcriptional analysis, and the levels of PQS, which included intracellular, extracellular, and membrane-associated PQS, were examined using TLC. Figure 2 demonstrates that PQS was detected in cell extracts prepared from the wild-type strain in early-stationary-phase LB medium cultures at 6 h of growth (OD595, ∼0.6); however, less PQS was detected in the ΔrpoN mutant at the same time point, as determined by UV inspection. In contrast, PQS levels were higher in the ΔrpoN mutant than in wild-type PAO1 at 10 h postinoculation, and this difference was even more pronounced at the 24-h time point, where the ΔrpoN mutant demonstrated significantly higher levels of PQS.
FIG 2.
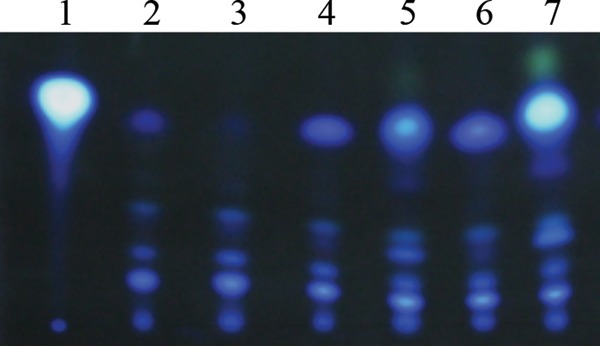
PQS production in wild-type PAO1 and the ΔrpoN mutant as measured by TLC. Overnight cultures of PAO1 and the ΔrpoN mutant were diluted to an OD595 of 0.01 in LB medium supplemented with 1 mM glutamine, grown at 37°C, harvested at an OD595 of ∼0.6 (6 h), 0.9 (10 h), and 1.1 (24 h), and then assayed for PQS production. Lane 1, 2 μl (10 mM) synthetic PQS; lane 2, PAO1 (6 h of growth); lane 3, ΔrpoN mutant (6 h of growth); lane 4, PAO1 (10 h of growth); lane 5, ΔrpoN mutant (10 h of growth); lane 6, PAO1 (24 h of growth); lane 7, ΔrpoN mutant (24 h of growth). A representative photograph of TLC-separated extracts from PAO1 and the ΔrpoN mutant is shown.
Somewhat surprisingly, although transcriptional analysis demonstrated increased expression of pqsA, pqsR, and pqsH at the 6-h time point, this did not correlate with the PQS levels observed. Although we cannot offer a conclusive explanation for this discrepancy, we hypothesize that the amount of substrate for PQS synthesis might be limited in the ΔrpoN mutant at this point during growth. Furthermore, it is possible that at this time point, the regulation of pqsA might be under the control of some intermediary regulatory network(s) other than PqsR-PQS. The increased levels of pqsA could be a consequence of the effect that RhlR has on pqsA expression. The pqsA promoter region has a distinct transcriptional site controlled by RhlR that exerts posttranscriptional regulation of pqsA to fine-tune the production of PQS (51). However, we do not exclude the possibility that a low level of expression of pqsH in the early growth phase may be responsible for undetectable levels of PQS in the ΔrpoN mutant, despite increased pqsA expression. In addition, the PQS levels observed at the 24-h time point were significantly higher than those found in the wild type, even though the ΔrpoN mutant demonstrated a modest 1.3-fold upregulation of pqsA expression relative to the wild type.
We favor the proposed negative autoregulatory model for PQS regulation in which, once maximal physiological levels of PQS are reached, PQS leads to downregulation of the pqsA operon (20). These results additionally confirm that RpoN negatively affects the synthesis of PQS through the downregulation of pqsA, pqsR, and pqsH expression.
pqsA, pqsE, and pqsH confer tolerance to carbapenems in the ΔrpoN mutant.
Our previous observation demonstrated that stationary-phase cells of the ΔrpoN mutant display a significant tolerant phenotype in the presence of carbapenems (37). One of the characteristics of antibiotic-tolerant cells is that their mechanism usually does not reflect MIC values, which remain unchanged. The MIC values of biapenem and doripenem for the mutant strains remained comparable to those observed for the wild-type strain PAO1 and were 0.5 μg/ml for both antibiotics for the wild-type PAO1 strain and the PAO1 ΔrpoN, ΔrpoN ΔvqsR, ΔrpoN ΔpqsA, ΔrpoN ΔpqsE, and ΔrpoN ΔpqsH mutant strains.
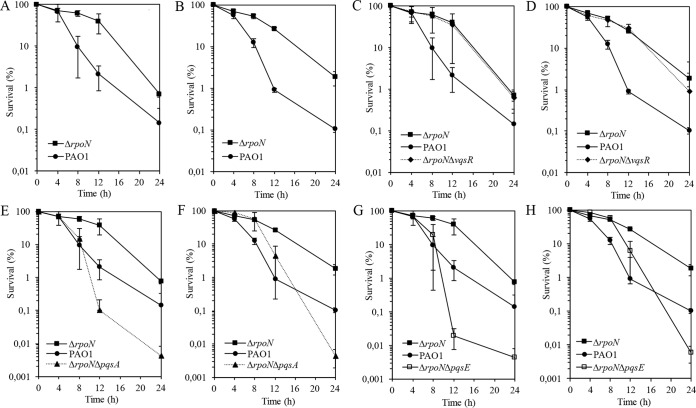
The goal of this study was to find the target(s) through which RpoN modulates the effect of carbapenems. The ΔrpoN mutant displayed a higher degree of tolerance to 32 μl/ml biapenem (Fig. 3A) and doripenem (Fig. 3B) than the wild-type strain.
FIG 3.
Killing assays of wild-type PAO1, the ΔrpoN mutant, and the ΔrpoN mutant-derived strains in the presence of biapenem (A, C, E, G) and doripenem (B, D, F, H) at 32 μg/ml. Under the assumption that the survival at time zero was 100%, the numbers of CFU were converted to percentages. The experiment was performed in triplicate. Error bars, SDs from three experiments.
The increased expression of vqsR in the ΔrpoN mutant prompted us to look at the role of and requirement for VqsR in surviving antibiotic stress. The ΔrpoN ΔvqsR mutant demonstrated a trend of susceptibility to carbapenems similar to that of the ΔrpoN mutant, suggesting that VqsR is not directly involved in the antibiotic tolerance observed in the ΔrpoN mutant (Fig. 3C and D).
To integrate the information gained in our transcriptional studies indicating that the ΔrpoN mutant has increased expression of pqsA and, consequently, increased production of PQS, we sought to determine whether pqsA is a target for the interaction with carbapenems in the ΔrpoN mutant. To assess the role of pqsA in the susceptibility to carbapenems in the ΔrpoN mutant, we performed the killing assay with the ΔrpoN ΔpqsA mutant in the presence of biapenem (Fig. 3E) and doripenem (Fig. 3F). The ΔrpoN ΔpqsA mutant exhibited a steep decrease in viability, measured as a decrease in the number of CFU, indicating the complete loss of the tolerant phenotype observed in the ΔrpoN mutant.
The observations suggesting a direct role of pqsA in tolerance to carbapenems in the ΔrpoN mutant prompted us to further address the significance of pqsE in the interaction with carbapenems. PqsE, which is coregulated together with the pqsABCD genes, is not required for HAQ synthesis and instead controls the production of PQS-regulated factors, such as pyocyanin (20). The loss of pqsE in the ΔrpoN mutant background resulted in a decline in viability in the presence of carbapenems (Fig. 3G and H).
To confirm that the loss of pqsE does not affect PQS production in the ΔrpoN mutant background and to gain additional information regarding the effect of vqsR on PQS production, we examined the production of PQS in the ΔrpoN ΔpqsE and ΔrpoN ΔvqsR mutants using TLC. The results demonstrated that neither the mutation in pqsE nor the mutation in vqsR affected the levels of PQS in the ΔrpoN mutant (Fig. 4). From these data, we concluded that the mechanism underlying the tolerance of stationary-phase ΔrpoN mutant cells is linked with the upregulation of the pqsA gene and the consequent PQS production.
FIG 4.
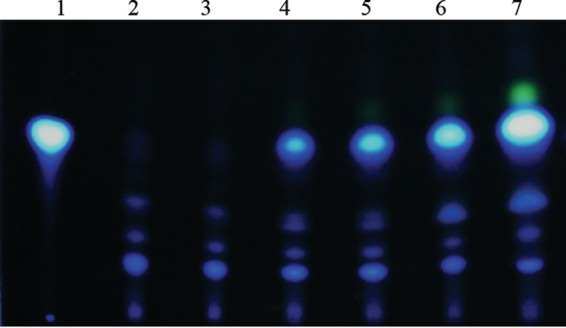
PQS production in the ΔrpoN ΔpqsE, and ΔrpoN ΔvqsR mutants as measured by TLC. Overnight cultures of the ΔrpoN ΔpqsE and ΔrpoN ΔvqsR mutants were diluted to an OD595 of 0.01 in LB medium supplemented with 1 mM glutamine, grown at 37°C, harvested at an OD595 of ∼0.6 (6 h), 0.9 (10 h), and 1.1 (24 h), and then assayed for PQS production. Lane 1, 2 μl (10 mM) synthetic PQS; lane 2, ΔrpoN ΔvqsR mutant (6 h of growth); lane 3, ΔrpoN ΔpqsE mutant (6 h of growth); lane 4, ΔrpoN ΔvqsR mutant (10 h of growth); lane 5, ΔrpoN ΔpqsE mutant (10 h of growth); lane 6, ΔrpoN ΔvqsR mutant (24 h of growth); lane 7, ΔrpoN ΔpqsE mutant (24 h of growth). A representative photograph of TLC-separated extracts from the ΔrpoN ΔvqsR and ΔrpoN ΔpqsE mutants is shown.
We also demonstrated that pqsE does not require PQS synthesis to promote survival in the presence of carbapenems in the ΔrpoN mutant, suggesting that PqsE might affect the response to carbapenems through the regulation of PQS-controlled virulence factors, such as pyocyanin. Therefore, we sought to define the correlation between pyocyanin production and carbapenem tolerance in the wild-type and mutant strains. Whereas mutations in pqsA and pqsE in the rpoN mutant background profoundly affected pyocyanin production, the ΔrpoN and the ΔrpoN ΔvqsR mutants demonstrated levels of pyocyanin equal to those observed in wild-type PAO1 (see Fig. S1 in the supplemental material).
Finally, we addressed the question of whether HHQ, a direct precursor of PQS that produces PQS through the action of PqsH, which is positively regulated by lasR (15), might play a role in the response to carbapenems. The sensitive phenotype of the ΔrpoN ΔpqsA mutant might be due to the loss of PQS or any other HAQ because, upon deletion of pqsA, the production of PQS was abolished, as was the production of many other HAQs, such as HQNO and HHQ. To address the role of HHQ production on survival upon carbapenem exposure, we constructed an ΔrpoN ΔpqsH mutant and performed a time-kill assay using biapenem at a concentration of 32 μl/ml. Assessment of the susceptibility of the stationary-phase cells of the ΔrpoN ΔpqsH mutant to biapenem compared to the survival rate of the ΔrpoN mutant displayed a clear difference; the rpoN and pqsH deletions abolished the biapenem-tolerant phenotype of the ΔrpoN mutant (Fig. 5). The growth of the wild-type and mutant strains was shown to be comparable (see Fig. S2 in the supplemental material). These results indicate that the loss of PQS synthesis through the mutation in the pqsH contributes to carbapenem tolerance.
FIG 5.
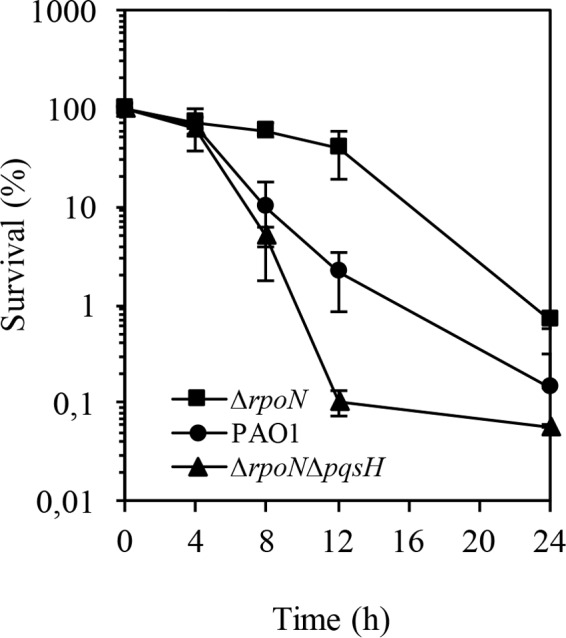
Biapenem (32 μg/ml) killing assay of wild-type PAO1, the ΔrpoN mutant, and the ΔrpoNΔpqsH mutant. Under the assumption that the survival at time zero was 100%, the numbers of CFU were converted to percentages. The experiment was performed in triplicate. Error bars, SDs from three experiments.
RpoN requires the contribution of both PQS and PqsE to overcome the effect of carbapenems.
To determine if the sensitive phenotype of the ΔrpoN ΔpqsA mutant is a consequence of a loss of PQS or PqsE, since the mutation in pqsA negatively affects the expression of pqsE (19), we initially addressed this question by performing a time-kill assay using wild-type PAO1 and the ΔrpoN ΔpqsA mutant with 32 μl/ml biapenem in the presence of 50 μM PQS, which is a biologically relevant concentration. The killing curve demonstrated that the addition of PQS completely restored the susceptibility of the ΔrpoN ΔpqsA mutant, increasing the survival by 100-fold. In wild-type PAO1, susceptibility to biapenem also decreased in the presence of exogenously supplied PQS (Fig. 6A). The biapenem killing curve observed for the ΔpqsA mutant was similar to that observed for the wild type (Fig. 6A).
FIG 6.
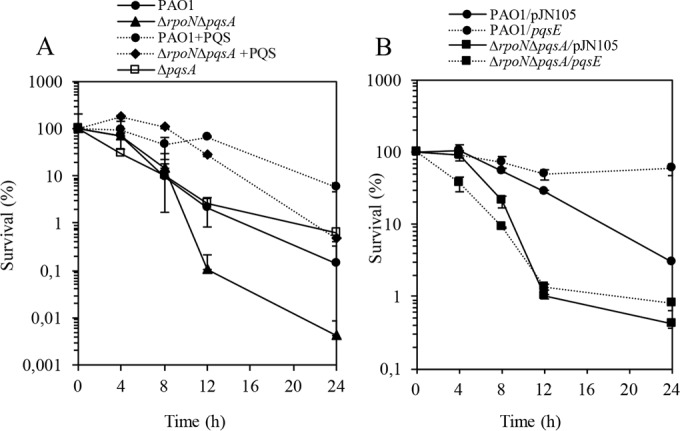
Biapenem (32 μg/ml) killing assay of wild-type PAO1 and the ΔrpoN ΔpqsA mutant in the presence of PQS (50 μM) (A) and pqsE overexpression using an arabinose-inducible pJN105-pqsE construct (B). (A) Time-kill assay for the ΔpqsA mutant in the presence of biapenem (32 μg/ml). Arabinose (0.2%) was added 30 min before the addition of biapenem. The viability of wild-type PAO1, the ΔrpoN ΔpqsA mutant, and the ΔpqsA mutant is expressed as percent survival.
Next, we evaluated the contribution of PqsE overexpression to the survival of the ΔrpoN ΔpqsA mutant in the presence of biapenem. We introduced pJN105-pqsE, expressing PqsE under the control of the arabinose promoter, in wild-type PAO1 and the ΔrpoN ΔpqsA mutant. The biapenem time-kill assay profiles of wild-type PAO1 and the ΔrpoN ΔpqsA mutant, which were compared in the presence and absence of a vector carrying pqsE induced by 0.2% arabinose in the medium, demonstrated that the inducible overexpression of pqsE completely abrogated the susceptibility to biapenem in wild-type PAO1 and produced a minor effect in the ΔrpoN ΔpqsA mutant (Fig. 6B). Because exogenously provided PQS positively affects the expression of the entire pqs operon, including pqsE, the results clearly demonstrate that RpoN, through the contribution of both PQS and the PqsE-mediated network, defines the response to carbapenems.
rhlR modulates the response to carbapenems in the ΔrpoN mutant.
The evidence that las signaling is placed at the top of the QS hierarchical system exerting transcriptional control over both rhlR and rhlI and that the production of PQS depends on lasR, which positively regulates pqsR and directly controls the expression of pqsH (13, 15, 50), prompted us to extend our studies investigating the importance of lasR in mediating carbapenem tolerance in the ΔrpoN mutant. To address this question, we tried to construct a ΔrpoN ΔlasR mutant but were unable to create this mutant after multiple attempts.
Throughout growth, the ΔrpoN mutant demonstrated significant increases in the levels of the transcriptional regulator rhlR. To further establish the importance of RhlR in carbapenem tolerance, we examined the effect of an rhlR mutation in the ΔrpoN mutant on biapenem survival. Treatment with biapenem at 32 μl/ml led to an increase in the sensitivity of the ΔrpoN ΔrhlR mutant after 12 h of growth (Fig. 7). Assessing the PQS levels in the ΔrpoN ΔrhlR mutant, we observed that the ΔrpoN ΔrhlR mutant expressed wild-type levels of PQS (Fig. 8). All together, these results confirm that RhlR is an important participant in mediating tolerance to carbapenems.
FIG 7.
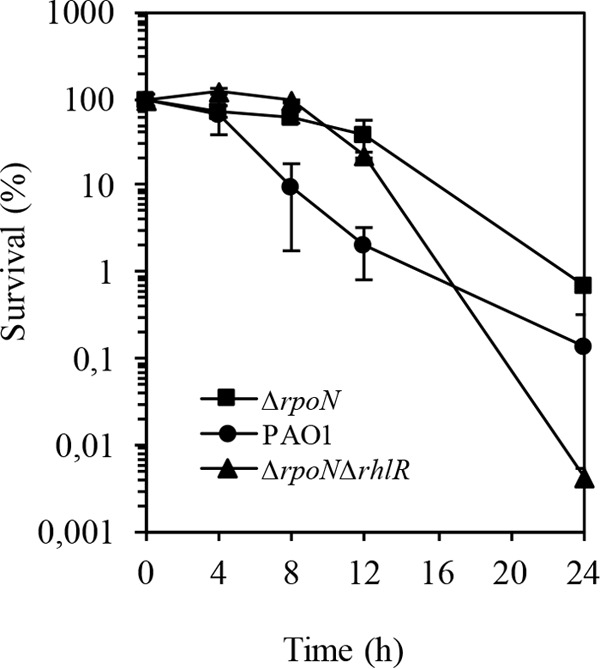
Biapenem (32 μg/ml) killing assay of wild-type PAO1, the ΔrpoN mutant, and the ΔrpoNΔrhlR mutant. Under the assumption that the survival at time zero was 100%, the numbers of CFU were converted to percentages. The experiment was performed in triplicate. Error bars, SDs from three experiments.
FIG 8.
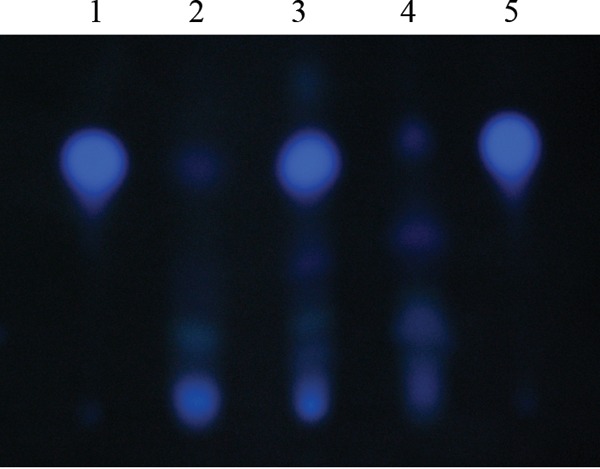
PQS production in wild-type PAO1, the ΔrpoN mutant, and the ΔrpoN ΔrhlR mutant as measured by TLC. Overnight cultures of PAO1, the ΔrpoN mutant, and the ΔrpoN ΔrhlR mutant were diluted to an OD595 of 0.01 in LB medium supplemented with 1 mM glutamine, grown at 37°C, harvested at an OD595 of ∼1.1 (24 h), and then assayed for PQS production. Lane 1, 2 μl (10 mM) synthetic PQS; lane 2, PAO1 (24 h of growth); lane 3, ΔrpoN mutant (24 h of growth); lane 4, ΔrpoN ΔrhlR mutant (24 h of growth); lane 5, 2 μl (10 mM) synthetic PQS. A representative photograph of TLC-separated extracts from PAO1, the ΔrpoN mutant, and the ΔrpoN ΔrhlR mutant is shown.
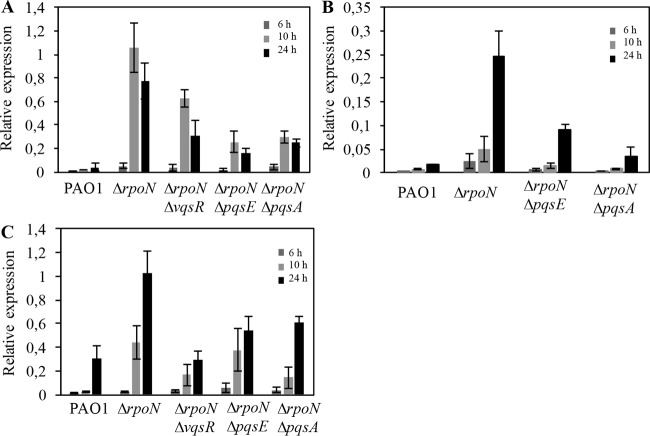
Transcriptional expression of the rhlR, vqsR, and rpoS genes in the ΔrpoN mutant and ΔrpoN mutant-derived strains.
Given that pqsA and pqsE mediate the response to carbapenems through interactions with RpoN, we further asked whether some of the major regulators of P. aeruginosa QS (RhlR, VqsR, and RpoS) previously shown to be negatively regulated by RpoN might also aid in modulating the response to carbapenems. To do this, we used qRT-PCR to compare the transcriptional expression of rhlR, vqsR, and rpoS in the wild-type PAO1, ΔrpoN mutant, and ΔrpoN mutant-derived strains. The levels of the rhlR, vqsR, and rpoS transcripts (Fig. 9A to C, respectively) were consistently higher in the ΔrpoN mutant than in the wild type at all tested time points, with the most pronounced difference being observed at the 24-h time point. These results confirm previously obtained data that indicate that RpoN predominantly exerts a negative regulation of rhlR, vqsR, and rpoS throughout growth (28, 35, 37).
FIG 9.
Expression levels of rhlR (A), vqsR (B), and rpoS (C) in wild-type PAO1, the ΔrpoN mutant, and the ΔrpoN mutant-derived strains. Cultures of PAO1, the ΔrpoN mutant, and the ΔrpoN mutant-derived strains grown overnight were diluted to an OD595 of 0.01, grown at 37°C in LB medium, and harvested at an OD595 of ∼0.6 (6 h), 0.9 (10 h), and 1.1 (24 h) for total RNA preparation. Transcript levels were measured by quantitative RT-PCR and normalized to the level of omlA expression. All results are averages from at least three independent experiments, and the error bars represent SDs.
Next, we investigated the contribution of the quorum-sensing transcriptional regulator VqsR on the expression of rhlR and rpoS in the ΔrpoN mutant background. In the ΔrpoN ΔvqsR mutant, rhlR levels were 2-fold lower than those in the ΔrpoN mutant at the 24-h time point (Fig. 9A). This confirms that VqsR plays a role in RhlR regulation and suggests that RpoN exerts part of its negative effect on RhlR by downregulating VqsR. Given the evidence that RpoN negatively affects the expression of sigma factor RpoS in stationary phase (28) and that VqsR is involved in the positive regulation of RpoS (45), we investigated the expression of rpoS in the ΔrpoN ΔvqsR mutant. At the 24-h time point, rpoS levels in the ΔrpoN ΔvqsR mutant were 3-fold lower than those in the ΔrpoN mutant (Fig. 9C). These results suggest that some of the interaction between RpoN and RpoS may be mediated through VqsR; however, the exact role of VqsR in the response to carbapenems remains to be elucidated, particularly in light of the fact that carbapenem susceptibility remained unchanged in the ΔrpoN ΔvqsR mutant, despite the downregulation of both RhlR and RpoS.
To gain more insights into how the RpoN network connects with pqsA, we also examined the transcript levels of rhlR, vqsR, and rpoS in the ΔrpoN ΔpqsA mutant and compared them to the transcript levels observed in the wild type and the ΔrpoN mutant. The effect of the pqsA deletion in the ΔrpoN mutant on rhlR expression was obvious at all time points, as rhlR levels decreased by 3.6- and 3-fold at the 10-h and 24-h time points, respectively, relative to those in the ΔrpoN mutant (Fig. 9A). The most pronounced difference in vqsR expression between the ΔrpoN ΔpqsA and ΔrpoN mutants was observed at the 24-h time point, with a 7-fold difference in expression being detected (Fig. 9B). At the 10-h time point, rpoS transcript levels were 3.1-fold lower in the ΔrpoN ΔpqsA mutant than in the ΔrpoN mutant (Fig. 9C). These results demonstrate that elimination of the synthesis of PQS and the activity of RhlR, VqsR, and RpoS modulates the response to carbapenems.
Using the same approach, we analyzed the effects of deleting pqsE in a ΔrpoN mutant background on the expression of rhlR, vqsR, and rpoS. All three genes, which are involved in QS regulation, followed a similar profile of downregulation in the ΔrpoN ΔpqsE mutant and the ΔrpoN mutant. At the 24-h time point, rhlR transcript levels were 5-fold lower (Fig. 9A) and vqsR levels were 2.6-fold lower (Fig. 9B) in the ΔrpoN ΔpqsE mutant than in the ΔrpoN mutant, and for rpoS levels, the same pattern seen with vqsR levels was observed, in which rpoS levels decreased by 2-fold in the ΔrpoN ΔpqsE mutant compared with those in the ΔrpoN mutant (Fig. 9C).
All together, these results demonstrate that RpoN acts on QS regulators, including RhlR, VqsR, and RpoS, and that these elements of QS may define the mechanism by which RpoN mediates the response of P. aeruginosa to carbapenems.
DISCUSSION
To escape the bactericidal effect of antibiotics, P. aeruginosa employs its complex regulatory network to coordinate and promote adaptation to antibiotic stress. Of the several genes in P. aeruginosa known to modulate the response to antimicrobials (5, 8, 24, 28, 29, 37), an important role is played by sigma factor RpoN. This sigma factor is involved in the regulation of nitrogen assimilation, motility, QS, biofilm formation, secretion systems, and many others (30–36).
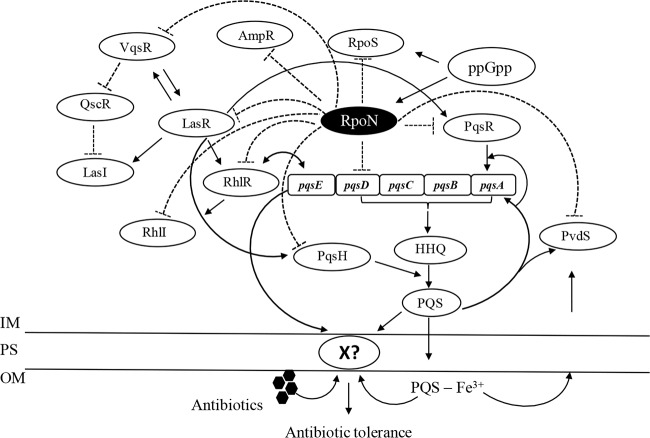
One important mechanism through which RpoN modulates the bactericidal effect of antimicrobials stems from its role in the regulation of QS, a target for antimicrobial therapy (52). In this study, we demonstrated that RpoN, in addition to its ascribed role in the regulation of the las and rhl QS systems, interacts with the PQS QS system, negatively regulates the expression of pqsA, pqsR, and pqsH, and, consequently, negatively affects the production of PQS. The regulatory network intercepted between RpoN and the elements of the QS system is presented in Fig. 10.
FIG 10.
Diagram of the proposed model of RpoN regulation in P. aeruginosa. The detailed network of regulation is described in the Discussion. Solid lines with arrows and dashed lines with blunt ends, genes that are positively and negatively affected, respectively. IM, inner membrane; OM, outer membrane; PS, periplasmic space.
Our initial studies on RpoN demonstrated that RpoN has a marked negative effect on the QS transcriptional regulator VqsR (37). Available evidence suggests that VqsR regulates QS systems (las, rhl, and pqs) by directly downregulating the expression of qscR (44), and the las system seems to be the dominant regulator of vqsR, pqsR, and pqsH (15, 53). We postulate that the observed activation of pqsR and vqsR in the ΔrpoN mutant is due to the upregulation of lasR, which in turn enhances pqsA expression. Furthermore, PqsR-PQS autoinduces PQS synthesis and further activates rhlR and rhlI expression.
In addition to increased expression of vqsR, the ΔrpoN mutant demonstrated significantly increased levels of the rpoS transcript throughout growth, suggesting that the absence of σ54 facilitates an increase in σS RNA polymerase binding, thus triggering the survival mode in the presence of an antibiotic. The fact that the RpoS-dependent pathway is involved in the response to carbapenems suggested that the carbapenem-tolerant phenotype of the ΔrpoN mutant in the stationary phase may be due to increased levels of RpoS (29). VqsR positively affects the expression of the pqsABCDE operon and of pqsH (46), and it has been demonstrated that PQS enhances RpoS expression (21); therefore, we presume that VqsR might modulate the expression of RpoS through PQS, reinforcing mutual control between PQS, VqsR, and RpoS.
We have previously reported that RpoN has an impact on iron-regulated genes irrespective of iron availability in the growth medium, and consequently, RpoN negatively affects the expression of the alternative sigma factor PvdS (37). Other findings also point to the fact that iron, QS, and RpoN are closely connected (54). To integrate the RpoN network into the regulation of PvdS, we postulate that both the control of the expression of iron-regulated genes, such as pvdS and pchA to pchG, by VqsR (44, 45, 55) and the iron-chelating properties of PQS (47, 48) could account for the increased expression of pvdS.
Using a time-kill assay, we were further able to demonstrate that pqsA, pqsE, pqsH, and rhlR play a role in mediating the increased survival of the ΔrpoN mutant in the presence of carbapenems because their inactivation in the ΔrpoN mutant altered the carbapenem-tolerant phenotype of the ΔrpoN mutant. The evidence that RpoN regulates several transcriptional regulators involved in the regulation of QS, including VqsR, RpoS, and PqsR, in addition to LasRI and RhlRI (35), suggests that the complex network between the elements of the QS systems contributes to the increased survival of the ΔrpoN mutant in the presence of carbapenems. This observation is consistent with a previous finding that the number of P. aeruginosa persister cells increases in response to QS and virulence factors (6). Additionally, it has been reported that the loss of rpoN through the deregulation of glucose metabolism increases gluconate production, which correlates with reduced susceptibility to several antibiotics (56).
A strategy for overcoming the effects of carbapenems requires RpoN to mediate this response through the regulators, which are likely to be membrane associated because carbapenems gain entrance into the cell through the outer membrane proteins (OMPs), and once they are located in the periplasmic space, they trigger lethality through interactions with transmembrane penicillin binding proteins (PBPs), leading to cell wall disintegration (57). One of the targets might be the transcriptional regulator AmpR, a potential inner membrane-associated protein (58). This hypothesis is supported by the observations that in the presence of β-lactams, the ΔrpoN mutant stimulated a significant increase in the levels of the ampR gene and the gene encoding β-lactamase, ampC (58), suggesting that this increase in ampR and ampC levels might aid in explaining the carbapenem-tolerant phenotype of the ΔrpoN mutant. Additionally, the ΔampR mutant exerted a negative effect on the expression of the major QS regulators LasR, RhlR, PqsR, and PQS and the pyochelin and pyoverdine genes (59).
Another potential strategy to overcome the effect of carbapenems is through the role of PQS and its association with the outer membrane. Because PQS has membrane-altering activities linked with the formation of outer membrane vesicles (OMVs) (60, 61) and more than 62.5% of the PQS produced is deposited in OMVs (62), we assume that the increased production of PQS and its association with the cell membrane would lower the effective concentration of carbapenems taken up by the cells.
An additional explanation for the role of PQS in modulating the response to carbapenems might come from the observation that OprF, a major outer membrane protein, is important in virulence factor production and in the formation of OMVs through modulation of PQS levels (63). Therefore, any perturbation in this network might affect the susceptibility to carbapenems.
Growing evidence suggests that one of the mechanisms by which cells react to antibiotic stress is through increased vesiculation (64). Adding to the complexity of PQS regulation, PQS has both antioxidant and pro-oxidant activities (5). The pro-oxidant activity of PQS increases the sensitivity of P. aeruginosa to peroxide and various antibiotics (5, 24), possibly resulting in cell lysis and DNA release. Our results with exogenously supplemented PQS demonstrate that PQS protected the cells from the effect of carbapenems. We presume that the difference in the survival potential between the cells exposed to PQS and those not exposed could be explained, in part, by the action of the alternative sigma factor RpoS, which could counterbalance the pro-oxidant effect of PQS by eliciting a stress response.
Furthermore, it is important to note that treatment with β-lactams leads to activation of the SOS response, which halts bacterial cell division and allows survival (65). Efflux pumps have been implicated as an important component of oxidative stress, providing protection against hydroxyl radicals by expelling damaged proteins outside the cells and promoting tolerance (66). To support this hypothesis, PQS is associated with the MexGHI-OpmD efflux pump, which controls antibiotic susceptibility through the production of PQS and N-acyl-l-homoserine lactone QS signal molecules and is also regulated by VqsR (44, 67). A recent study demonstrates that MexG is capable of binding PQS (68). The studies by Wu et al. demonstrated that the pro-oxidant paraquat promoted antibiotic tolerance, underscoring the importance of oxidative stress in antibiotic tolerance (69).
The fact that the induction of pqsE in the ΔrpoN ΔpqsA mutant produced merely a basal decrease in carbapenem susceptibility suggests that at least two factors, PQS and PqsE, cooperate to alleviate the effect of carbapenems in the ΔrpoN mutant. In addition to pqsA, the information that we gathered from the time-kill assay for the ΔrpoN ΔpqsH mutant reveals the direct involvement of PQS in the response to carbapenems in the ΔrpoN mutant. The fact that the ΔpqsH mutant is defective in vesicle formation (15, 61) additionally accentuates the importance of PQS in the interaction with the cell wall-targeting antibiotics. Furthermore, pqsH is directly controlled by LasR and not by PqsR (15). It is therefore conceivable that the LasR regulatory control over pqsH is important in tolerance to carbapenems. In the same line of experiments, we demonstrated that pqsE plays a role in tolerance to carbapenems in the ΔrpoN mutant and that its role in tolerance is not dependent on PQS levels because they remained as high in the ΔrpoN ΔpqsE mutant as in the ΔrpoN mutant. These observations led us to conclude that the loss of tolerance to carbapenems might also be accounted for by the loss of PQS-mediated virulence factors because PqsE influences the production of the secondary metabolites, such as pyocyanin, through interactions with RhlR (18, 20). Given that rhlR is controlled by RpoN and the regulation of RhlR and PqsE is interdependent, we addressed the possibility of whether suppressing the production of virulence factors via a mutation in rhlR in the ΔrpoN mutant would correspond to the susceptibility observed in the ΔrpoN ΔpqsE mutant. The loss of rhlR in the ΔrpoN mutant background affected survival in the presence of carbapenems and led to the decreased production of PQS, indicating that PQS production is important for biapenem tolerance mediated through RhlR.
In this study, we demonstrates that RpoN shuts down major metabolic systems, such as the QS systems, to communicate environmental cues between networks and to reduce energy costs. In this way, RpoN promotes the activation of only those cellular functions required for cells to enter into a less metabolically active state and persist. In addition, it was demonstrated that during growth under nutrient-restricted conditions the ΔrpoN mutant downregulates the expression of pqs signaling (70). Low metabolic activity and stressed environmental conditions are connected with the increase in the levels of the alarmone ppGpp, a regulator of the stringent response, which is involved in the regulation of QS systems, the nitrogen stress response, and antibiotic tolerance (5, 8, 71, 72) and is essential for sustaining the survival of the cells. Therefore, we postulate that another level of RpoN regulation is mediated through ppGpp. Finally, the observations presented herein contribute to a better understanding regarding the way in which P. aeruginosa integrates multiple regulatory signals to alleviate the effect of carbapenems. A complete understanding of the regulatory pathways that interfere with RpoN during antibiotic stress remains to be elucidated.
Supplementary Material
ACKNOWLEDGMENTS
D.V. was a recipient of a fellowship from the Fujii-Otsuka Fellowship for International Exchange Program. This work was supported by Grant-in-Aid for Scientific Research (C) (no. 2646278700) from the Japan Society for the Promotion of Science.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00260-16.
REFERENCES
- 1.Lewis K. 2012. Persister cells: molecular mechanisms related to antibiotic tolerance. Handb Exp Pharmacol 211:121–133. doi: 10.1007/978-3-642-28951-4_8. [DOI] [PubMed] [Google Scholar]
- 2.Juan C, Macia MD, Gutierrez O, Vidal C, Perez JL, Oliver A. 2005. Molecular mechanisms of β-lactam resistance mediated by AmpC hyperproduction in Pseudomonas aeruginosa clinical strains. Antimicrob Agents Chemother 49:4733–4738. doi: 10.1128/AAC.49.11.4733-4738.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashizume T, Ishino F, Nakagawa J, Tamaki S, Matsuhashi M. 1984. Studies on the mechanism of action of imipenem (N-formimidoylthienamycin) in vitro: binding to the penicillin-binding proteins (PBPs) in Escherichia coli and Pseudomonas aeruginosa, and inhibition of enzyme activities due to the PBPs in E. coli. J Antibiot (Tokyo) 37:394–400. doi: 10.7164/antibiotics.37.394. [DOI] [PubMed] [Google Scholar]
- 4.Monahan LG, Turnbull L, Osvath SR, Birch D, Charles IG, Whitchurch CB. 2014. Rapid conversion of Pseudomonas aeruginosa to a spherical cell morphotype facilitates tolerance to carbapenems and penicillins but increases susceptibility to antimicrobial peptides. Antimicrob Agents Chemother 58:1956–1962. doi: 10.1128/AAC.01901-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen D, Joshi-Datar A, Lepine F, Bauerle E, Olakanmi O, Beer K, McKay G, Siehnel R, Schafhauser J, Wang Y, Britigan BE, Singh PK. 2011. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 334:982–986. doi: 10.1126/science.1211037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Möker N, Dean CR, Tao J. 2010. Pseudomonas aeruginosa increases formation of multidrug-tolerant persister cells in response to quorum-sensing signaling molecules. J Bacteriol 192:1946–1955. doi: 10.1128/JB.01231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dörr T, Lewis K, Vulic M. 2009. SOS response induces persistence to fluoroquinolones in Escherichia coli. PLoS Genet 5:e1000760. doi: 10.1371/journal.pgen.1000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viducic D, Ono T, Murakami K, Susilowati H, Kayama S, Hirota K, Miyake Y. 2006. Functional analysis of spoT, relA and dksA genes on quinolone tolerance in Pseudomonas aeruginosa under nongrowing condition. Microbiol Immunol 50:349–357. doi: 10.1111/j.1348-0421.2006.tb03793.x. [DOI] [PubMed] [Google Scholar]
- 9.de Kievit TR, Iglewski BH. 2000. Bacterial quorum sensing in pathogenic relationships. Infect Immun 68:4839–4849. doi: 10.1128/IAI.68.9.4839-4849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 11.Passador L, Cook JM, Gambello MJ, Rust L, Iglewski BH. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260:1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- 12.Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A. 1996. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol Microbiol 21:1137–1146. doi: 10.1046/j.1365-2958.1996.00063.x. [DOI] [PubMed] [Google Scholar]
- 13.Pesci EC, Pearson JP, Seed PC, Iglewski BH. 1997. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol 179:3127–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pesci EC, Milbank JB, Pearson JP, McKnight S, Kende AS, Greenberg EP, Iglewski BH. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Déziel E, Lépine F, Milot S, He J, Mindrinos MN, Tompkins RG, Rahme LG. 2004. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc Natl Acad Sci U S A 101:1339–1344. doi: 10.1073/pnas.0307694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu S, Jensen V, Seeliger J, Feldmann I, Weber S, Schleicher E, Häussler S, Blankenfeldt W. 2009. Structure elucidation and preliminary assessment of hydrolase activity of PqsE, the Pseudomonas quinolone signal (PQS) response protein. Biochemistry 48:10298–10307. doi: 10.1021/bi900123j. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher LA, McKnight SL, Kuznetsova MS, Pesci EC, Manoil C. 2002. Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa. J Bacteriol 184:6472–6480. doi: 10.1128/JB.184.23.6472-6480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrow JM III, Sund ZM, Ellison ML, Wade DS, Coleman JP, Pesci EC. 2008. PqsE functions independently of PqsR-Pseudomonas quinolone signal and enhances the rhl quorum-sensing system. J Bacteriol 190:7043–7051. doi: 10.1128/JB.00753-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rampioni G, Pustelny C, Fletcher MP, Wright VJ, Bruce M, Rumbaugh KP, Heeb S, Cámara M, Williams P. 2010. Transcriptomic analysis reveals a global alkyl-quinolone-independent regulatory role for PqsE in facilitating the environmental adaptation of Pseudomonas aeruginosa to plant and animal hosts. Environ Microbiol 12:1659–1673. doi: 10.1111/j.1462-2920.2010.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hazan R, He J, Xiao G, Dekimpe V, Apidianakis Y, Lesic B, Astrakas C, Déziel E, Lépine F, Rahme LG. 2010. Homeostatic interplay between bacterial cell-cell signaling and iron in virulence. PLoS Pathog 6:e1000810. doi: 10.1371/journal.ppat.1000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diggle SP, Winzer K, Chhabra SR, Worrall KE, Cámara M, Williams P. 2003. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol Microbiol 50:29–43. doi: 10.1046/j.1365-2958.2003.03672.x. [DOI] [PubMed] [Google Scholar]
- 22.McKnight SL, Iglewski BH, Pesci EC. 2000. The Pseudomonas quinolone signal regulates rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol 182:2702–2708. doi: 10.1128/JB.182.10.2702-2708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wade DS, Calfee MW, Rocha ER, Ling EA, Engstrom E, Coleman JP, Pesci EC. 2005. Regulation of Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa. J Bacteriol 187:4372–4380. doi: 10.1128/JB.187.13.4372-4380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Häussler S, Becker T. 2008. The pseudomonas quinolone signal (PQS) balances life and death in Pseudomonas aeruginosa populations. PLoS Pathog 4:e1000166. doi: 10.1371/journal.ppat.1000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murakami KS, Masuda S, Darst SA. 2002. Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 Å resolution. Science 296:1280–1284. doi: 10.1126/science.1069594. [DOI] [PubMed] [Google Scholar]
- 26.Suh SJ, Silo-Suh L, Woods DE, Hassett DJ, West SE, Ohman DE. 1999. Effect of rpoS mutation on the stress response and expression of virulence factors in Pseudomonas aeruginosa. J Bacteriol 181:3890–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whiteley M, Bangera MG, Bumgartner RE, Parsek MR, Teitzel GM, Lory S, Greenberg EP. 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860–864. doi: 10.1038/35101627. [DOI] [PubMed] [Google Scholar]
- 28.Kayama S, Murakami K, Ono T, Ushimaru M, Yamamoto A, Hirota K, Miyake Y. 2009. The role of rpoS gene and quorum-sensing system in ofloxacin tolerance in Pseudomonas aeruginosa. FEMS Microbiol Lett 298:184–192. doi: 10.1111/j.1574-6968.2009.01717.x. [DOI] [PubMed] [Google Scholar]
- 29.Murakami K, Ono T, Viducic D, Kayama S, Mori M, Hirota K, Nemoto K, Miyake Y. 2005. Role for rpoS gene of Pseudomonas aeruginosa in antibiotic tolerance. FEMS Microbiol Lett 242:161–167. doi: 10.1016/j.femsle.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Jishage M, Iwata A, Ueda S, Ishihama A. 1996. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of four species of sigma subunit under various growth conditions. J Bacteriol 178:5447–5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merrick MJ, Coppard JR. 1989. Mutations in genes downstream of the rpoN gene (encoding sigma 54) of Klebsiella pneumoniae affect expression from sigma 54-dependent promoters. Mol Microbiol 3:1765–1775. doi: 10.1111/j.1365-2958.1989.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 32.Totten PA, Lara JC, Lory S. 1990. The rpoN gene product of Pseudomonas aeruginosa is required for expression of diverse genes, including the flagellin gene. J Bacteriol 172:389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woods DE, Straus DC, Johanson WG, Berry WK Jr, Bass JA. 1980. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun 29:1146–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sana TG, Soscia C, Tonglet CM, Garvis S, Bleves S. 2013. Divergent control of two type VI secretion systems by RpoN in Pseudomonas aeruginosa. PLoS One 8:e76030. doi: 10.1371/journal.pone.0076030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heurlier K, Dénervaud V, Pessi G, Reimmann C, Haas D. 2003. Negative control of quorum sensing by RpoN (sigma54) in Pseudomonas aeruginosa PAO1. J Bacteriol 185:2227–2235. doi: 10.1128/JB.185.7.2227-2235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson LS, Webb JS, Rice SA, Kjelleberg S. 2003. The alternative sigma factor RpoN regulates the quorum sensing gene rhlI in Pseudomonas aeruginosa. FEMS Microbiol Lett 220:187–195. doi: 10.1016/S0378-1097(03)00097-1. [DOI] [PubMed] [Google Scholar]
- 37.Viducic D, Ono T, Murakami K, Katakami M, Susilowati H, Miyake Y. 2007. rpoN gene of Pseudomonas aeruginosa alters its susceptibility to quinolones and carbapenems. Antimicrob Agents Chemother 51:1455–1462. doi: 10.1128/AAC.00348-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogel HJ, Bonner DM. 1956. Acetyl-ornithinase of Escherichia coli: partial purification and some properties. J Biol Chem 218:97–106. [PubMed] [Google Scholar]
- 39.Miyake Y, Fujiwara S, Usui T, Suginaka H. 1992. Simple method for measuring the antibiotic concentration required to kill adherent bacteria. Chemotherapy 38:286–290. doi: 10.1159/000239015. [DOI] [PubMed] [Google Scholar]
- 40.Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86. doi: 10.1016/S0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 41.Schweizer HP. 1992. Allelic exchange in Pseudomonas aeruginosa using novel ColE1-type vectors and a family of cassettes containing a portable oriT and the counter-selectable Bacillus subtilis sacB marker. Mol Microbiol 6:1195–1204. doi: 10.1111/j.1365-2958.1992.tb01558.x. [DOI] [PubMed] [Google Scholar]
- 42.Newman JR, Fuqua C. 1999. Broad-host-range expression vectors that carry the l-arabinose-inducible Escherichia coli araBAD promoter and the araC regulator. Gene 227:197–203. doi: 10.1016/S0378-1119(98)00601-5. [DOI] [PubMed] [Google Scholar]
- 43.Essar DW, Eberly L, Hadero A, Crawford IP. 1990. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol 172:884–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang H, Deng X, Ji Q, Sun F, Shen T, He C. 2012. The Pseudomonas aeruginosa global regulator VqsR directly inhibits QscR to control quorum-sensing and virulence gene expression. J Bacteriol 194:3098–3108. doi: 10.1128/JB.06679-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Juhas M, Wiehlmann L, Huber B, Jordan D, Lauber J, Salunkhe P, Limpert AS, von Götz F, Steinmetz I, Eberl L, Tümmler B. 2004. Global regulation of quorum sensing and virulence by VqsR in Pseudomonas aeruginosa. Microbiology 150:831–841. doi: 10.1099/mic.0.26906-0. [DOI] [PubMed] [Google Scholar]
- 46.Juhas M, Wiehlmann L, Salunkhe P, Lauber J, Buer J, Tümmler B. 2005. GeneChip expression analysis of the VqsR regulon of Pseudomonas aeruginosa TB. FEMS Microbiol Lett 242:287–295. doi: 10.1016/j.femsle.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 47.Bredenbruch F, Geffers R, Nimtz M, Buer J, Häussler S. 2006. The Pseudomonas aeruginosa quinolone signal (PQS) has an iron-chelating activity. Environ Microbiol 8:1318–1329. doi: 10.1111/j.1462-2920.2006.01025.x. [DOI] [PubMed] [Google Scholar]
- 48.Diggle SP, Matthijs S, Wright VJ, Fletcher MP, Chhabra SR, Lamont IL, Kong X, Hider RC, Cornelis P, Cámara M, Williams P. 2007. The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem Biol 14:87–96. doi: 10.1016/j.chembiol.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Xiao G, Deziel E, He J, Lepine F, Lesic B, Castonguay MH, Milot S, Tampakaki AP, Stachel SE, Rahme LG. 2006. MvfR, a key Pseudomonas aeruginosa pathogenicity LTTR-class regulatory protein, has dual ligands. Mol Microbiol 62:1689–1699. doi: 10.1111/j.1365-2958.2006.05462.x. [DOI] [PubMed] [Google Scholar]
- 50.Xiao G, He J, Rahme LG. 2006. Mutation analysis of the Pseudomonas aeruginosa mvfR and pqsABCDE gene promoters demonstrates complex quorum-sensing circuitry. Microbiology 152:1679–1686. doi: 10.1099/mic.0.28605-0. [DOI] [PubMed] [Google Scholar]
- 51.Brouwer S, Pustelny C, Ritter C, Klinkert B, Narberhaus F, Häussler S. 2014. The PqsR and RhlR transcriptional regulators determine the level of Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa by producing two different pqsABCDE mRNA isoforms. J Bacteriol 196:4163–4171. doi: 10.1128/JB.02000-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rasmussen TB, Givskov M. 2006. Quorum-sensing inhibitors as anti-pathogenic drugs. Int J Med Microbiol 296:149–161. doi: 10.1016/j.ijmm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Li LL, Malone JE, Iglewski BH. 2007. Regulation of the Pseudomonas aeruginosa quorum-sensing regulator VqsR. J Bacteriol 189:4367–4374. doi: 10.1128/JB.00007-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Damron FH, Owings JP, Okkotsu Y, Varga JJ, Schurr JR, Goldberg JB, Schurr MJ, Yu HD. 2012. Analysis of the Pseudomonas aeruginosa regulon controlled by the sensor kinase KinB and sigma factor RpoN. J Bacteriol 194:1317–1330. doi: 10.1128/JB.06105-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cornelis P, Aendekerk S. 2004. A new regulator linking quorum sensing and iron uptake in Pseudomonas aeruginosa. Microbiology 150:752–756. doi: 10.1099/mic.0.27086-0. [DOI] [PubMed] [Google Scholar]
- 56.Behrends V, Bell TJ, Liebeke M, Cordes-Blauert A, Ashraf SN, Nair C, Zlosnik JE, Williams HD, Bundy JG. 2013. Metabolite profiling to characterize disease-related bacteria: gluconate excretion by Pseudomonas aeruginosa mutants and clinical isolates from cystic fibrosis patients. J Biol Chem 288:15098–15109. doi: 10.1074/jbc.M112.442814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. 2011. Carbapenems: past, present, and future. Antimicrob Agents Chemother 55:4943–4960. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caille O, Zincke D, Merighi M, Balasubramanian D, Kumari H, Kong K, Silva-Herzog E, Narasimhan G, Schneper L, Lory S, Mathee K. 2014. Structural and functional characterization of Pseudomonas aeruginosa global regulator AmpR. J Bacteriol 196:3890–3902. doi: 10.1128/JB.01997-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balasubramanian D, Schneper L, Kumari H, Mathee K. 2013. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res 41:1–20. doi: 10.1093/nar/gks1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mashburn LM, Whiteley M. 2005. Membrane vesicles traffic signals and facilitate group activities in a procaryote. Nature 437:422–425. doi: 10.1038/nature03925. [DOI] [PubMed] [Google Scholar]
- 61.Mashburn-Warren L, Howe J, Garidel P, Richter W, Steiniger F, Roessle M, Brandenburg K, Whiteley M. 2008. Interaction of quorum signals with outer membrane lipids: insights into prokaryotic membrane vesicle formation. Mol Microbiol 69:491–502. doi: 10.1111/j.1365-2958.2008.06302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lépine F, Déziel E, Milot S, Rahme LG. 2003. A stable isotope dilution assay for the quantification of the Pseudomonas quinolone signal in Pseudomonas aeruginosa cultures. Biochim Biophys Acta 1622:36–41. doi: 10.1016/S0304-4165(03)00103-X. [DOI] [PubMed] [Google Scholar]
- 63.Fito-Boncompte L, Chapalain A, Bouffartigues E, Chaker H, Lesouhaitier O, Gicquel G, Bazire A, Madi A, Connil N, Véron W, Taupin L, Toussaint B, Cornelis P, Wei Q, Shioya K, Déziel E, Feuilloley MG, Orange N, Dufour A, Chevalier S. 2011. Full virulence of Pseudomonas aeruginosa requires OprF. Infect Immun 79:1176–1186. doi: 10.1128/IAI.00850-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cirz RT, O'Neill BM, Hammond JA, Head SR, Romesberg FE. 2006. Defining the Pseudomonas aeruginosa SOS response and its role in the global response to the antibiotic ciprofloxacin. J Bacteriol 188:7101–7110. doi: 10.1128/JB.00807-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller C, Thomsen LE, Gaggero C, Mosseri R, Ingmer H, Cohen SN. 2004. SOS response induction by β-lactams and bacterial defense against antibiotic lethality. Science 305:1629–1631. doi: 10.1126/science.1101630. [DOI] [PubMed] [Google Scholar]
- 66.Poole K. 2008. Bacterial multidrug efflux pumps serve other functions. Microbe 3:179–185. [Google Scholar]
- 67.Aendekerk S, Diggle SP, Song Z, Høiby N, Cornelis P, Williams P, Cámara M. 2005. The MexGHI-OpmD multidrug efflux pump controls growth, antibiotic susceptibility and virulence in Pseudomonas aeruginosa via 4-quinolone-dependent cell-to-cell communication. Microbiology 151:1113–1125. doi: 10.1099/mic.0.27631-0. [DOI] [PubMed] [Google Scholar]
- 68.Hodgkinson JT, Gross J, Baker YR, Springa DR, Welch M. 2016. A new Pseudomonas quinolone signal (PQS) binding partner: MexG. Chem Sci 7:2553–2562. doi: 10.1039/C5SC04197J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu Y, Vulić M, Keren I, Lewis K. 2012. Role of oxidative stress in persister tolerance. Antimicrob Agents Chemother 56:4922–4926. doi: 10.1128/AAC.00921-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cai Z, Liu Y, Chen Y, Yam JK, Chew SC, Chua SL, Wang K, Givskov M, Yang L. 2015. RpoN regulates virulence factors of Pseudomonas aeruginosa via modulating the PqsR quorum sensing regulator. Int J Mol Sci 16:28311–28319. doi: 10.3390/ijms161226103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brown DR, Barton G, Pan Z, Buck M, Wigneshweraraj S. 2014. Nitrogen stress response and stringent response are coupled in Escherichia coli. Nat Commun 5:4115. doi: 10.1038/ncomms5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schafhauser J, Lepine F, McKay G, Ahlgren HG, Khakimova M, Nguyen D. 2014. The stringent response modulates 4-hydroxy-2-alkylquinoline biosynthesis and quorum-sensing hierarchy in Pseudomonas aeruginosa. J Bacteriol 196:1641–1650. doi: 10.1128/JB.01086-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.