Abstract
It is now well established that bacterial infections are often associated with biofilm phenotypes that demonstrate increased resistance to common antimicrobials. Further, due to the collective attrition of new antibiotic development programs by the pharmaceutical industries, drug repurposing is an attractive alternative. In this work, we screened 1,280 existing commercially available drugs in the Prestwick Chemical Library, some with previously unknown antimicrobial activity, against Staphylococcus aureus, one of the commonly encountered causative pathogens of burn and wound infections. From the primary screen of the entire Prestwick Chemical Library at a fixed concentration of 10 μM, 104 drugs were found to be effective against planktonic S. aureus strains, and not surprisingly, these were mostly antimicrobials and antiseptics. The activity of 18 selected repurposing candidates, that is, drugs that show antimicrobial activity that are not already considered antimicrobials, observed in the primary screen was confirmed in dose-response experiments. Finally, a subset of nine of these drug candidates was tested against preformed biofilms of S. aureus. We found that three of these drugs, niclosamide, carmofur, and auranofin, possessed antimicrobial activity against preformed biofilms, making them attractive candidates for repurposing as novel antibiofilm therapies.
INTRODUCTION
Staphylococcus aureus is an opportunistic human commensal that colonizes the skin and respiratory tract. S. aureus infections have risen significantly as a result of advances in medicine, such as implantable devices and intravenous (i.v.) catheters (1). Furthermore, the resistance of S. aureus to methicillin has now become widespread in hospital settings (2), and infections caused by methicillin-resistant S. aureus (MRSA) strains kill an estimated 19,000 patients in hospitals every year (3). Exacerbating the problem of resistance is the fact that S. aureus can form biofilms, which are matrix-enclosed bacterial communities that are inherently more resistant to antibiotics due to their dense exopolymeric matrix and reduced metabolism (4). Therefore, new drugs for the treatment of drug-resistant S. aureus biofilm infections are still desperately needed. However, due to the high cost, long incubation period, and low success rate of new drug development, the private pharmaceutical sector has withdrawn from antibiotic discovery (5). In response, individual or collaborative efforts in not-for-profit organizations and academic institutions focusing on novel or nontraditional approaches for antibiotic discovery have increased. One such approach is to explore the potential use of existing drugs in disease areas outside their original indications, otherwise known as “drug repositioning” or “drug repurposing” (5). Finding new uses for existing drugs can be achieved either on the basis of a detailed understanding of the chemistry of individual compounds and their potential targets from in silico modeling followed by in vitro cell culture and in vivo animal studies or directly by screening a large library of compounds in disease-relevant phenotypic assays, thus allowing an unbiased approach to obtain successful hits (5). The latter approach can accelerate and bring down the costs of the drug discovery process if an appropriate library of FDA-approved compounds with substantial diversity, drug-like properties, and acceptable safety profiles is chosen and a relevant phenotypic assay that closely mimics the in vivo conditions is used. In this work, we used a phenotypic, high-throughput biofilm assay approach to screen the FDA-approved, off-patent Prestwick Chemical Library to identify compounds that display novel antibiofilm activity against S. aureus biofilms.
MATERIALS AND METHODS
Strains and culture conditions.
The TCH1516 methicillin-resistant strain of S. aureus was used in this study. Stocks were stored in vials with cryogenic beads soaked in glycerol at −80°C. For every experiment, overnight cultures were prepared by transferring a single bead into 20 ml of tryptic soy broth (TSB) medium (Becton, Dickinson and Company, Franklin Lakes, NJ) and incubating in a shaking incubator (Thermo Fisher, Waltham, MA) at 150 rpm and 37°C. For the preliminary screen and dose-response experiments, 100 μl of the overnight culture was transferred to 10 ml of fresh TSB and incubated for 3 h at 150 rpm and 37°C to allow the subculture to reach the log phase of growth. This log-phase culture was used for the following steps. For all experiments, 1-ml aliquots of the overnight culture (or log-phase culture) were transferred into 1.5-ml microcentrifuge tubes and centrifuged at 14,000 rpm for 5 min (or 4,000 rpm for 15 min). After centrifugation, the supernatant was removed and the cells were resuspended in 1 ml phosphate-buffered saline (PBS; Sigma-Aldrich, St. Louis, MO). After repeating the centrifugation process once more, the cells were resuspended in 1 ml PBS for quantification using turbidometry in a BioPhotometer (Eppendorf, Hauppauge, NY) set to an optical density at 600 nm (OD600), using PBS alone as a blank. The cell density of the overnight culture (or log-phase culture) was calculated using a standard curve formula. Finally, the cell density was adjusted to 4 × 106 cells/ml in 2× Muller-Hinton broth (MHB) medium (Becton, Dickinson and Company, Franklin Lakes, NJ) for the preliminary and dose-response experiments or 1 × 108 cells/ml in biofilm medium (45% brain heart infusion [BHI], 45% tryptic soy broth, 10% human serum) for the preformed biofilm experiments.
Primary screen.
First, we performed an initial screen of all 1,280 off-patent FDA-approved drugs in the Prestwick Chemical Library (Prestwick Chemical, Illkirch-Graffenstaden, France) at 10 μM in 96-well plates against planktonic cultures of S. aureus. Briefly, seeding solutions were prepared at a density of 4 × 106 cells/ml in 2× MHB, and 50 μl was dispensed into 96-well plates using a Hamilton Microlab STARlet robotic liquid-handling system (Hamilton Robotics, Reno, NV). Next, working solutions of the compounds from the Prestwick Chemical Library were prepared in sterile Milli-Q H2O at a concentration of 20 μM. Then, 50 μl of the working solutions of the compounds was combined with the preseeded 96-well plates using the STARlet system to create duplicate test plates with cell and compound concentrations of 2 × 106 cells/ml and 10 μM, respectively. The test plates were then incubated at 37°C for 24 h. Cell viability was determined using turbidometry at OD600. Compounds that reduced the turbidity of the culture by more than 55% relative to that for an untreated control were classified as hits and selected for subsequent dose-response experiments.
Dose-response of selected repositionable candidates.
For confirmatory purposes and initial determination of potency, active compounds selected from the initial screen were serially diluted over a range of concentrations (1.25 μM to 40 μM) and 50 μl of each compound was dispensed using the STARlet system into duplicate rows of a 96-well plate. Next, as in the primary screen experiments, a cell suspension was prepared at a concentration of 4 × 106 cells/ml in 2× MHB, and 50 μl was combined using the STARlet system with the predispensed drug dilution plates to create duplicate test plates with a dilution range of 0.625 μM to 20 μM of the selected compounds and cells at 2 × 106 cells/ml. Test plates were incubated overnight at 37°C to allow compound action. Cell viability was determined using turbidometry at OD600, from which dose-response profiles were obtained. The 50% inhibitory concentration (IC50) was estimated as the concentration of any given compound that reduced the viability of the biofilm by 50% compared to that of an untreated control (growth medium only). Furthermore, standard viable cell counts were performed on a subset of nine drugs with novel antimicrobial activity to determine their MICs and minimal bactericidal concentrations (MBCs). Briefly, 100 μl from each well was diluted in 900 μl of PBS. Next, five 10-fold serial dilutions were prepared from the initial dilution, and 50 μl of each of these was plated in triplicate on blood agar plates. The plates were incubated at 37°C for 24 h. Finally, colonies were counted in an automated colony counter (ProtoCOL-Synbiosis; Microbiology International, Frederick, MD).
Activity against preformed biofilms.
We evaluated the efficacy against preformed biofilms of a subset of 9 drugs that were selected on the basis of their novelty as antimicrobials.
We adapted a 96-well-plate biofilm model that has been previously validated and used in other similar assays (6). In our modified protocol, seeding solutions were prepared at a density of 1 × 108 cells/ml in biofilm medium, consisting of 45% BHI medium (Becton, Dickinson and Company, Franklin Lakes, NJ), 45% yeast extract-peptone-dextrose (YPD) medium (Becton, Dickinson and Company, Franklin Lakes, NJ), and 10% human serum (Thermo Fisher, Waltham, MA). We have previously determined that this particular composition is optimal for maximum biofilm yield (7). One hundred microliters of the seeding solution was dispensed into the wells of a collagen-coated 96-well plate (Thermo Fisher, Waltham, MA) and incubated at 37°C for 4 h to allow cell attachment onto the surface. Then, the suspension was removed and the wells were carefully rinsed twice with 100 μl of sterile PBS to remove any unbound cells. The wells were replenished with 100 μl of fresh biofilm medium, and the plates were incubated at 37°C for 24 h to allow biofilm formation. After maturation, the medium was removed and the biofilms were carefully and gently rinsed twice with sterile PBS to remove any unbound cells. One hundred microliters of serial dilutions of the nine selected compounds over a concentration range of 0.625 μM to 40 μM in the biofilm medium was prepared and was added to the rinsed biofilms. Duplicate plates were prepared for every compound. The biofilms were then incubated at 37°C for 24 h to allow compound action. After 24 h, the biofilms were gently and carefully rinsed with sterile PBS, and the viability of the biofilms was determined using a 1:10 dilution of the Presto Blue cell viability reagent (Thermo Fisher, Waltham, MA) in biofilm medium. Presto Blue is a fluorescent metabolic assay that correlates the number of viable bacteria immobilized in the biofilm to the fluorescent signal generated. Presto Blue has been extensively used in other studies to determine the viability of bacteria within biofilms (8–12). After a 30-min incubation at 37°C, the fluorescence at 530 nm and 590 nm (excitation and emission, respectively) was read in a microplate reader. In addition, time-kill studies and viable cell counts were performed to determine the reduction in the number of viable cells within the biofilms at early and late time points. Briefly, at 2 and 24 h posttreatment, the medium was removed and the biofilms were resuspended by repeatedly dispensing and removing 100 μl of PBS while scraping the sides and bottom of the well with a pipette tip. Five 10-fold serial dilutions of the resuspended biofilms were prepared, plated, and enumerated as described above.
Finally, in order to confirm the presence of a biofilm, untreated biofilms were grown, rinsed, and stained with 100 μl of FilmTracer Sypro Ruby biofilm matrix stain (Thermo Fisher, Waltham, MA). The biofilms were stained at 37°C for 30 min and rinsed, and the fluorescence at 450 nm and 610 nm (excitation and emission, respectively) was read in a microplate reader. The Sypro Ruby stain selectively labels the glycoproteins, lipoproteins, phosphoproteins, and other difficult-to-stain proteins that are present in the biofilm matrix.
RESULTS AND DISCUSSION
Primary screen.
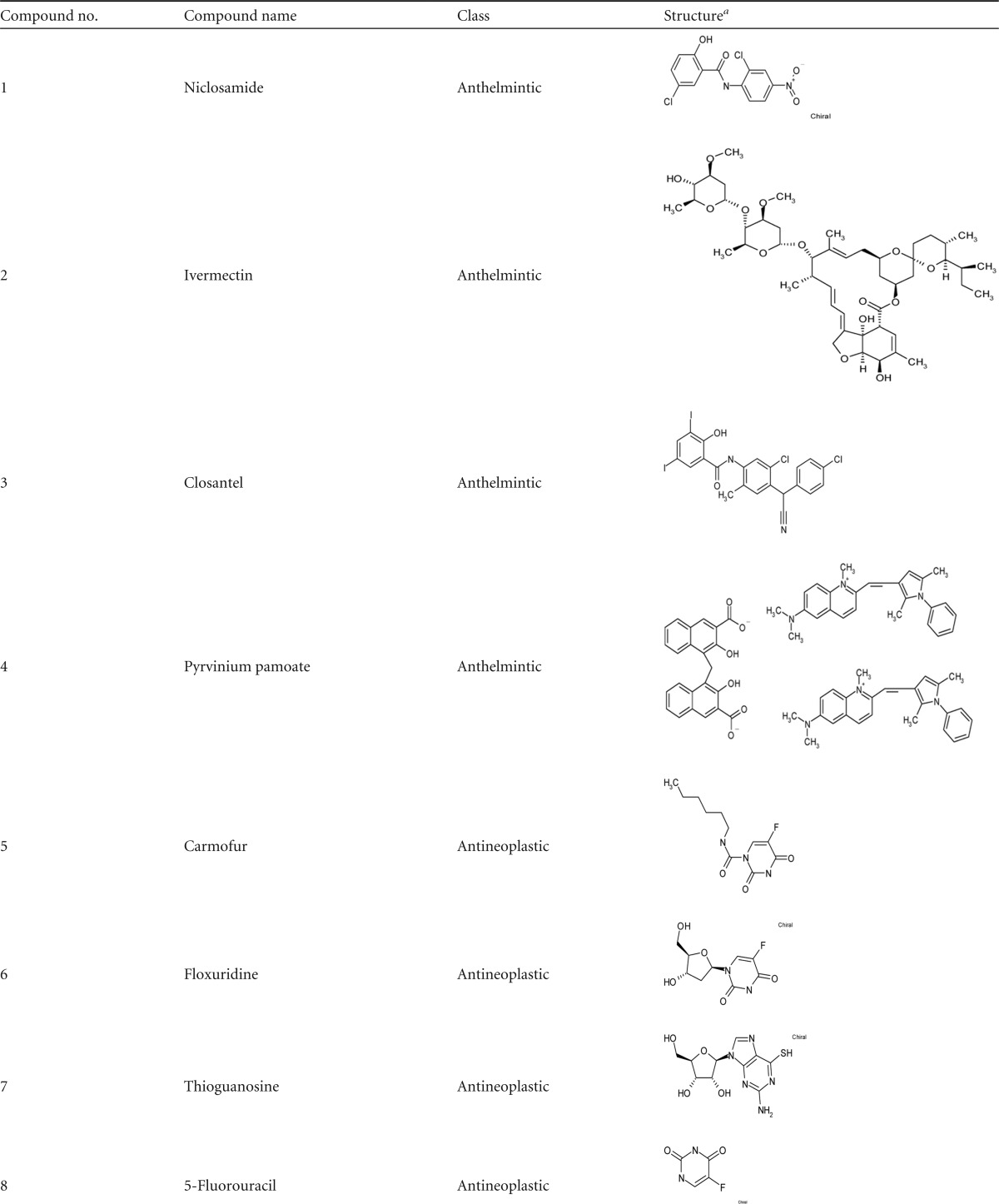
From the initial screen of all 1,280 compounds in the Prestwick Chemical Library, we identified 104 compounds that reduced the viability of S. aureus cultures by more than 55%, as determined by turbidometry (Fig. 1A). Based on their known pharmacological profiles, we classified these 104 compounds either as antimicrobials/antiseptics (n = 86) or as miscellaneous drug candidates (n = 18) (Fig. 1B). Because the purpose of this work was to find new repositionable drugs, we decided to move forward with only those drugs that were not antimicrobials. The 18 repositionable candidates, their original or currently known classifications, and their structures are shown in Table 1.
FIG 1.
Preliminary screen and breakdown of library by category. (A) A total of 1,280 compounds were tested against planktonic cultures of S. aureus at 10 μM. A total of 104 drugs inhibited the growth of bacteria by more than 55%. (B) The Prestwick Chemical Library consists of 212 antimicrobials, antiseptics, and antifungals and 1,068 miscellaneous drugs. Of the 104 hits, 86 were antimicrobials and 18 were miscellaneous (Misc.) repositionable drug candidates.
TABLE 1.
Summary of selected nonantimicrobials with antimicrobial activity
The illustrations of the chemical structures were adapted from the technical documentation provided with the Prestwick Chemical Library.
Dose-response assays of selected repositionable candidates.
The 18 selected candidates were tested over a concentration range of 0.625 μM to 20 μM (2-fold dilutions) against planktonic cultures of S. aureus both to confirm the activity observed in the primary screen and to establish their potency using the IC50 as a metric. Most of the drugs tested showed a gradual change in antimicrobial activity in a dose-dependent fashion, while some drugs were very potent and the S. aureus cultures were nonviable even at the lowest concentration tested (Fig. 2). Table 2 summarizes the IC50, MICs, and MBCs of all 18 selected candidates tested against planktonic cultures, and these are discussed below.
FIG 2.
Selected candidates were tested in 2-fold dilutions ranging from 0.625 μM to 20 μM. The antimicrobial activity of the selected drugs was confirmed for the anthelmintics niclosamide, pyrvinium pamoate, ivermectin, and closantel (A); the antineoplastics carmofur, thioguanosine, floxuridine, 5-fluorouracil, gemcitabine, and epirubicin HCl (B); and the miscellaneous drugs benzbromarone, pinaverium bromide (Br), dicumarol, ebselen, auranofin, verteporfin, 1,8-dihydroyanthraquinone, and tolcapone (C and D). Ctrl, control.
TABLE 2.
Summary of antimicrobial activities of candidates
| Drug class and druga | IC50 (μM) for planktonic S. aureus | MIC (μM) | MBC (μM) |
|---|---|---|---|
| Anthelmintics | |||
| Niclosamide | 1.2 | 5 | >20 |
| Pyrvinium pamoate | 1.1 | 2.5 | >20 |
| Ivermectin | 6.8 | >20 | >20 |
| Closantel | <0.6 | ||
| Antineoplastics | |||
| Carmofur | 0.6 | 2.5 | >20 |
| Thioguanosine | 1.9 | ||
| Floxuridine | <0.6 | ||
| 5-Fluorouracil | 0.6 | ||
| Gemcitabine | ≪0.6 | ||
| Epirubicin hydrochloride | 2.4 | >20 | >20 |
| Miscellaneous | |||
| Benzbromarone (antianginal) | 1.7 | ||
| Pinaverium bromide (antispastic) | 4.5 | 20 | >20 |
| Dicumarol (anticoagulant) | 3.0 | ||
| Ebselen (anti-inflammatory) | 1.3 | ||
| Auranofin (antirheumatic) | ≪0.6 | 0.6 | >20 |
| Verteporfin (photosensitizer) | 1.6 | 10 | >20 |
| 1,8-Dihydroxyanthraquinone (laxative) | 5.1 | ||
| Tolcapone (antiparkinsonian) | 7.5 | >20 | >20 |
Drugs that were selected for testing against preformed biofilms are in bold.
Anthelmintics are drugs that are primarily used for the treatment of helminth infestations in both animals and humans. We observed that, in agreement with recent reports by Rajamuthiah et al. (13), niclosamide showed strong antimicrobial activity against planktonic S. aureus with an IC50 of 1.2 μM. Furthermore, closantel, a homologue of niclosamide, also showed significant antimicrobial activity against planktonic S. aureus with an IC50 below the lowest concentration tested (0.6 μM), in agreement with the findings of Macielag et al. (14).
Another drug, auranofin, has recently been reported to have strong antimicrobial activity against several Gram-positive organisms (15–17), and our results agree well with these reports, as the IC50 of auranofin was well below the lowest concentration tested (0.6 μM).
Antineoplastics are drugs that prevent or inhibit the abnormal growth of human cells and are commonly used as anticancer treatments. Gemcitabine, floxuridine, carmofur, and 5-fluorouracil showed significant antimicrobial activity against planktonic S. aureus with IC50s equal to or below the lowest concentration tested (0.6 μM). The antimicrobial activity of gemcitabine against several strains of S. aureus has previously been reported by Jordheim et al. (18). An average MIC of 0.9 μM was reported in that study for most clinical MRSA strains, and that finding agrees very well with our observation of an IC50 below 0.6 μM. Huczynski et al. (19) reported on the antimicrobial activity of floxuridine and several conjugates against several reference and clinical strains of S. aureus. In their studies, they found that floxuridine alone was very effective against all the strains tested, reporting an MIC equal to or below 0.016 μM. In our experiments, we observed an IC50 well below 0.6 μM for floxuridine. Unfortunately, this activity correlates with the cytotoxicity of the drug, likely making it unsuitable for repurposing (19). Gieringer et al. (20) determined that 5-fluorouracil could inhibit the growth of S. aureus at an MIC of 6.15 μM, which was greater than the IC50 of 0.6 μM that we observed.
Interestingly, they also demonstrated that 5-fluorouracil could inhibit the growth of several Gram-negative bacteria when 5-fluorouracil was combined with β-lactam antibiotics, which are not as effective on their own. In our study, we found that carmofur was highly effective against S. aureus, with an IC50 of 0.6 μM. To our knowledge, there are no reports of the antimicrobial activity of carmofur against S. aureus. The IC50s observed in our study for the drugs tested correlate well with previous reports, as all values fell below the reported MIC values, which correspond to 100% visual inhibition (i.e., the IC100).
Interestingly, benzbromarone, ebselen, and verteporfin, an antianginal, anti-inflammatory, and photosensitizer, respectively, showed significant antimicrobial activity with IC50s below 2 μM. Gordon et al. (21) reported that benzbromarone can inhibit S. aureus strain RN6290 with an IC50 of 0.13 μM. In our experiments, benzbromarone inhibited S. aureus with an IC50 of 1.7 μM, which was approximately 10-fold higher than the value reported by Gordon et al. (21). This discrepancy in IC50s is likely due to the difference in the strains tested, as different strains may carry different levels of the drug target, which, in this case, is the quorum-sensing membrane receptor AgrC. Ebselen, as reported by Chan et al. (22), can inhibit S. aureus with an IC50 of approximately 4.3 μM, which compares well with the results of our experiments, which found an IC50 of 1.3 μM. To the best of our knowledge, there are no reports on the antimicrobial activity of verteporfin against either planktonic cultures or preformed biofilms of S. aureus. In our experiments, verteporfin had significant antimicrobial activity with an IC50 of 1.6 μM. Using another photoactive compound unrelated to verteporfin (methylene blue), Tubby et al. (23) demonstrated that the photodynamic inactivation of bacteria remains a viable antimicrobial strategy, especially for localized infections.
Activity against preformed biofilms.
Based on a thorough literature search and the lack of previously published reports regarding their antibiofilm activity, we selected the following compounds to test against preformed biofilms of S. aureus: niclosamide, ivermectin, carmofur, pyrvinium pamoate, epirubicin HCl, auranofin, pinaverium bromide, verteporfin, and tolcapone. We observed that most of the drugs capable of inhibiting planktonic growth were ineffective against preformed biofilms (see Fig. S1 in the supplemental material), with the exception of niclosamide (Fig. 3A), carmofur (Fig. 3B), and auranofin (Fig. 3C). These three drugs showed antibiofilm activity at higher concentrations, with IC50s being within the concentration range tested. We observed a 1-log-unit reduction in the amount of viable bacteria in the biofilms after only 2 h of treatment (see Fig. S2 in the supplemental material) with niclosamide, carmofur, and auranofin. It is evident from Fig. S2 in the supplemental material that none of these drugs have bactericidal effects at the concentration tested (40 μM). The drop in antimicrobial activity of the remaining drugs may likely be due to the ineffectiveness of the drugs against metabolically inactive biofilm cells (24), increased resistance as a result of the hypermutability of the cells (25), and potential interactions of resistance factors with the biofilm matrix (26). Table 3 summarizes the antibiofilm activity of the selected compounds. Finally, we observed an increase in the production of biofilm matrix of an untreated biofilm over a period of 40 h (see Fig. S3 in the supplemental material). This increase in the production of glycoproteins suggests that the cells that were attached to the surface initially developed into a mature biofilm.
FIG 3.
A subset of the candidates was tested in 2-fold dilutions ranging from 0.625 μM to 40 μM against preformed biofilms of Staphylococcus aureus. Most lost their activity. Niclosamide (A), carmofur (B), and auranofin (C), however, were still effective at inhibiting Staphylococcus growth in the biofilms, albeit to a lesser extent.
TABLE 3.
Summary of antibiofilm activity of selected drugs
| Drug (drug class) | IC50 (μM) for S. aureus biofilm |
|---|---|
| Anthelmintics | |
| Niclosamide | 5.9 |
| Pyrvinium pamoate | >20 |
| Ivermectin | >20 |
| Antineoplastics | |
| Carmofur | 17.2 |
| Epirubicin hydrochloride | >20 |
| Miscellaneous | |
| Pinaverium bromide (antispastic) | >20 |
| Auranofin (antirheumatic) | 11.7 |
| Verteporfin (photosensitizer) | >20 |
| Tolcapone (antiparkinsonian) | >20 |
Niclosamide.
Niclosamide is an FDA-approved anthelmintic commonly used to treat tapeworms in humans. It is in the salicylanilide family of compounds (a large family with a range of medical applications) and is an analogue of closantel. The antimicrobial activity of some derivatives and analogues of niclosamide has been documented (27, 28). It has received increased attention in recent years due to its anticancer activity (29) and antidiabetic activity (30), highlighting its potential as a candidate for repurposing. The typical dosage is 2 g taken orally every 2 to 7 days, as required. We found that niclosamide inhibited the growth of S. aureus in planktonic cultures, as previously reported (13), with an IC50 of 1.2 μM (Fig. 2A). In the previously mentioned study, niclosamide was reported to have antistaphylococcal activity against planktonic cultures in vitro and in an in vivo model of Caenorhabditis elegans infection, although the infections were due to planktonic cells and were not due to biofilms. We observed that niclosamide displayed a somewhat reduced activity against preformed biofilms compared to that of cells in suspension, with an increase in the IC50 to approximately 6 μM (Fig. 3A; Table 3), possibly indicating a decreased ability of the compound to diffuse into the depths of the biofilm or an increase in the resistance of the cells grown in the biofilm mode. Such a low dose of niclosamide, even if applied directly to an open wound site (as a topical formulation) with direct access to the bloodstream, would be inconsequential in terms of toxicity yet would be sufficient to inhibit the growth of S. aureus biofilms. The mechanism of action of niclosamide on biofilms is not known. It has been shown that closantel, an analog of niclosamide also commonly used as an anthelmintic, targets the KinA/Spo0F two-component regulatory system (TCS) in Bacillus subtilis that regulates cell homeostasis, the cell's adaptive response, and sporulation (14). Hence, it is likely that niclosamide acts in a similar manner on the multitude of TCSs of S. aureus, such as the LytSR and GraRS systems, as these have been shown to modulate biofilm formation and resistance to cationic antimicrobial peptides (31, 32), respectively, by inhibiting the autophosphorylation of the sensory histidine kinase component. Since TCSs are largely conserved mechanisms of signal transduction in bacteria and have no homology to signal transduction systems in higher eukaryotes, they remain an attractive target for new (33) or repositioned antimicrobials. For example, the VraRS and GraRS systems, which confer resistance to vancomycin (in vancomycin-resistant S. aureus [VRSA] strains) (34) and the cationic peptides of the mammalian innate immune response (32), respectively, have been described in S. aureus. Therefore, niclosamide may have an added advantage in its ability not only to suppress TCSs that regulate cell growth and biofilm formation (as we have suggested herein) but also to suppress TCSs that regulate antimicrobial resistance, such as VraRS and GraRS, opening the door for effective combinatorial strategies.
Carmofur.
Carmofur, an antineoplastic commonly used to treat colorectal cancers, is another compound that showed activity against S. aureus planktonic and biofilm cultures. It is an analogue of pyrimidine and is derived from 5-fluorouracil. Typical dosages range from 200 mg to 600 mg per day, administered orally. Carmofur targets the activity of thymidylate synthase (TS), an enzyme essential for the conversion of dUMP to dTMP during S phase of the eukaryotic cell cycle, as well as inhibits intracellular ceramidase (35, 36). Okino et al. (37) described the first ever bacterial ceramidase in Pseudomonas aeruginosa as well as several potential homologues in other species, such as Mycobacterium tuberculosis and S. aureus, and hence, it is possible that carmofur may elicit cell death in S. aureus by inhibiting bacterial ceramidase. Consistent with the increased resistance of biofilms, carmofur inhibited the growth of planktonic S. aureus but had lower activity against preformed biofilms of the same, with IC50s of 0.6 μM and 17.2 μM, respectively (Fig. 2A and 3C; Table 3).
Auranofin.
Auranofin is a gold thiolate compound that has been used to treat rheumatoid arthritis for decades, and it has recently received significant attention due to its antimicrobial potential. A typical dosage for arthritis treatment is 6 mg per day, and the peak plasma concentration after 2 h is 0.025 μg/ml; the steady-state blood concentration of gold is 3.5 μM. In two separate studies, Hokai et al. (15) and Cassetta et al. (16) discovered that auranofin significantly inhibits the growth of S. aureus USA300, and they found MICs of 0.2 μg/ml and 0.25 μg/ml, respectively (∼0.3 μM). Recently, Siles et al. (6) showed that auranofin can inhibit the growth and the formation of Candida albicans biofilms. Furthermore, Harbut et al. (17) showed that auranofin is capable of inhibiting both actively dividing cells and nondividing persisters of Mycobacterium tuberculosis. We found that, in agreement with previous publications, auranofin has a strong antimicrobial effect against planktonic S. aureus with an IC50 of ≪0.265 μM. As expected, although it was still active, the antimicrobial effect of auranofin was reduced when it was tested against preformed biofilms, with the observed IC50 being 11.7 μM (Table 3). This concentration is significantly higher than the peak plasma concentration of the drug achieved when it is administered orally, but depending on the mode of application (oral, topical, or i.v.) and the formulation used, this difference may not be clinically significant. To our knowledge, there are no published studies investigating the antibacterial activity of auranofin against biofilms of S. aureus. It has been shown that auranofin acts by strongly binding the active-site cysteine in the thioredoxin receptor (TrxR), thus disrupting the cycle of thioredoxin (Trx) renewal, leaving the cell susceptible to oxidative stresses.
Overall, in this work we identified 18 repositionable candidate drugs that display antimicrobial activity against planktonic S. aureus. Furthermore, we identified three drugs, niclosamide, carmofur, and auranofin, that retain their antimicrobial activity against preformed biofilms of a methicillin-resistant strain of S. aureus, and we believe that these findings constitute the first step in the repurposing/repositioning process. Previous studies had described the antimicrobial activity of only some of the above-described compounds against planktonic cultures but not against biofilms, which are more clinically relevant due to their increased recalcitrance and high association with implantable devices, burns, and open wounds. Their antibiofilm activity makes these drugs attractive candidates for repurposing as novel anti-biofilm therapies, especially in combination with traditional antimicrobials, and warrant further attention to determine their modes of action, indications for use, methods of delivery, dosing, and any potential synergies with existing antimicrobials.
Supplementary Material
ACKNOWLEDGMENTS
The opinions and assertions contained herein are the private view of the authors and are not to be construed as official or as reflecting the views of the U.S. Department of the Army or the U.S. Department of Defense. One of the authors (K.P.L.) is a federal employee, and the work reported is part of his official duties.
This work was supported in part by the Naval Medical Research Center's Advanced Medical Development Program (MIPR N3239815MHX040) and the U.S. Army Medical Research and Materiel Command (USAMRMC), Combat Casualty Care Research Directorate. One of the authors (N.S.T.) was supported by an appointment to the Postgraduate Research Participation Program at the U.S. Army Institute of Surgical Research, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and USAMRMC.
Funding Statement
This work was supported in part by the Naval Medical Research Center's Advanced Medical Development Program (MIPR N3239815MHX040) and the U.S. Army Medical Research and Materiel Command (USAMRMC), Combat Casualty Care Research Directorate. N.S.T. was supported by an appointment to the Postgraduate Research Participation Program at the U.S. Army Institute of Surgical Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and USAMRMC.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00377-16.
REFERENCES
- 1.Fowler VG Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, Corey GR, Spelman D, Bradley SF, Barsic B, Pappas PA, Anstrom KJ, Wray D, Fortes CQ, Anguera I, Athan E, Jones P, van der Meer JT, Elliott TS, Levine DP, Bayer AS, ICE Investigators . 2005. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293:3012–3021. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 2.Diekema DJ, BootsMiller BJ, Vaughn TE, Woolson RF, Yankey JW, Ernst EJ, Flach SD, Ward MM, Franciscus CL, Pfaller MA, Doebbeling BN. 2004. Antimicrobial resistance trends and outbreak frequency in United States hospitals. Clin Infect Dis 38:78–85. doi: 10.1086/380457. [DOI] [PubMed] [Google Scholar]
- 3.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK, Active Bacterial Core surveillance (ABCs) MRSA Investigators . 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 4.Bales PM, Renke EM, May SL, Shen Y, Nelson DC. 2013. Purification and characterization of biofilm-associated EPS exopolysaccharides from ESKAPE organisms and other pathogens. PLoS One 8:e67950. doi: 10.1371/journal.pone.0067950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkinson GF, Pritchard K. 2015. In vitro screening for drug repositioning. J Biomol Screen 20:167–179. doi: 10.1177/1087057114563024. [DOI] [PubMed] [Google Scholar]
- 6.Siles SA, Srinivasan A, Pierce CG, Lopez-Ribot JL, Ramasubramanian AK. 2013. High-throughput screening of a collection of known pharmacologically active small compounds for identification of Candida albicans biofilm inhibitors. Antimicrob Agents Chemother 57:3681–3687. doi: 10.1128/AAC.00680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen P, Abercrombie JJ, Jeffrey NR, Leung KP. 2012. An improved medium for growing Staphylococcus aureus biofilm. J Microbiol Methods 90:115–118. doi: 10.1016/j.mimet.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Baker CN, Tenover FC. 1996. Evaluation of Alamar colorimetric broth microdilution susceptibility testing method for staphylococci and enterococci. J Clin Microbiol 34:2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pettit RK, Weber CA, Pettit GR. 2009. Application of a high throughput Alamar blue biofilm susceptibility assay to Staphylococcus aureus biofilms. Ann Clin Microbiol Antimicrob 8:28. doi: 10.1186/1476-0711-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettit RK, Weber CA, Kean MJ, Hoffmann H, Pettit GR, Tan R, Franks KS, Horton ML. 2005. Microplate Alamar blue assay for Staphylococcus epidermidis biofilm susceptibility testing. Antimicrob Agents Chemother 49:2612–2617. doi: 10.1128/AAC.49.7.2612-2617.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peeters E, Nelis HJ, Coenye T. 2008. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Methods 72:157–165. doi: 10.1016/j.mimet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Elkhatib WF, Khairalla AS, Ashour HM. 2014. Evaluation of different microtiter plate-based methods for the quantitative assessment of Staphylococcus aureus biofilms. Future Microbiol 9:725–735. doi: 10.2217/fmb.14.33. [DOI] [PubMed] [Google Scholar]
- 13.Rajamuthiah R, Fuchs BB, Conery AL, Kim W, Jayamani E, Kwon B, Ausubel FM, Mylonakis E. 2015. Repurposing salicylanilide anthelmintic drugs to combat drug resistant Staphylococcus aureus. PLoS One 10:e0124595. doi: 10.1371/journal.pone.0124595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macielag MJ, Demers JP, Fraga-Spano SA, Hlasta DJ, Johnson SG, Kanojia RM, Russell RK, Sui Z, Weidner-Wells MA, Werblood H, Foleno BD, Goldschmidt RM, Loeloff MJ, Webb GC, Barrett JF. 1998. Substituted salicylanilides as inhibitors of two-component regulatory systems in bacteria. J Med Chem 41:2939–2945. doi: 10.1021/jm9803572. [DOI] [PubMed] [Google Scholar]
- 15.Hokai Y, Jurkowicz B, Fernandez-Gallardo J, Zakirkhodjaev N, Sanau M, Muth TR, Contel M. 2014. Auranofin and related heterometallic gold(I)-thiolates as potent inhibitors of methicillin-resistant Staphylococcus aureus bacterial strains. J Inorg Biochem 138:81–88. doi: 10.1016/j.jinorgbio.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cassetta MI, Marzo T, Fallani S, Novelli A, Messori L. 2014. Drug repositioning: auranofin as a prospective antimicrobial agent for the treatment of severe staphylococcal infections. Biometals 27:787–791. doi: 10.1007/s10534-014-9743-6. [DOI] [PubMed] [Google Scholar]
- 17.Harbut MB, Vilcheze C, Luo X, Hensler ME, Guo H, Yang B, Chatterjee AK, Nizet V, Jacobs WR Jr, Schultz PG, Wang F. 2015. Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis. Proc Natl Acad Sci U S A 112:4453–4458. doi: 10.1073/pnas.1504022112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordheim LP, Ben Larbi S, Fendrich O, Ducrot C, Bergeron E, Dumontet C, Freney J, Doleans-Jordheim A. 2012. Gemcitabine is active against clinical multiresistant Staphylococcus aureus strains and is synergistic with gentamicin. Int J Antimicrob Agents 39:444–447. doi: 10.1016/j.ijantimicag.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Huczynski A, Antoszczak M, Kleczewska N, Lewandowska M, Maj E, Stefanska J, Wietrzyk J, Janczak J, Celewicz L. 2015. Synthesis and biological activity of salinomycin conjugates with floxuridine. Eur J Med Chem 93:33–41. doi: 10.1016/j.ejmech.2015.01.045. [DOI] [PubMed] [Google Scholar]
- 20.Gieringer JH, Wenz AF, Heinz-M J, Daschner FD. 1986. Effect of 5-fluorouracil, mitoxantrone, methotrexate, and vincristine on the antibacterial activity of ceftriaxone, ceftazidime, cefotiam, piperacillin, and netilmicin. Chemotherapy 32:418–424. [DOI] [PubMed] [Google Scholar]
- 21.Gordon CP, Williams P, Chan WC. 2013. Attenuating Staphylococcus aureus virulence gene regulation: a medicinal chemistry perspective. J Med Chem 56:1389–1404. doi: 10.1021/jm3014635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan G, Hardej D, Santoro M, Lau-Cam C, Billack B. 2007. Evaluation of the antimicrobial activity of ebselen—role of the yeast plasma membrane H+-ATPase. J Biochem Mol Toxicol 21:252–264. doi: 10.1002/jbt.20189. [DOI] [PubMed] [Google Scholar]
- 23.Tubby S, Wilson M, Nair SP. 2009. Inactivation of staphylococcal virulence factors using a light-activated antimicrobial agent. BMC Microbiol 9:211. doi: 10.1186/1471-2180-9-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K. 2004. Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett 230:13–18. doi: 10.1016/S0378-1097(03)00856-5. [DOI] [PubMed] [Google Scholar]
- 25.Molin S, Tolker-Nielsen T. 2003. Gene transfer occurs with enhanced efficiency in biofilms and induces enhanced stabilisation of the biofilm structure. Curr Opin Biotechnol 14:255–261. doi: 10.1016/S0958-1669(03)00036-3. [DOI] [PubMed] [Google Scholar]
- 26.Nichols WW, Evans MJ, Slack MPE, Walmsley HL. 1989. The penetration of antibiotics into aggregates of mucoid and non-mucoid Pseudomonas aeruginosa. J Gen Microbiol 135:13. [DOI] [PubMed] [Google Scholar]
- 27.Cheng TJ, Wu YT, Yang ST, Lo KH, Chen SK, Chen YH, Huang WI, Yuan CH, Guo CW, Huang LY, Chen KT, Shih HW, Cheng YS, Cheng WC, Wong CH. 2010. High-throughput identification of antibacterials against methicillin-resistant Staphylococcus aureus (MRSA) and the transglycosylase. Bioorg Med Chem 18:8512–8529. doi: 10.1016/j.bmc.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 28.Pauk K, Zadrazilova I, Imramovsky A, Vinsova J, Pokorna M, Masarikova M, Cizek A, Jampilek J. 2013. New derivatives of salicylamides: preparation and antimicrobial activity against various bacterial species. Bioorg Med Chem 21:6574–6581. doi: 10.1016/j.bmc.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 29.Ye T, Xiong Y, Yan Y, Xia Y, Song X, Liu L, Li D, Wang N, Zhang L, Zhu Y, Zeng J, Wei Y, Yu L. 2014. The anthelmintic drug niclosamide induces apoptosis, impairs metastasis and reduces immunosuppressive cells in breast cancer model. PLoS One 9:e85887. doi: 10.1371/journal.pone.0085887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao H, Zhang Y, Zeng X, Shulman GI, Jin S. 2014. Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat Med 20:1263–1269. doi: 10.1038/nm.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma-Kuinkel BK, Mann EE, Ahn JS, Kuechenmeister LJ, Dunman PM, Bayles KW. 2009. The Staphylococcus aureus LytSR two-component regulatory system affects biofilm formation. J Bacteriol 191:4767–4775. doi: 10.1128/JB.00348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang SJ, Bayer AS, Mishra NN, Meehl M, Ledala N, Yeaman MR, Xiong YQ, Cheung AL. 2012. The Staphylococcus aureus two-component regulatory system, GraRS, senses and confers resistance to selected cationic antimicrobial peptides. Infect Immun 80:74–81. doi: 10.1128/IAI.05669-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsushita M, Janda KD. 2002. Histidine kinases as targets for new antimicrobial agents. Bioorg Med Chem 10:855–867. doi: 10.1016/S0968-0896(01)00355-8. [DOI] [PubMed] [Google Scholar]
- 34.Kuroda M, Kuwahara-Arai K, Hiramatsu K. 2000. Identification of the up- and down-regulated genes in vancomycin-resistant Staphylococcus aureus strains Mu3 and Mu50 by cDNA differential hybridization method. Biochem Biophys Res Commun 269:485–490. doi: 10.1006/bbrc.2000.2277. [DOI] [PubMed] [Google Scholar]
- 35.Kitchens ME, Forsthoefel AM, Barbour KW, Spencer HT, Berger FG. 1999. Mechanisms of acquired resistance to thymidylate synthase inhibitors—the role of enzyme stability. Mol Pharmacol 56:1062–1070. [DOI] [PubMed] [Google Scholar]
- 36.Realini N, Solorzano C, Pagliuca C, Pizzirani D, Armirotti A, Luciani R, Costi MP, Bandiera T, Piomelli D. 2013. Discovery of highly potent acid ceramidase inhibitors with in vitro tumor chemosensitizing activity. Sci Rep 3:1035. doi: 10.1038/srep01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okino N, Tani M, Imayama S, Ito M. 1998. Purification and characterization of a novel ceramidase from Pseudomonas aeruginosa. J Biol Chem 273:6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.