Abstract
Staphylococci are a leading cause of catheter-related infections (CRIs) due to biofilm formation. CRIs are typically managed by either device removal or systemic antibiotics, often in combination with catheter lock solutions (CLSs). CLSs provide high concentrations of the antimicrobial agent at the site of infection. However, the most effective CLSs against staphylococcal biofilm-associated infections have yet to be determined. The purpose of this study was to evaluate the efficacy and suitability of two newly described antimicrobial agents, ML:8 and Citrox, as CLSs against Staphylococcus aureus biofilms. ML:8 (1% [vol/vol]) and Citrox (1% [vol/vol]), containing caprylic acid and flavonoids, respectively, were used to treat S. aureus biofilms grown in vitro using newly described static and flow biofilm assays. Both agents reduced biofilm viability >97% after 24 h of treatment. Using a rat model of CRI, ML:8 was shown to inactivate early-stage S. aureus biofilms in vivo, while Citrox inactivated established, mature in vivo biofilms. Cytotoxicity and hemolytic activity of ML:8 and Citrox were equivalent to those of other commercially available CLSs. Neither ML:8 nor Citrox induced a cytokine response in human whole blood, and exposure of S. aureus to either agent for 90 days was not associated with any increase in resistance. Taken together, these data reveal the therapeutic potential of these agents for the treatment of S. aureus catheter-related biofilm infections.
INTRODUCTION
The effective treatment of staphylococcal catheter-related infections (CRIs) represents a significant clinical challenge, mainly due to the ability of staphylococci to form biofilms (1). The surfaces of intravascular catheters (IVCs) can rapidly become coated with host matrix proteins (2). Staphylococci express numerous surface proteins that can bind to these proteins (3). Current treatment for staphylococcal IVC infection involves device removal, but this is not always possible due to clinical circumstances. The Infectious Disease Society of America (IDSA) guidelines on the management of CRIs recommend the use of catheter lock solutions (CLSs) for the attempted salvage of an IVC associated with a CRI (4). However, there is no consensus on the most appropriate agent for use as a CLS in the treatment of staphylococcal CRIs. Several commonly used antibiotics and antiseptics were recently shown to be ineffective for the treatment of Staphylococcus aureus infections involving biofilms (5). Because current options are limited, the need for novel therapeutic agents for use as CLSs or as antistaphylococcal biofilm treatment options for other device-related infections is of great clinical importance (6).
We examined the antimicrobial effects and suitability for in vivo use of two newly described antimicrobial agents, namely, ML:8 and Citrox. ML:8 includes components previously approved for parenteral nutrition; the fatty acid caprylic acid is the main component and has been shown to be effective in the treatment of periodontal pathogens (7). The efficacy of caprylic acid against biofilms was also reported previously (8). Citrox is an antimicrobial formulation composed of flavonoids. Flavonoids are secondary metabolites that are found in plants, and their antimicrobial activities have been confirmed against a wide variety of bacterial species (9). The flavonoids in Citrox are neoeriocitrin, isonaringin, naringin, hesperidin, neohesperidin, neodiosmin, naringenin, poncirin, and rhiofolin (10). The efficacy of Citrox against biofilm-producing oral microorganisms has been reported previously (11).
The aim of this study was to examine if these two newly described antimicrobial agents can potentially be used for the treatment of S. aureus-mediated CRIs. We therefore investigated the effectiveness of ML:8 and Citrox to eradicate biofilms produced by methicillin-susceptible (MSSA) and -resistant (MRSA) S. aureus strains, using clinically relevant in vitro and in vivo-like models of CRIs, and examined their potential clinical use as CLSs.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The main bacterial strains and clinical isolates used in this study are described in Table 1. S. aureus strains were grown with aeration at 37°C in either Mueller-Hinton (MH) broth (Sigma) or RPMI 1640 medium (Gibco). MH broth was used for MIC testing and resistance testing, while RPMI 1640 medium was used for all biofilm-related experiments.
TABLE 1.
Bacterial strains used in this study
| S. aureus strain | Characteristics | Reference |
|---|---|---|
| SH1000 | MSSA reference strain; functional rsbU derivative of 8325-4 rsbU+; serotype (ST) 8, CC8 | 34 |
| BH48 (04) | MSSA clinical isolate; ST 8, CC8 | 35 |
| BH1CC | MRSA clinical isolate; SCCmec type II, ST 8, CC8 | 35 |
| USA300 JE2 | MRSA strain USA300 derivative lacking plasmids P01 and P03; JE2 LAC ST 8, CC 8 | 36 |
| USA300 lux | MRSA strain USA300 LAC constitutively expressing luciferase (lux) from Photorhabdus luminescens; CC8 | 37 |
MIC testing.
MICs for planktonic cells were established using a broth microdilution method. Serial dilutions of test agents (0.007 to 2% [vol/vol]) were prepared and exposed to bacterial strains grown in MH broth. Each strain was prepared to a 0.5 McFarland standard. Solutions were incubated for 24 h at 37°C before the results were examined to determine the MICs.
Static biofilm formation and treatment.
S. aureus biofilms were formed as previously described (5, 12). Platelet-poor plasma was obtained from healthy volunteers. Plasma was diluted to 20% (vol/vol) in carbonate buffer (pH 9.6) and used to precondition the wells of a 96-well microtiter plate (Nunc, Denmark) at 37°C for 2 h (13). The plasma solution was removed, and an overnight culture of the test organism in RPMI 1640 was diluted 1:1, to an optical density at 600 nm (OD600) of 0.7, in RPMI 1640. From this suspension, 100-μl aliquots were inoculated into the microtiter plate wells and incubated at 37°C for 24 h, 3 days, or 5 days, as indicated. The RPMI 1640 medium was changed daily for mature biofilms. Following the initial incubation and washing, 100 μl of the test solution (ML:8 or Citrox [0.025 to 1% {vol/vol}] diluted in RPMI 1640) was added to each test well at 37°C for 24 h and then washed twice with sterile distilled water. RPMI 1640 medium without bacteria was used as a negative control and was held in the well during incubations; washing steps were carried out as described above.
Resazurin conversion assay.
One hundred microliters of a redox indicator dye, either alamarBlue (Biosource, Invitrogen, United Kingdom) (20% [vol/vol] alamarBlue in RPMI 1640 medium) or resazurin (88 μM resazurin in water), was added to each well as described previously (14). Plates were incubated for a further 60 min at 37°C in the dark to determine biofilm viability after antimicrobial treatment. Biofilm viability was determined using a fluorimeter with an excitation wavelength of 544 nm and an emission wavelength of 590 nm. The fluorescence produced was proportional to the number of living cells present. Each experiment was performed using three technical and biological replicates; results represent biofilm OD values (means ± standard deviations [SD]).
Measurement of antibiofilm activity of antimicrobial agents under flow conditions.
A flow cell and pump were used to create a microfluidic model (Cellix Ltd., Ireland). Vena8 Fluoro+ flow chambers were coated with 100% plasma for 2 h at 37°C. Exponentially growing S. aureus cultures were adjusted to an OD600 of 0.2 in RPMI 1640 and injected into each chamber to attach for 1 h at 37°C on an inverted microscope. The pump was activated, and RPMI 1640 at a shear rate of 6.25 dynes (200 μl/min) was infused through the chambers of the chip for 24 h. Treatment agents (1% [vol/vol]) were then injected into each chamber and allowed to treat the biofilm statically for a further 24 h. Chambers were analyzed using bright-field and confocal microscopy.
Confocal microscopy.
Biofilm structure and treatment efficacy were analyzed using an inverted confocal microscope (LSM 510 Meta; Zeiss) and LSM510 image capture software. Bacterial cells were visualized using Syto 9 green (3.34 mM) and propidium iodide (20 mM). Optimized lasers (argon [488 nm] and HeNe [632.8 nm]) were used to excite the dyes and to capture the fluorescence emitted from the cells, under a magnification of ×40. ImageJ software was used to calculate the fluorescence intensity for each chamber.
Cell culture.
HaCaT cells, a human keratinocyte cell line, were cultured in Dulbecco's modified Eagle's medium (DMEM) (Bio-Sciences, Ireland) with 2 mM l-glutamine supplemented with 10% (vol/vol) fetal bovine serum (FBS). THP-1 cells, a human acute monocyte leukemic cell line, were cultured in RPMI 1640 (Bio-Sciences, Ireland) supplemented with 10% (vol/vol) FBS.
MTT assay for assessing cytotoxicity.
Cells were seeded into the wells of 96-well microtiter plates (Nunc, Denmark) at a density of 1 × 105 cells/ml. THP-1 and HaCaT cells were incubated in serum-free medium containing increasing concentrations of test agent for 24 h. Control wells were treated with Triton X-100 (1%) or medium free of test agents. MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] (500 μg/ml) was then added to each well, incubated for 4 h, and washed with phosphate-buffered saline (PBS). MTT fixative solution (isopropanol) was added to each well, and the plates were fixed for 4 min with shaking. The absorbance was measured at 595 nm in a Thermo Multiskan Ex plate reader (Thermo Fisher, United Kingdom). All incubations were done at 37°C in a 5% CO2 humidified incubator. Three technical replicates as well as three biological replicates were tested.
Determining the hemolytic activity of ML:8 and Citrox.
Potential hemolytic activity of ML:8 and Citrox was determined using fresh human erythrocytes from healthy donors in a modified version of the method originally described by Cantisani et al. (15). Blood was drawn and centrifuged, plasma was removed, and the remaining erythrocytes were washed three times with PBS. Erythrocytes were diluted in 40 ml PBS, and 50-μl aliquots were added to a 96-well plate (Nunc, Denmark). Test agents (0.007 to 2% [vol/vol]) were prepared in RPMI 1640, and 50 μl of each solution was added to erythrocytes. Plates were incubated at 37°C for 24 h. Supernatant was then withdrawn from the top of each well and placed in a new plate. The release of hemoglobin was monitored by measuring the absorbance of the supernatant at 490 nm. Negative and positive controls consisted of erythrocytes suspended in RPMI 1640 and erythrocytes in RPMI 1640 with 1% Triton X-100, respectively.
Investigation of immune response to ML:8 and Citrox.
Whole blood was exposed to either ML:8, Citrox, or an untreated medium control for 2 h at 37°C in 96-well microtiter plates (Nunc, Denmark). Exposed blood was collected into Eppendorf tubes and centrifuged at 150 × g for 10 min. After centrifugation, serum was collected and used immediately or stored at −20°C. A Bio-Plex Pro human cytokine 8-plex assay plate containing eight cytokines was used; these included both proinflammatory and anti-inflammatory cytokines and chemokines (interleukin-2 [IL-2], IL-4, IL-6, IL-8, IL-10, granulocyte macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor alpha [TNF-α], and gamma interferon [IFN-γ]). Serum samples were diluted 1:4, and the plate was analyzed according to the manufacturer's instructions. The plate was read to assess fluorescence using a Bio-Plex 200 plate reader. DuoSet IL-8 and TNF-α enzyme-linked immunosorbent assay (ELISA) kits were used to validate the cytokine results. ELISAs were carried out in accordance with the manufacturer's instructions. The plate was read to determine the optical density of each well immediately after the addition of the stop solution; plates were read at 450 nm using a Thermo Multiskan-Ex plate reader (Thermo Fisher, United Kingdom).
Investigation of potential of ML:8 and Citrox to induce antimicrobial resistance.
Antimicrobial resistance testing was performed using Mueller-Hinton (MH) agar plates supplemented with antimicrobial agents at concentrations of 0.125 and 0.0625% (vol/vol) for ML:8 and 0.015 and 0.0075% (vol/vol) for Citrox. MSSA strain SH1000 and MRSA strain BH1CC were adjusted to a 0.5 McFarland standard; 100-μl aliquots were plated in triplicate on plates containing test agents. Plates were incubated for 48 h at 37°C and then transferred to 4°C for 48 h to allow bacterial cells to recover from the challenge. Bacterial cells were harvested, adjusted to a 0.5 McFarland standard, and cultured on fresh agar plates with test agents added. This process was repeated in triplicate twice weekly for 12 weeks.
Animal studies. (i) Husbandry and care.
Male Sprague-Dawley rats with preimplanted jugular vein central venous catheters (polyurethane, 0.025-in. internal diameter, and 40-μl volume) were obtained from Charles River Labs (Kent, United Kingdom). Initial weights were 200 to 250 g per rat. Rats received water and Agway rodent chow ad libitum throughout the experiments. All work was performed in accordance with statutory instrument no. 543 (2012) issued by the Irish government. Animals were held in quarantine for 1 week on arrival to monitor health status; animals were examined for signs of illness, pains, and infection at the end of the week. Catheters were flushed with heparin saline (500 IU) every second day of the quarantine week to maintain patency.
(ii) Rat intravascular catheter model of infection.
A rat IVC model of infection was developed as described previously by Ulphani and Rupp (16), van Praagh et al. (17), and Chauhan et al. (18, 19), with the following modifications. Briefly, rats were anesthetized with isoflurane (5% for induction and 2% for maintenance) for all catheter manipulations. Jugular vein central venous catheters were inoculated by instillation of 0.2 ml of saline containing 1 × 106 CFU/ml (high inoculum) or 1 × 104 CFU/ml (low inoculum) of MRSA strain USA300 lux. The bacteria remained in the lumen of the catheter for 1 or 5 days. After 1 or 5 days of infection (early or mature biofilm, respectively), 50 μl of treatment agent (ML:8 or Citrox) was instilled into the lumen of the catheter daily for a further 4 days. Subcutaneous injections of vancomycin (50 mg/kg of body weight) were administered every 12 h for the duration of the experiment. Three rats were used per treatment group. Bioluminescence intensity at the site of infection was monitored daily using a PerkinElmer IVIS imaging system (exposure, 20 s; binning, level 4, setting f1).
(iii) Terminal harvest and analysis of results.
Rats were anesthetized, blood was collected via cardiac puncture and serially diluted, and CFU counts were determined. Rats were euthanized by cervical dislocation. Catheters were removed, and their bioluminescence intensities were analyzed via an IVIS imaging system. Catheters were then transferred to sterile tubes. Biofilms were harvested from the lumens of the catheters by using TrpLE (1×; Gibco). Bacterial numbers were determined using a broth dilution and spread plate method on tryptone soy agar (TSA) (Fannin, LIP, Ireland).
Ethical approvals.
Blood donations were obtained from healthy adult donors. Written, informed consent was obtained from participants at the time of collection. Ethics approval for collection and use of blood was granted by the Ethics Committee of the Royal College of Surgeons in Ireland (REC820). Animal experiments were conducted under Health Products Regulatory Authority Ireland guidelines, with ethical approval from the RCSI Ethics Committee (REC931).
Statistical analysis.
Two-tailed, paired Student's t tests were used to determine the statistical significance of data by using Microsoft Excel. Significance was defined as having a P value of ≤0.05. For the animal experiments, descriptive statistics (including means and standard deviations) were calculated for each test agent. Log10 transformation of CFU data from catheters was used to ensure a normal distribution. A two-sample t test was conducted to compare the log-transformed CFU values.
RESULTS
ML:8 and Citrox inactivate early and mature in vitro S. aureus biofilms.
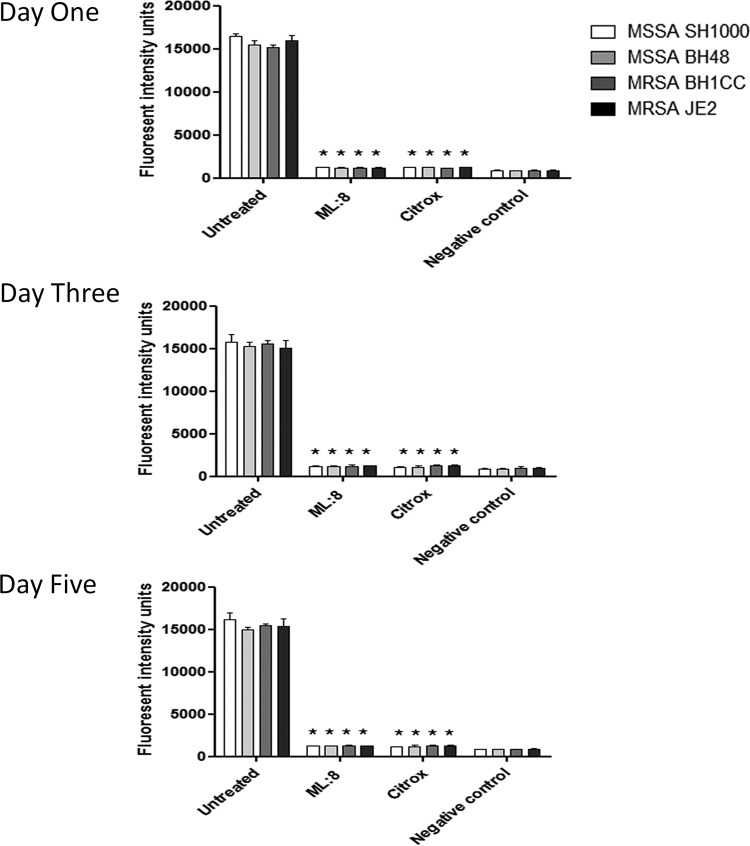
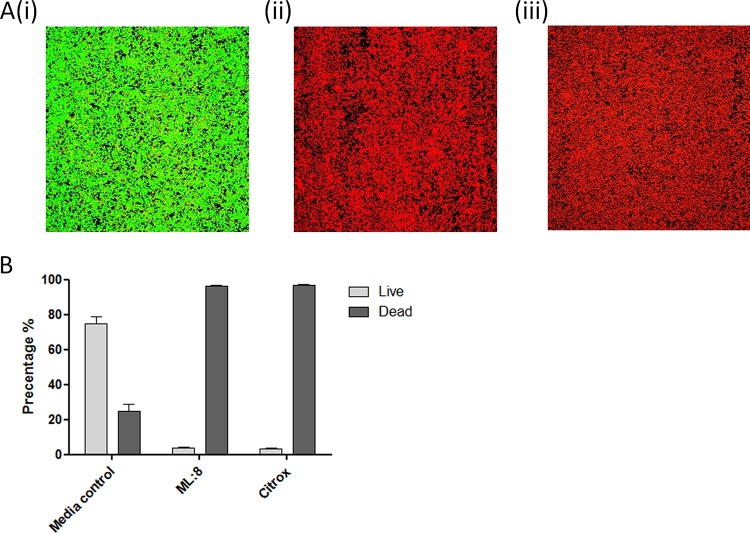
The MICs of ML:8 and Citrox against planktonic bacteria are shown in Table 2. The results indicate an MIC of 0.125% (vol/vol) for ML:8 and one of 0.015% (vol/vol) for Citrox for all strains listed in Table 1. No difference in susceptibility was observed between the MRSA and MSSA strains examined. Initial in vitro biofilm testing was carried out against S. aureus coagulase-mediated biofilms grown on surfaces coated with human plasma (5, 12). Twenty-four-hour treatments with ML:8 and Citrox at 1% (vol/vol) were both effective for the inactivation of biofilms that had been developed over 1, 3, and 5 days as described in Materials and Methods (Fig. 1). No significant difference (P ≤ 0.05) in bacterial susceptibility to ML:8 or Citrox was observed between strains or stages of biofilm maturity. The effects of ML:8 and Citrox were further investigated on biofilms formed on plasma-coated surfaces under shear force by using live/dead staining and confocal microscopy. Experiments under shear force were carried out using the four lead bacterial strains, and no variation between strains was observed. Results shown are for MSSA strain SH1000. Upon visual inspection, 24-h treatment with ML:8 (Fig. 2A, panel ii) and Citrox (Fig. 2A, panel iii) resulted in predominantly red cells, indicating cell death. Quantitative analysis revealed that treatment with ML:8 (1% [vol/vol]) and Citrox (1% [vol/vol]) resulted in 97% and 98% reductions in cell viability, respectively (Fig. 2B). To ensure that our treatment times and doses were not limited to a small number of S. aureus strains, a larger group of biofilm-positive isolates representing various genetic backgrounds was also included in the study. These strains and isolates represent other clonal complexes (CCs) commonly associated with S. aureus infection, namely, CC5, CC8, CC15, CC22, CC30, CC45, CC122, and CC398 (20, 21). Significant variation between clonal complexes was not observed (see Fig. S1 in the supplemental material). The optimum dose against biofilms (ODB) formed by S. aureus was determined to be 1% (vol/vol) for both ML:8 and Citrox.
TABLE 2.
MICs of ML:8 and Citrox for Staphylococcus aureus strains used in this study
| Agent | Concn range tested (% [vol/vol]) | MIC (% [vol/vol]) for MSSA strain |
MIC (% [vol/vol]) for MRSA strain |
||
|---|---|---|---|---|---|
| SH1000 | BH48 | BH1CC | USA300 JE2 | ||
| ML:8 | 0.007–2 | 0.125 | 0.125 | 0.125 | 0.125 |
| Citrox | 0.007–2 | 0.015 | 0.015 | 0.015 | 0.015 |
FIG 1.
Susceptibility of mature S. aureus biofilms to ML:8 and Citrox. Biofilms formed by MSSA strains SH1000 and BH48 and MRSA strains BH1CC and JE2 were grown in RPMI 1640 for 1, 3, or 5 days and then treated with ML:8 (1% [vol/vol]) or Citrox (1% [vol/vol]) at 37°C for 24 h. RPMI 1640 without bacteria was used as a negative control. Viability of biofilms was measured using the resazurin conversion assay. Assays were performed in triplicate, and data represent mean fluorescence intensities and SD. Statistically significant results are indicated (*, P ≤ 0.05; two-tailed Student t tests).
FIG 2.
Effects of ML:8 and Citrox on biofilms formed under shear conditions. (A) MSSA strain SH1000 biofilms were formed under conditions of flow in RPMI 1640 and then treated statically with medium (i), ML:8 (1% [vol/vol]) (ii), or Citrox (1% [vol/vol]) (iii) for 24 h at 37°C. Live/dead staining was carried out and visualized using a confocal microscope. (B) ImageJ software was used to calculate the percentages of pixels representing live (green) and dead (red) bacteria. Data represent mean percentages and SD. Assays were performed in triplicate, and representative images are shown.
ML:8 and Citrox demonstrate compatibility with host cells equivalent to that for comparative agents in in vitro testing.
The cytotoxicity of ML:8 and Citrox and two existing CLSs in clinical use, namely, Duralock-C and ethanol, was investigated using monocyte and keratinocyte cell lines, THP-1 and HaCaT cells, respectively. The 50% inhibitory concentration (IC50) of each agent was determined using an MTT assay as described in Materials and Methods, and the results are presented in Table 3. While cytotoxicity was observed for ML:8 and Citrox at concentrations below that seen to be effective against biofilms, similar results were also observed for the CLSs ethanol and Duralock-C, which are currently in clinical use. Cytotoxicity of ML:8 was evident at concentrations between 6- and 17-fold below the ODB; results for Citrox showed IC50s 24- and 26-fold lower than the ODB. Ethanol exposure resulted in cytotoxicity at concentrations 12.5- and 21-fold below the ODB, while Duralock-C resulted in IC50s 97- and 202-fold below the optimum concentrations for use against bacterial biofilms. The hemolytic properties of ML:8, Citrox, and comparative agents were also assessed in human whole blood. Hemolysis was evident after exposure to high concentrations of ML:8 (≥0.25% [vol/vol]) and Citrox (≥0.125% [vol/vol]); hemolysis was also observed for the comparative agents Duralock-C and ethanol, at concentrations of ≥11.6% and ≥3.75% (vol/vol), respectively. Continuous subculturing with concentrations of ML:8 and Citrox 1-fold below the MICs shown in Table 2 was carried out over 90 days to assess the potential to induce resistance. No increase in resistance was detected after exposure of the MSSA strain SH1000 and the MRSA strain BH1CC over this period. An investigation into the host immune response to ML:8 and Citrox revealed no significant induction of the cytokines tested compared to a medium control, indicating that ML:8 and Citrox did not trigger an immune response in the exposure time examined (2 h) (see Fig. S2 in the supplemental material). Results for IL-8 and TNF-α were confirmed by ELISA.
TABLE 3.
Comparison of IC50s of treatment agents against THP-1 monocytes and HaCaT keratinocytes
| Treatment agent | IC50 (% [vol/vol]) |
|
|---|---|---|
| THP1 cells | HaCaT cells | |
| ML:8 | 0.058 | 0.149 |
| Citrox | 0.041 | 0.038 |
| Ethanol | 2.4 | 1.7 |
| Duralock-C | 0.48 | 0.21 |
ML:8 and Citrox are effective for treatment of in vivo S. aureus biofilm infection.
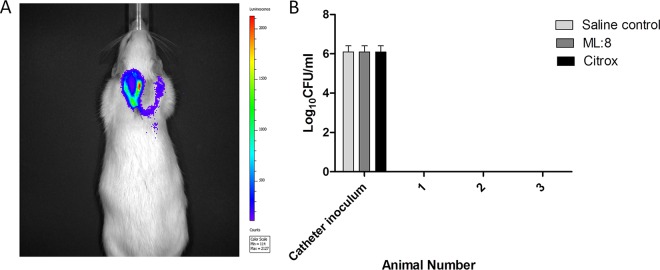
Sprague-Dawley rats with implanted IVCs were used for in vivo testing. Infection was established in the IVC, and the treatment agents ML:8 and Citrox were examined as CLSs in this model. Figure 3A shows a representative image of a Sprague-Dawley rat 5 days after infection of the IVC with MRSA strain USA300 lux. This image was captured prior to the initiation of CLS treatment, and high levels of luminescence are shown within the implanted catheter. Subcutaneous injections of vancomycin prevented systemic infection; this was confirmed by the negative blood cultures obtained at the experimental endpoint (Fig. 3B).
FIG 3.
Monitoring infection development and dissemination. Optimized doses of high-inoculum (106 CFU/ml) MRSA USA300 lux in 40 μl were injected into catheters, and infection was allowed to develop for 5 days. (A) An IVIS imaging system was used to monitor the development of infection. Bioluminescence activity in a Sprague-Dawley rat was acquired using an IVIS 100 camera at 5 days postinfection. The color scale indicates the degree of luminescence, from high (red) to low (purple). A representative animal is shown (n = 3). (B) Catheter inoculation and dissemination into blood were assessed at the experiment endpoint. Animals were euthanized at day 9, blood was harvested, and log10 CFU counts were determined. Assays were performed in triplicate, and the data represent mean log10 CFU per milliliter and SD.
Images of explanted catheters were captured using IVIS imaging, and representative images are shown in Fig. 4A for the untreated (i) as well as ML:8 (ii)- and Citrox (iii)-treated catheters. While luminescence remained high (maximum value, 1 × 107) in catheters treated with ML:8 (1% [vol/vol]), it was shown to be lower than the level for the untreated control (maximum value, 4 × 107). No detectable luminescence was observed in the Citrox-treated catheters. Bacterial biofilms harvested from the explanted catheters resulted in a CFU count of 8.73 × 1010 CFU/catheter for the untreated control, with an SD of 0.89, and 8.69 × 1010 CFU/catheter for the ML:8 (1% [vol/vol])-treated catheters, with an SD of 1.04 (Fig. 4B). Catheters treated with Citrox (1% [vol/vol]) did not contain any detectable viable bacteria (Fig. 4B).
FIG 4.
Effects of ML:8 and Citrox on in vivo biofilms formed over 5 days. Optimized doses of 106 CFU/ml (A and B) and 104 CFU/ml (C and D) MRSA USA300 lux in 40 μl were injected into catheters, and infection was allowed to develop for 1 (C and D) or 5 (A and B) days. This was followed by treatment for 4 days with saline (control), ML:8 (1%), or Citrox (1%). Animals were euthanized at day 9. Catheters were explanted, bioluminescence activity was acquired using an IVIS 100 camera (A and C), and log10 CFU counts were determined (B and D). Representative catheters are shown. Three animals were used per group, and data represent mean log10 CFU per milliliter and SD. Statistically significant results are indicated (*, P ≤ 0.05; two-tailed Student t tests versus untreated animals).
The effect of ML:8 on lower-inoculum (104 CFU/ml) biofilms formed over 1 day was also investigated. Results are shown in Fig. 4C. High-level luminescence was observed in the untreated catheter (Fig. 4C, panel i), while no detectable luminescence was observed for the ML:8-treated catheter (Fig. 4C, panel ii). Harvested bacterial biofilms resulted in a CFU count of 7.58 × 1010 CFU/catheter for the untreated control, with an SD of 0.95, and no bacteria were detected for the ML:8 treatment group (Fig. 4D).
DISCUSSION
Current treatment for staphylococcal CRIs often involves device removal. However, this is not always possible due to clinical circumstance and can also result in considerable patient morbidity and mortality (22). Systemic antibiotics are usually administered to treat CRIs, but although they are generally effective at eliminating circulating planktonic bacteria, they frequently fail to sterilize the device or IVC, leaving the patient at risk of complications or recurrence. One treatment approach involves the use of CLSs in combination with systemic antibiotics to eradicate the S. aureus biofilm within the IVC. However, recent research has highlighted that a large number of antibiotics and antiseptic agents are not effective as CLSs for the treatment of biofilm-mediated S. aureus infection (5). Therefore, there is an urgent need for novel and effective agents for the treatment of device-related infections involving staphylococcal biofilms (6, 23).
In this study, two agents, ML:8 and Citrox, were investigated to determine their potential use as novel antistaphylococcal biofilm treatments. At concentrations of 0.125% (vol/vol) and 0.015% (vol/vol), respectively, ML:8 and Citrox were effective against planktonic cells. Investigations also found concentrations as low as 1% (vol/vol) for both ML:8 and Citrox to be optimum for the inactivation of bacterial cells in a sessile state under both static and flow conditions. This is significant because staphylococcal biofilms under conditions of flow have previously been shown to be more tolerant to treatment than those formed under static conditions, and treatment of patients with conventional antimicrobials against staphylococcal biofilm infections, such as CRI, frequently results in treatment failure due to the antimicrobial resistance of organisms within a biofilm and failure of antimicrobial agents to reach the desired MIC within this environment (24, 25). Both agents also killed biofilms formed over 1, 3, and 5 days, suggesting the potential for ML:8 and Citrox to be used in the treatment of established staphylococcal biofilm infections, such as those that occur clinically when CRIs are diagnosed after establishment of biofilm. Our findings are consistent with research published on the antimicrobial potential of caprylic acid (7, 8). A study examining the effect of ML:8-X10, a formulation similar to ML:8, and vancomycin showed equivalent activities of both agents against S. aureus (26). This is in contrast to our study, in which we found the activity of ML:8 against biofilm-forming S. aureus to be superior to those of many commonly used antimicrobials (5). The antistaphylococcal biofilm properties of Citrox are reported for the first time in this study. A previous study described the antimicrobial properties of two formulations of Citrox versus oral microorganisms; while it demonstrated broad antimicrobial properties against a range of pathogens, that study did not include staphylococci and was limited to artificial in vitro planktonic and biofilm assays, which did not replicate the in vivo-like environment described in this study (11).
To investigate further the potential therapeutic use of ML:8 and Citrox, the cytotoxic and hemolytic potentials of these agents were examined. Cytotoxicity to the HaCaT and THP-1 cell lines was evident at a concentration shown to be effective against bacterial biofilms (1% [vol/vol]). The effects of ML:8 and Citrox on erythrocytes were also examined to determine if either agent caused hemolysis of human blood. Hemolysis was observed after exposure to high concentrations (≥0.25% [vol/vol]) of the agents. However, compared to currently used CLSs, such as ethanol and sodium citrate solutions (Duralock-C), cytotoxicity and hemolytic levels were found to be no worse than those of the currently used treatments, which would therefore warrant further investigation of these agents as potential CLSs. For agents used as CLSs, if leakage from the IVC into the systemic circulation did occur, the solution would be diluted rapidly, dramatically minimizing the risk of toxicity described for the concentrations tested in this study. Taken together, this rapid dilution in conjunction with the small volume in a catheter reduces the risk of harm to a patient.
Short-chain fatty acids, including acetic, butyric, and propionic acids, have previously been linked to increased levels of IL-1β, IL-6, and IL-8, leading to a proinflammatory response in humans (27), while some flavonoids, including quercetin, have previously been shown to inhibit expression of the proinflammatory cytokine TNF-α (28, 29). The potential of ML:8 or Citrox to induce or inhibit an innate immune response involving a range of cytokines was therefore investigated. Reassuringly, no cytokine response was detected in fresh whole blood following exposure to either novel agent. Interestingly, no resistance to ML:8 or Citrox arose in the MSSA strain SH1000 or the MRSA strain BH1CC over a 90-day period of exposure to sublethal concentrations of either agent. In contrast, bacterial resistance to antibiotics has previously been reported after 2 to 5 days of exposure to sublethal concentrations (30). Resistance to other agents, such as tea tree oil, have also been shown to emerge within 90 days (31).
To ensure that our initial results were not confined to limited genetic backgrounds, we expanded our study to include many other S. aureus clonal complexes (CCs) commonly isolated from patients within hospitals. Previous studies have shown an association between S. aureus clonality, invasive disease, and antimicrobial resistance (32, 33). Significant variation in the susceptibility of biofilms produced by strains from various genetic lineages was not shown after treatment with 1% (vol/vol) ML:8 or Citrox.
A rat model of IVC infection assessed the effects of these novel agents in vivo. Citrox (1% [vol/vol]) was effective in the eradication of MRSA biofilms formed over 5 days in this model, using an initial inoculum of 106 CFU/ml. In comparison, ML:8 (1% [vol/vol]) did not result in a significant reduction in biofilm viability against biofilms formed under the same conditions. ML:8 eradicated biofilm viability in this model when biofilms were formed over 1 day, using a lower inoculum of 104 CFU/ml. Biofilm maturity may be an important contributing factor in the resistance of 5-day in vivo biofilms to ML:8 treatment. Increased resistance of mature 5-day biofilms was previously reported for a number of commonly used antimicrobials (5, 12).
Importantly, the findings with these novel agents could be investigated further in other S. aureus models of infection, such as prosthetic joint infections, surgical site infections, and wound infections; this is of particular significance and importance given the increasing use of medical devices, with their associated risk of infection, within modern health care. While many antimicrobial agents kill bacteria in a planktonic state, only a limited number are effective at treating device-related staphylococcal infections involving biofilms, and therefore these agents represent additional treatment options versus staphylococci. Taken together, our findings demonstrate that ML:8 and Citrox have the potential to be used therapeutically for the treatment of CRIs involving S. aureus biofilms.
Supplementary Material
ACKNOWLEDGMENTS
J.P.O. was a past recipient of an Irish Research Council for Science, Engineering and Technology (IRCSET)/Citrox Biosciences Enterprise Partnership Scheme award, between 2008 and 2011. S.H. was previously an employee and a recipient of research funding from Marvao Medical (previous partial license holder of ML:8), from 2010 to 2012. Neither affiliation was ongoing while the research presented was carried out or at the time of publication. All other authors declare no conflicts of interest.
This research was funded by a major grant award from the Healthcare Infection Society and Irish Health Research Board to E.O., J.P.O., and H.H.
Funding Statement
This work, including the efforts of Hilary Humphreys, James P. O'Gara, and Eoghan O’Neill, was funded by Health Research Board (HRB) (HRA_POR/2012/52) and by Healthcare Infection Society (HIS) (HIS Major Award 2011). The funders had no role in the design, execution, or interpretation of this study and were not involved in the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00910-16.
REFERENCES
- 1.Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Götz F. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol 20:1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 2.François P, Vaudaux P, Lew PD. 1998. Role of plasma and extracellular matrix proteins in the physiopathology of foreign body infections. Ann Vasc Surg 12:34–40. doi: 10.1007/s100169900112. [DOI] [PubMed] [Google Scholar]
- 3.Foster TJ, Höök M. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol 6:484–488. doi: 10.1016/S0966-842X(98)01400-0. [DOI] [PubMed] [Google Scholar]
- 4.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogan S, Zapotoczna M, Stevens NT, Humphreys H, O'Gara JP, O'Neill E. 2016. In vitro approach for identification of the most effective agents for antimicrobial lock therapy in the treatment of intravascular catheter-related infections caused by Staphylococcus aureus. Antimicrob Agents Chemother 60:2923–2931. doi: 10.1128/AAC.02885-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan S, Stevens NT, Humphreys H, O'Gara JP, O'Neill E. 2015. Current and future approaches to the prevention and treatment of staphylococcal medical device-related infections. Curr Pharm Des 21:100–113. [DOI] [PubMed] [Google Scholar]
- 7.Laverty G, Gilmore BF, Jones DS, Coyle L, Folan M, Breathnach R. 2015. Antimicrobial efficacy of an innovative emulsion of medium chain triglycerides against canine and feline periodontopathogens. J Small Anim Pract 56:253–263. doi: 10.1111/jsap.12344. [DOI] [PubMed] [Google Scholar]
- 8.Rosenblatt J, Reitzel RA, Raad I. 2015. Caprylic acid and glyceryl trinitrate combination for eradication of biofilm. Antimicrob Agents Chemother 59:1786–1788. doi: 10.1128/AAC.04561-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandalari G, Bennett RN, Bisignano G, Trombetta D, Saija A, Faulds CB, Gasson MJ, Narbad A. 2007. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J Appl Microbiol 103:2056–2064. doi: 10.1111/j.1365-2672.2007.03456.x. [DOI] [PubMed] [Google Scholar]
- 10.Ripley I, Darlington G. August 2010. Compound and compositions for treatment of disease. US patent 20100210573.
- 11.Hooper SJ, Lewis MA, Wilson MJ, Williams DW. 2011. Antimicrobial activity of Citrox bioflavonoid preparations against oral microorganisms. Br Dent J 210:E22. doi: 10.1038/sj.bdj.2010.1224. [DOI] [PubMed] [Google Scholar]
- 12.Zapotoczna M, McCarthy H, Rudkin JK, O'Gara JP, O'Neill E. 2015. An essential role for coagulase in Staphylococcus aureus biofilm development reveals new therapeutic possibilities for device-related infections. J Infect Dis 212:1883–1893. doi: 10.1093/infdis/jiv319. [DOI] [PubMed] [Google Scholar]
- 13.Walker JN, Horswill AR. 2012. A coverslip-based technique for evaluating Staphylococcus aureus biofilm formation on human plasma. Front Cell Infect Microbiol 2:39. doi: 10.3389/fcimb.2012.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waters EM, McCarthy H, Hogan S, Zapotoczna M, O'Neill E, O'Gara JP. 2014. Rapid quantitative and qualitative analysis of biofilm production by Staphylococcus epidermidis under static growth conditions. Methods Mol Biol 1106:157–166. doi: 10.1007/978-1-62703-736-5_14. [DOI] [PubMed] [Google Scholar]
- 15.Cantisani M, Leone M, Mignogna E, Kampanaraki K, Falanga A, Morelli G, Galdiero M, Galdiero S. 2013. Structure-activity relations of myxinidin, an antibacterial peptide derived from the epidermal mucus of hagfish. Antimicrob Agents Chemother 57:5665–5673. doi: 10.1128/AAC.01341-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulphani JS, Rupp ME. 1999. Model of Staphylococcus aureus central venous catheter-associated infection in rats. Lab Anim Sci 49:283–287. [PubMed] [Google Scholar]
- 17.Van Praagh AD, Li T, Zhang S, Arya A, Chen L, Zhang XX, Bertolami S, Mortin LI. 2011. Daptomycin antibiotic lock therapy in a rat model of staphylococcal central venous catheter biofilm infections. Antimicrob Agents Chemother 55:4081–4089. doi: 10.1128/AAC.00147-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chauhan A, Lebeaux D, Ghigo JM, Beloin C. 2012. Full and broad-spectrum in vivo eradication of catheter-associated biofilms using gentamicin-EDTA antibiotic lock therapy. Antimicrob Agents Chemother 56:6310–6318. doi: 10.1128/AAC.01606-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chauhan A, Lebeaux D, Decante B, Kriegel I, Escande MC, Ghigo JM, Beloin C. 2012. A rat model of central venous catheter to study establishment of long-term bacterial biofilm and related acute and chronic infections. PLoS One 7:e37281. doi: 10.1371/journal.pone.0037281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Neill E, Pozzi C, Houston PH, Humphreys H, Robinson DA, Loughman A, Foster TJ, O'Gara JP. 2008. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J Bacteriol 190:3835–3850. doi: 10.1128/JB.00167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNicholas S. 2012. Investigation of bacterial virulence and host response in blood stream infections caused by methicillin resistant Staphylococcus aureus (MRSA) and methicillin-susceptible Staphylococcus aureus (MRSA). Royal College of Surgeons Ireland, Dublin, Ireland. [Google Scholar]
- 22.Kim EY, Saunders P, Yousefzadeh N. 2010. Usefulness of anti-infective lock solutions for catheter-related bloodstream infections. Mt Sinai J Med 77:549–558. doi: 10.1002/msj.20213. [DOI] [PubMed] [Google Scholar]
- 23.Kiedrowski MR, Horswill AR. 2011. New approaches for treating staphylococcal biofilm infections. Ann N Y Acad Sci 1241:104–121. doi: 10.1111/j.1749-6632.2011.06281.x. [DOI] [PubMed] [Google Scholar]
- 24.Kostenko VM, Salek M, Sattari P, Martinuzzi RJ. 2010. Staphylococcus aureus biofilm formation and tolerance to antibiotics in response to oscillatory shear stresses of physiological levels. FEMS Immunol Med Microbiol 59:421–431. doi: 10.1111/j.1574-695X.2010.00694.x. [DOI] [PubMed] [Google Scholar]
- 25.Olson ME, Ceri H, Morck DW, Buret AG, Read RR. 2002. Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can J Vet Res 66:86–92. [PMC free article] [PubMed] [Google Scholar]
- 26.Luther MK, Mermel LA, LaPlante KL. 2014. Comparison of ML8-X10 (a prototype oil-in-water micro-emulsion based on a novel free fatty acid), taurolidine/citrate/heparin and vancomycin/heparin antimicrobial lock solutions in the eradication of biofilm-producing staphylococci from central venous catheters. J Antimicrob Chemother 69:3263–3267. doi: 10.1093/jac/dku281. [DOI] [PubMed] [Google Scholar]
- 27.Mirmonsef P, Zariffard MR, Gilbert D, Makinde H, Landay AL, Spear GT. 2012. Short-chain fatty acids induce pro-inflammatory cytokine production alone and in combination with Toll-like receptor ligands. Am J Reprod Immunol 67:391–400. doi: 10.1111/j.1600-0897.2011.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nair MP, Mahajan S, Reynolds JL, Aalinkeel R, Nair H, Schwartz SA, Kandaswami C. 2006. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-kappa beta system. Clin Vaccine Immunol 13:319–328. doi: 10.1128/CVI.13.3.319-328.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo SM. 2013. Flavanoids and gene expression in mammalian cells, p 191–200. In Buslig B, Manthey J (ed), Flavonoids in cell function. Springer Science & Business Media, New York, NY. [Google Scholar]
- 30.Kohanski MA, DePristo MA, Collins JJ. 2010. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol Cell 37:311–320. doi: 10.1016/j.molcel.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson RR. 2000. Selection of resistance to the essential oil of Melaleuca alternifolia in Staphylococcus aureus. J Antimicrob Chemother 45:549–550. doi: 10.1093/jac/45.4.549. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen G, Monecke S, Ehricht R, Soderquist B. 2013. Prevalence of clonal complexes and virulence genes among commensal and invasive Staphylococcus aureus isolates in Sweden. PLoS One 8:e77477. doi: 10.1371/journal.pone.0077477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fowler VG Jr, Nelson CL, McIntyre LM, Kreiswirth BN, Monk A, Archer GL, Federspiel J, Naidich S, Remortel B, Rude T, Brown P, Reller LB, Corey GR, Gill SR. 2007. Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. J Infect Dis 196:738–747. doi: 10.1086/520088. [DOI] [PubMed] [Google Scholar]
- 34.Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, Foster SJ. 2002. sigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J Bacteriol 184:5457–5467. doi: 10.1128/JB.184.19.5457-5467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Neill E, Pozzi C, Houston P, Smyth D, Humphreys H, Robinson DA, O'Gara JP. 2007. Association between methicillin susceptibility and biofilm regulation in Staphylococcus aureus isolates from device-related infections. J Clin Microbiol 45:1379–1388. doi: 10.1128/JCM.02280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4:e00537-12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo Y, Ramos RI, Cho JS, Donegan NP, Cheung AL, Miller LS. 2013. In vivo bioluminescence imaging to evaluate systemic and topical antibiotics against community-acquired methicillin-resistant Staphylococcus aureus-infected skin wounds in mice. Antimicrob Agents Chemother 57:855–863. doi: 10.1128/AAC.01003-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.