Abstract
There are no wholly successful chemotherapeutic strategies against Burkholderia cepacia complex (BCC) colonization in cystic fibrosis (CF). We assessed the impact of cysteamine (Lynovex) in combination with standard-of-care CF antibiotics in vitro against BCC CF isolates by the concentration at which 100% of bacteria were killed (MIC100) and checkerboard assays under CLSI standard conditions. Cysteamine facilitated the aminoglycoside-, fluoroquinolone- and folate pathway inhibitor-mediated killing of BCC organisms that were otherwise resistant or intermediately sensitive to these antibiotic classes. Slow-growing BCC strains are often recalcitrant to treatment and form biofilms. In assessing the impact of cysteamine on biofilms, we demonstrated inhibition of BCC biofilm formation at sub-MIC100s of cysteamine.
INTRODUCTION
Colonization of the airways with Burkholderia cepacia complex (BCC) is a major contributory factor to patient morbidity and mortality in cystic fibrosis (CF) (1–3), reducing life expectancy of those affected (4% of the adult CF population) by as much as 16 years (2, 4). Most, if not all, BCC strains (5) are clinically resistant or inherently insensitive to currently available CF antibiotics (2, 6, 7). BCC colonization is a significant clinical challenge in CF that will increase as survival rates for this condition continue to improve (8, 9). There remains a critical, unmet need for new chemotherapeutic approaches to resolving BCC colonization in CF.
We previously described the antimicrobial, antibiofilm, and mucolytic attributes of cysteamine against Pseudomonas aeruginosa and other CF bacterial pathogens (10–12) and report here that, more strikingly, the modification of currently available therapeutic strategies (2, 13) with the introduction of cysteamine as an adjunct antimicrobial agent brings about effective killing of BCC (both type strains and CF isolates).
MATERIALS AND METHODS
Bacterial strains and reagents.
The 36 Burkholderia strains employed for this study include representative strains from each species (or genomovar), including B. cenocepacia and B. multivorans, those most commonly associated with infection in cystic fibrosis (14). Sixteen of the BCC clinical isolates were sourced from Aberdeen Royal Infirmary, with seven from adult patients and nine pediatric isolates. Eight strains were sourced from the Glasgow adult patient cohort. An additional 12 type strains were included in this study. Cysteamine, trimethoprim, sulfamethoxazole, and ciprofloxacin were sourced from Sigma-Aldrich (United Kingdom), and tobramycin and ceftazidime were sourced from Discovery Fine Chemicals (United Kingdom). All other reagents were purchased from Sigma-Aldrich (United Kingdom).
MIC and checkerboard assays.
The concentration at which 100% of bacteria were killed (MIC100) for all BCC isolates was determined for cysteamine and the antibiotics tobramycin, ciprofloxacin, ceftazidime, and trimethoprim-sulfamethoxazole using the CLSI broth microdilution procedure (15). Checkerboard assays of cysteamine and antibiotics were conducted according to the method of Burkhart et al. (16). Antibiotic susceptibility profiling of BCC (resistant, intermediate, or sensitive to antibiotics) was performed using CLSI performance standards for antimicrobial susceptibility testing using interpretive standards for other non-Enterobacteriaceae (17).
Biofilm assays.
The crystal violet method for detecting the adherence of bacterial biomass to polypropylene 96-well plates was adapted from similar studies (18–20). Inocula were prepared using a McFarland standard equivalent of 5 × 105 CFU/ml from growing cultures (according to CLSI document M07-A9 [15]) into 100 μl of cation-adjusted Mueller-Hinton broth containing appropriate concentrations of test antibiotic. Cultures were incubated statically for 48 h in a humidified atmosphere at 37°C to establish biofilms. Culture medium containing planktonic bacteria was carefully removed and discarded, and the plates were washed gently three times with 150 μl of sterile phosphate-buffered saline (PBS) prior to air drying for 1 h. The remaining attached bacteria in wells were then stained with 200 μl of 1% crystal violet solution for 2 min, prior to 3 further washes with PBS and solubilization with 200 μl of ethanol. Plates were then read at 595 nm.
Assessment of cysteamine-mediated biofilm prevention in a shear-flow environment was performed using a BioFlux 200 automated microfluidic system (Fluxion Biosciences, CA). Inocula were prepared as described above (CLSI document M07-A9 [15]) from growing cultures and seeded into prewarmed cation-adjusted Mueller-Hinton broth with or without the sub-MIC100 concentration of 128 μg/ml of cysteamine. A 48-well Bioflux plate was primed for 1 min with prewarmed cation-adjusted Mueller-Hinton broth prior to addition of treated and untreated cultures. Medium was passed through capillaries at 37 μl/h (0.4 dyne) for 20 h, and images were captured using an Axiovert 40CFL microscope (Carl Zeiss, United Kingdom) and camera and Bioflux 200 software.
RESULTS
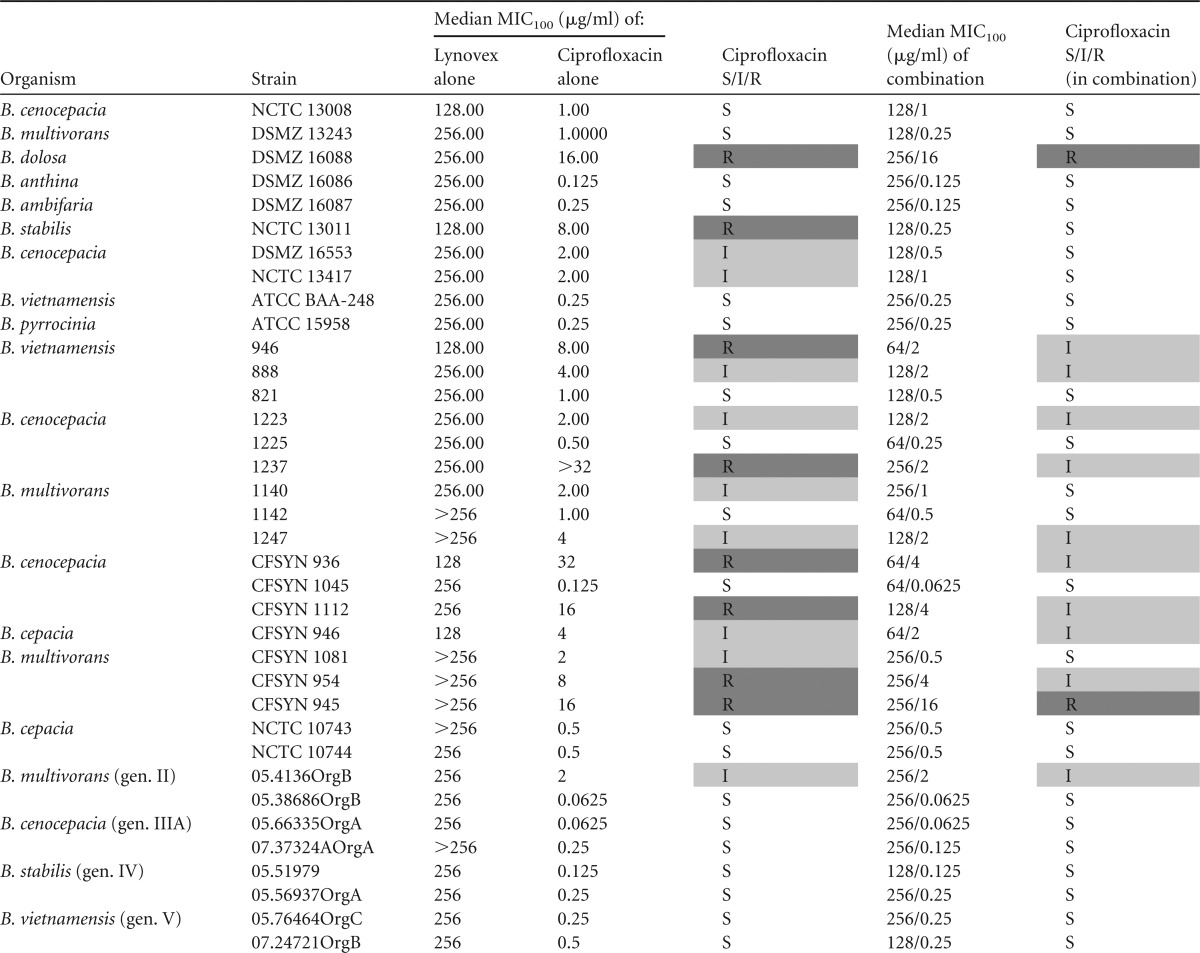
MIC assays with combinations of cysteamine and the recommended CF antibiotics were performed to assess the utility of cysteamine in vitro as an adjunct antibiotic therapy against BCC. A panel of BCC clinical isolates and type strains was tested to determine genomovar-specific effects and any differences in cysteamine-mediated effects between type strains and clinical isolates associated with colonization in two major United Kingdom patient cohorts. The 36 strains tested were resistant in vitro to at least one antibiotic selected from tobramycin, ciprofloxacin, trimethoprim-sulfamethoxazole, and ceftazidime (25 out of 36). The majority of clinical strains of CF origin were found to be resistant to tobramycin; perhaps this is not surprising considering the status of tobramycin as a mainstay, long-term antibiotic intervention in CF. Cysteamine potentiated the activity of tobramycin against 33 of 36 BCC isolates and type strains tested and reversed resistance/insensitivity in 17 of those strains (Table 1), all 17 being CF isolates. Cysteamine also potentiated the activity of ciprofloxacin against 21 of 36 BCC isolates tested and reversed resistance/insensitivity in 10 strains that were not sensitive to ciprofloxacin (Table 2). Only two isolates (B. dolosa DSMZ 16088 and a clinical isolate of B. multivorans CFSYN 945) remained resistant to ciprofloxacin in the presence of cysteamine.
TABLE 1.
Antimicrobial activities of tobramycin and tobramycin in combination with cysteamine against Burkholderia isolatesa
All results represent the MIC100s from triplicate samples from triplicate experiments. Determination of susceptibility, intermediate status, or resistance (S/I/R) was based upon CLSI interpretive criteria. All data manipulation was carried out in Microsoft Excel. gen., genomovar. Strains with the resistance or intermediate status are highlighted by shading.
TABLE 2.
Antimicrobial activities of ciprofloxacin and ciprofloxacin in combination with cysteamine against Burkholderia isolatesa
All results represent the MIC100s from triplicate samples from triplicate experiments. S/I/R determination was based upon CLSI interpretive criteria. All data manipulation was carried out in Microsoft Excel. Resistant or intermediate results are highlighted with shading.
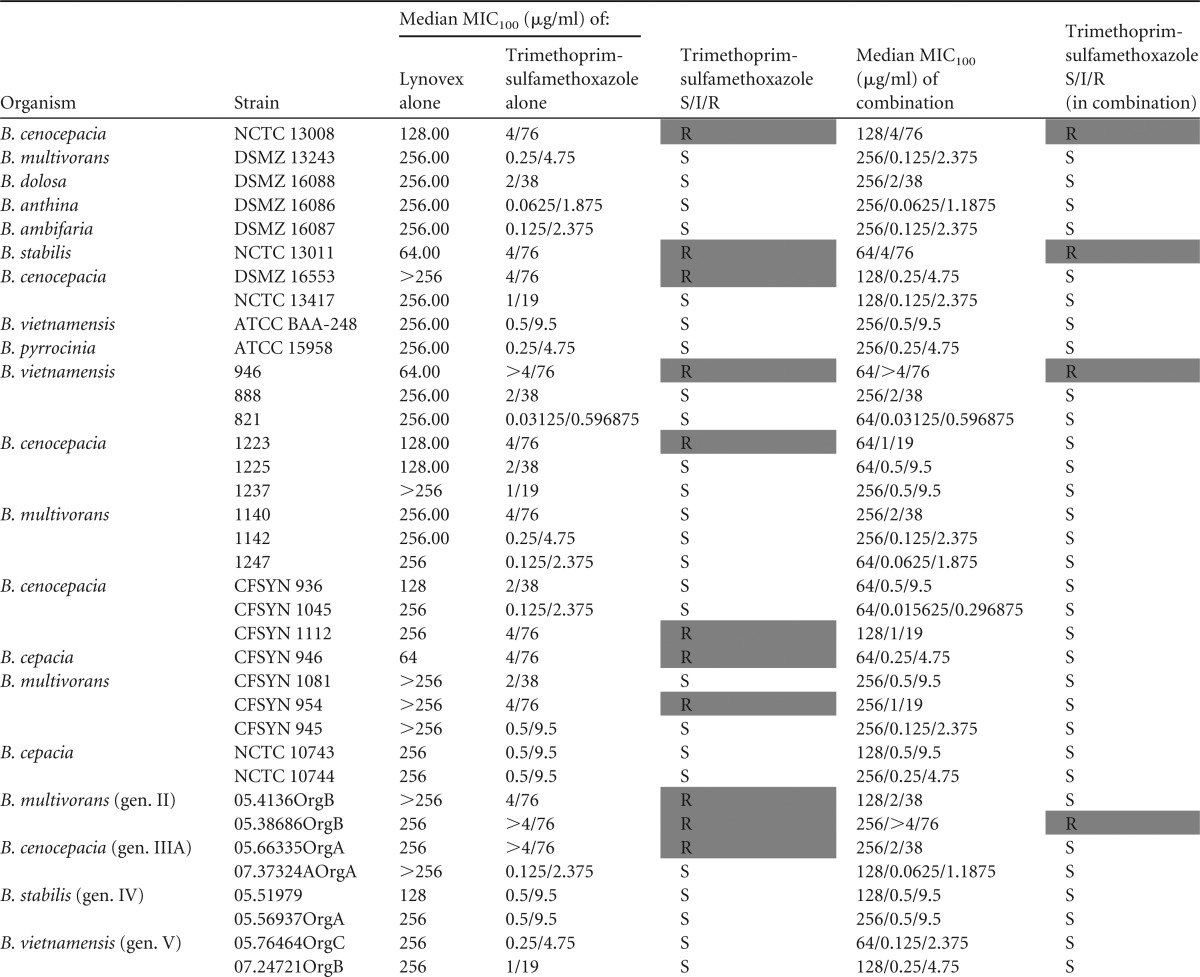
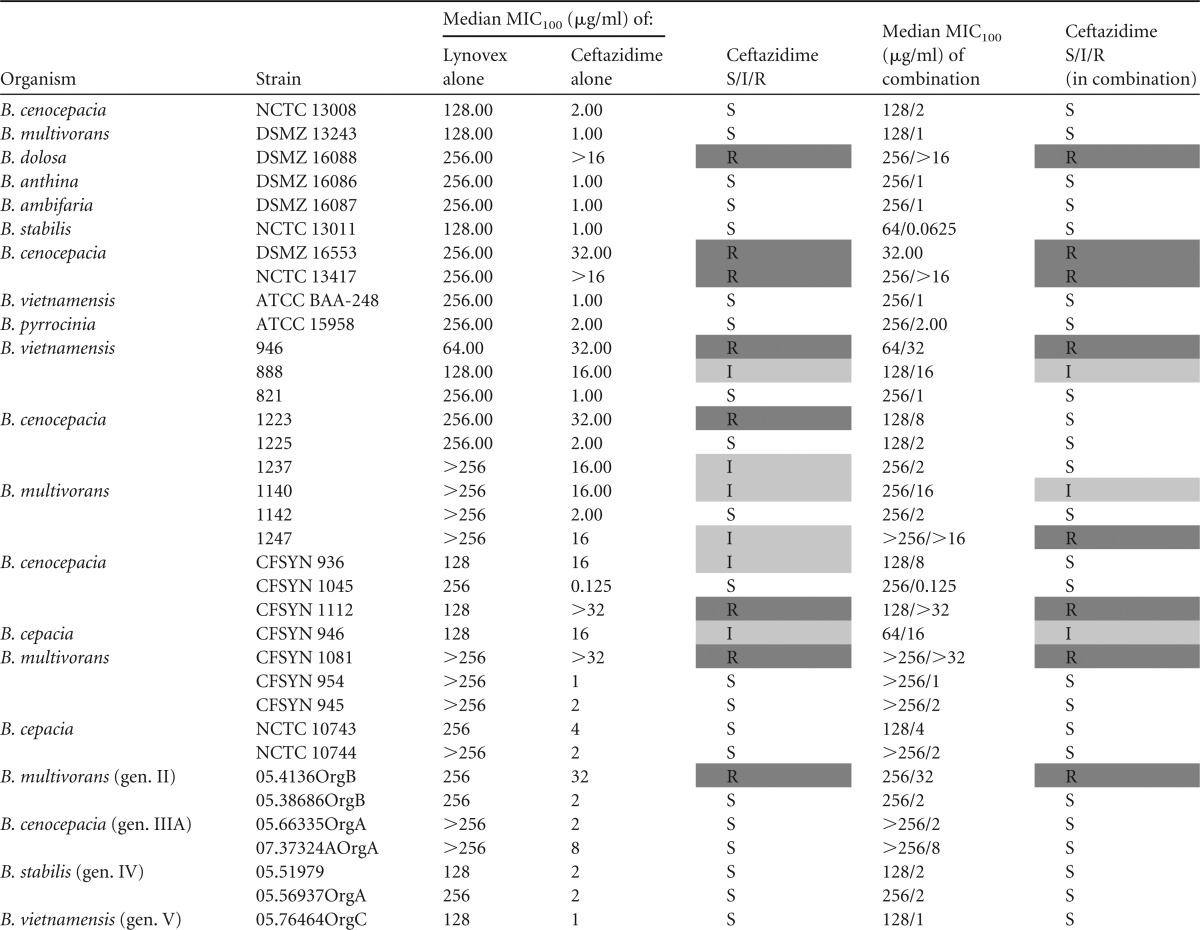
In addition to this impact on widely used antibiotics, the ability of cysteamine to enhance the activity of ceftazidime and trimethoprim-sulfamethoxazole, antibiotics used specifically to treat BCC, was also investigated (Tables 3 and 4). Interestingly, and pointing to an antibiotic class-specific effect, cysteamine had no major impact on ceftazidime susceptibility of the BCC strains tested in this system. In contrast, cysteamine potentiated the activity of trimethoprim-sulfamethoxazole against the majority (22 out of 36) of BCC isolates and type strains studied.
TABLE 3.
Antimicrobial activities of trimethoprim-sulfamethoxazole and trimethoprim-sulfamethoxazole in combination with cysteamine against Burkholderia isolates versus S/I/R determinations based upon CLSI interpretive criteriaa
All results represent the MIC100s from triplicate samples from triplicate experiments. All data manipulation was carried out in Microsoft Excel. Resistant or intermediate results are highlighted by shading.
TABLE 4.
Antimicrobial activities of ceftazidime and ceftazidime in combination with cysteamine against Burkholderia isolatesa
All results represent the MIC100s from triplicate samples from triplicate experiments. S/I/R determination was based upon CLSI interpretive criteria. All data manipulation was carried out in Microsoft Excel. Strains with resistant or intermediate results are highlighted by shading.
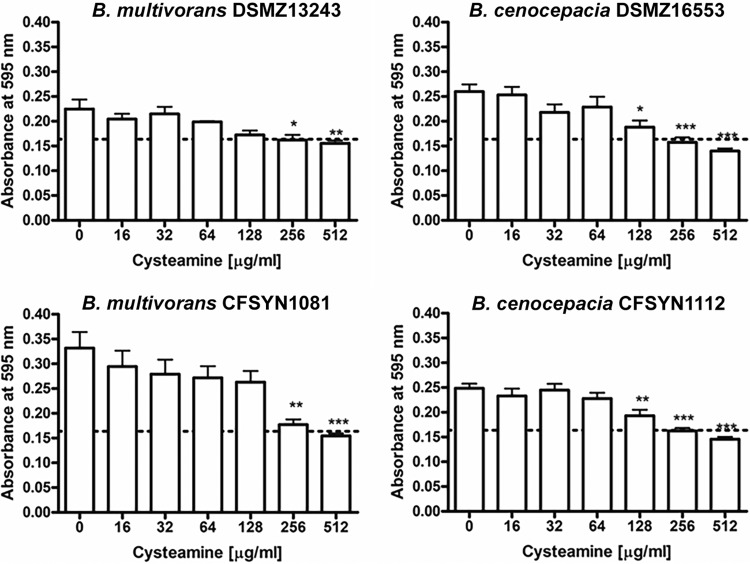
Planktonic cells were employed in our initial cysteamine adjunct assays, whereas in vivo, BCC colonizes the CF airway in biofilm form (21, 22). We have already described the antibiofilm properties of cysteamine against Pseudomonas (10–12). In order to confirm any activity of cysteamine specifically against BCC biofilm structures in vitro, we assessed its ability to prevent BCC biofilm formation using the Bioflux microfluidic system, and we used the crystal violet method for assessing biomass in 96-well microtiter plates. In both systems, the methodology was adapted to keep conditions as close as possible to CLSI standards for MIC testing rather than to favor the growth of biofilms. For example, the medium used was cation-adjusted Mueller-Hinton broth as opposed to other choices, such as tryptic soy broth, which would favor biofilm formation in BCC (23). This was to clearly demonstrate inhibition of attachment at sub-MIC100s. Crystal violet assessment demonstrated inhibition of bacterial attachment at 48 h in the presence of concentrations of cysteamine subinhibitory for planktonic growth (Fig. 1). Inhibition of biofilm formation was dose dependent, increasing with concentrations of cysteamine approaching the MIC, with significant inhibition of both strains of B. cenocepacia tested at 128 μg/ml. The antibiofilm activity of cysteamine against BCC was confirmed in real time in a dynamic-flow microfluidics system. The effect of a subinhibitory concentration of cysteamine was assessed on biofilm formation in the BioFlux microfluidic system on B. cenocepacia clinical strain CFSYN 1112. Cysteamine prevented biofilm formation in cells in channels that were exposed to cysteamine over 20 h compared to samples in wells not exposed to cysteamine (see Movie S1 in the supplemental material). Viable planktonic cells from the outlet well were cultured at the end of the experiment, confirming an effect on attachment and not bacterial viability at the test concentration.
FIG 1.
Cysteamine inhibits adherence of BCC strains at concentrations below the MIC100 as detected by crystal violet assay. One-way analysis of variance with Tukey's posttest analysis showed significant differences as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001 (compared to untreated medium-only controls [n = 6]).
DISCUSSION
The findings of our current study point to the potential of cysteamine as a means to resolve or prevent BCC colonization through a simple and sustainable modification to the current standard of care in CF. Tobramycin and ciprofloxacin are mainstays of the CF antibiotic regimen, and resistance to these antibiotics in CF BCC strains is common and inherent in some strains (24) and is readily selected for (25); indeed, we demonstrate that all but one of the strains (NCTC 13008) tested in this study which had been isolated from a patient with CF were resistant to tobramycin treatment. Cysteamine was able to reverse the tobramycin and ciprofloxacin resistance phenotype and improve sensitivity to co-trimoxazole treatment (Tables 1 to 3). Therefore, cysteamine has the potential to extend the target spectrum of these antibiotics to include BCC. This is timely as regards its potential use as an adjunct with tobramycin considering the recent increased interest in the reapplication of inhaled or high-dose tobramycin against BCC (26, 27). Furthermore, the activity of antibiotics specifically deployed against BCC, such as trimethoprim-sulfamethoxazole, can also be further potentiated by cysteamine. Interestingly, ceftazidime activity was not altered by cotreatment with cysteamine, which suggests an antibiotic class-specific effect, at least within the in vitro systems employed in this study to assess antimicrobial activity.
The slow-growing, biofilm-forming characteristic of BCC contributes to the recalcitrance of this organism to existing antibiotic chemotherapy. In this study, we followed our previous work on the interactions of cysteamine with biofilm (10) in addition to assessing any direct antimicrobial activity. Cysteamine inhibited bacterial attachment at concentrations below the MIC100 for each strain tested, with significant inhibition of B. cenocepacia type strain DSMZ 16553 and clinical isolate CFSYN 1112. Interestingly, although we previously demonstrated that combinations of tobramycin and cysteamine were more effective in biofilm prevention and eradication for Pseudomonas aeruginosa, the addition of antibiotics did not enhance the antibiofilm activity of cysteamine against BCC (data not shown). Cysteamine was not able to disrupt existing biofilms in the slower-growing BCC strains over 48 h over the same range of concentrations of the antibiotics as tested in this in vitro system. This may indicate that cysteamine adjunct maintenance therapy may be better at preventing the establishment of BCC colonization in CF than at removing existing biofilms in chronically infected patients; however, the enhancement of antimicrobial activity may prove to be the more important feature of this compound in this situation. Further research to determine optimum antibiotic combinations and concentrations to eradicate established BCC biofilms may yet prove efficacious.
We purposely did not use an exhaustive panel of BCC strains for this study. We instead employed a focused set of clinically relevant CF isolates from two of the United Kingdom's specialist CF centers (8 isolates from Glasgow and 16 from Aberdeen) and an additional 12 type strains in order to cover all known BCC genomovars, regardless of clinical relevance.
Cysteamine is in late-stage clinical trials for the treatment of cystic fibrosis and is being developed in oral and inhaled forms for acute exacerbations and chronic longer-term maintenance (10–12). An oral form of cysteamine was investigated in an open-label clinical study (28) in the United Kingdom in which tolerability, absorption, pK, and early evidence of efficacy were assessed in adult CF patients with stable disease. A global two-part registration study for oral cysteamine in acute exacerbations is now being initiated (EudraCT no. 2015-0004986-99) for which endpoints will include the reduction in sputum microbial burden over and above that achieved with standard of care therapy (SOCT) exacerbation interventions.
Thus far, cysteamine appears to be a promising candidate treatment for CF, but how its interactions with all components of the complex CF microbiome contribute to its clinical effects is yet to be determined. We have already have demonstrated the utility of cysteamine against other, more common CF pathogens that are known to drive acute infectious exacerbations (Pseudomonas in particular) (29, 30). We believe that this study is important in confirming the efficacy of cysteamine against the more insidious BCC and its colonization of the CF airway, which may be eradicated and perhaps prevented by long-term use of an adjunct to SOCT such as cysteamine, which is able to potentiate the effects of existing antibiotics and “switch” BCC to becoming sensitive and also prevent this organism from forming biofilms. Not all BCC isolates tested in this study responded to cotreatment. As well as any strain-specific nuances in cysteamine response, the antibiotic class-specific differences in responses to cysteamine coexposure we have underpinned for BCC (and other organisms in our previous work) are the subject of further study.
Supplementary Material
ACKNOWLEDGMENTS
We thank Gordon Ramage from the University of Glasgow (United Kingdom) and Deirdre O'Brien from Aberdeen Royal Infirmary for kindly supplying the BCC clinical isolates.
All of the authors made a significant contribution to the design, execution, and analysis of the work described herein and have seen and agreed to the submitted version of the manuscript.
All authors are current or previous employees of NovaBiotics. Deborah O'Neil is a director and shareholder of NovaBiotics Ltd. Derry Mercer and Jennifer Robertson hold share options in NovaBiotics Ltd.
All authors are named inventors on patents (regarding the antimicrobial and dispersant properties of cysteamine) which are wholly owned by NovaBiotics Ltd.
This study was supported solely by internal funding.
Funding Statement
This work was funded by NovaBiotics Ltd.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01198-16.
REFERENCES
- 1.Brüssow H. 2012. Pseudomonas biofilms, cystic fibrosis, and phage: a silver lining? mBio 3(2):e00061-12. doi: 10.1128/mBio.00061-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horsley A, Jones AM. 2012. Antibiotic treatment for Burkholderia cepacia complex in people with cystic fibrosis experiencing a pulmonary exacerbation. Cochrane Database Syst Rev 10:CD009529. [DOI] [PubMed] [Google Scholar]
- 3.O'Malley CA. 2009. Infection control in cystic fibrosis: cohorting, cross-contamination, and the respiratory therapist. Respir Care 54:641–657. doi: 10.4187/aarc0446. [DOI] [PubMed] [Google Scholar]
- 4.Regan KH, Bhatt J. 2014. Eradication therapy for Burkholderia cepacia complex in people with cystic fibrosis. Cochrane Database Syst Rev 10:CD009876. [DOI] [PubMed] [Google Scholar]
- 5.Mahenthiralingam E, Baldwin A, Dowson CG. 2008. Burkholderia cepacia complex bacteria: opportunistic pathogens with important natural biology. J Appl Microbiol 104:1539–1551. doi: 10.1111/j.1365-2672.2007.03706.x. [DOI] [PubMed] [Google Scholar]
- 6.Tseng SP, Tsai WC, Liang CY, Lin YS, Huang JW, Chang CY, Tyan YC, Lu PL. 2014. The contribution of antibiotic resistance mechanisms in clinical Burkholderia cepacia complex isolates: an emphasis on efflux pump activity. PLoS One 9:e104986. doi: 10.1371/journal.pone.0104986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou J, Chen Y, Tabibi S, Alba L, Garber E, Saiman L. 2007. Antimicrobial susceptibility and synergy studies of Burkholderia cepacia complex isolated from patients with cystic fibrosis. Antimicrob Agents Chemother 51:1085–1088. doi: 10.1128/AAC.00954-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drevinek P, Mahenthiralingam E. 2010. Burkholderia cenocepacia in cystic fibrosis: epidemiology and molecular mechanisms of virulence. Clin Microbiol Infect 16:821–830. doi: 10.1111/j.1469-0691.2010.03237.x. [DOI] [PubMed] [Google Scholar]
- 9.Zlosnik JE, Costa PS, Brant R, Mori PY, Hird TJ, Fraenkel MC, Wilcox PG, Davidson AG, Speert DP. 2011. Mucoid and nonmucoid Burkholderia cepacia complex bacteria in cystic fibrosis infections. Am J Respir Crit Care Med 183:67–72. doi: 10.1164/rccm.201002-0203OC. [DOI] [PubMed] [Google Scholar]
- 10.Charrier C, Rodger C, Robertson J, Kowalczuk A, Shand N, Fraser-Pitt D, Mercer DK, O'Neil DA. 2014. Cysteamine (Lynovex®), a novel mucoactive antimicrobial and antibiofilm agent for the treatment of cystic fibrosis. Orphanet J Rare Dis 9:189. doi: 10.1186/s13023-014-0189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charrier C, Rodger C, Shand N, Mercer DK, O'Neil DA. 2012. Lynovex®, a novel mucolytic-antimicrobial agent for the treatment of cystic fibrosis. J Cyst Fibros 11(Suppl 1):S74. [Google Scholar]
- 12.Mercer DK, Charrier C, Robertson J, Kowalczuk A, Devlin E, Fraser-Pitt D, Devereux G, O'Neil DA. 2014. Ex vivo efficacy of Lynovex®, a next generation tri-functional candidate cystic fibrosis therapy. J Cyst Fibros 13(Suppl 1):S58. [Google Scholar]
- 13.Flume PA, Van Devanter DR. 2012. State of progress in treating cystic fibrosis respiratory disease. BMC Med 10:88. doi: 10.1186/1741-7015-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LiPuma JJ. 2005. Update on the Burkholderia cepacia complex. Curr Opin Pulm Med 11:528–533. doi: 10.1097/01.mcp.0000181475.85187.ed. [DOI] [PubMed] [Google Scholar]
- 15.CLSI. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed, M07-A9 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 16.Burkhart CG, Burkhart CN, Isham N. 2006. Synergistic antimicrobial activity by combining an allylamine with benzoyl peroxide with expanded coverage against yeast and bacterial species. Br J Dermatol 154:341–344. doi: 10.1111/j.1365-2133.2005.06924.x. [DOI] [PubMed] [Google Scholar]
- 17.CLSI. 2012. Performance standards for antimicrobial susceptibility testing; 22nd informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 18.Cassat JE, Lee CT, Smeltzer MS. 2007. Investigation of biofilm formation in clinical isolates of Staphylococcus aureus. Methods Mol Biol 391:127–144. doi: 10.1007/978-1-59745-468-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conway BA, Venu V, Speert DP. 2002. Biofilm formation and acyl homoserine lactone production in the Burkholderia cepacia complex. J Bacteriol 184:5678–5685. doi: 10.1128/JB.184.20.5678-5685.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu WS, Chen CC, Chuang YC, Su BA, Chiu YH, Hsu HJ, Ko WC, Tang HJ. 2013. Efficacy of combination oral antimicrobial agents against biofilm-embedded methicillin-resistant Staphylococcus aureus. J Microbiol Immunol Infect 46:89–95. doi: 10.1016/j.jmii.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Bragonzi A, Farulla I, Paroni M, Twomey KB, Pirone L, Lore NI, Bianconi I, Dalmastri C, Ryan RP, Bevivino A. 2012. Modelling co-infection of the cystic fibrosis lung by Pseudomonas aeruginosa and Burkholderia cenocepacia reveals influences on biofilm formation and host response. PLoS One 7:e52330. doi: 10.1371/journal.pone.0052330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwab U, Abdullah LH, Perlmutt OS, Albert D, Davis CW, Arnold RR, Yankaskas JR, Gilligan P, Neubauer H, Randell SH, Boucher RC. 2014. Localization of Burkholderia cepacia complex bacteria in cystic fibrosis lungs and interactions with Pseudomonas aeruginosa in hypoxic mucus. Infect Immun 82:4729–4745. doi: 10.1128/IAI.01876-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomlin KL, Malott RJ, Ramage G, Storey DG, Sokol PA, Ceri H. 2005. Quorum-sensing mutations affect attachment and stability of Burkholderia cenocepacia biofilms. Appl Environ Microbiol 71:5208–5218. doi: 10.1128/AEM.71.9.5208-5218.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nzula S, Vandamme P, Govan JRW. 2002. Influence of taxonomic status on the in vitro antimicrobial susceptibility of the Burkholderia cepacia complex. J Antimicrob Chemother 50:265–269. doi: 10.1093/jac/dkf137. [DOI] [PubMed] [Google Scholar]
- 25.Jassem AN, Zlosnik JE, Henry DA, Hancock RE, Ernst RK, Speert DP. 2011. In vitro susceptibility of Burkholderia vietnamensis to aminoglycosides. Antimicrob Agents Chemother 55:2256–2264. doi: 10.1128/AAC.01434-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratjen A, Yau Y, Wettlaufer J, Matukas L, Zlosnik JE, Speert DP, LiPuma JJ, Tullis E, Waters V. 2015. In vitro efficacy of high-dose tobramycin against Burkholderia cepacia complex and Stenotrophomonas maltophilia isolates from cystic fibrosis patients. Antimicrob Agents Chemother 59:711–713. doi: 10.1128/AAC.04123-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy S, Beaudoin T, Yau YC, Caraher E, Zlosnik JE, Speert DP, LiPuma JJ, Tullis E, Waters V. 2016. Activity of tobramycin against cystic fibrosis isolates of Burkholderia cepacia complex grown as biofilms. Antimicrob Agents Chemother 60:348–355. doi: 10.1128/AAC.02068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devereux G, Steele S, Griffiths K, Devlin E, Fraser-Pitt D, Cotton S, Norrie J, Chrystyn H, O'Neil D. 6 May 2016. An open-label investigation of the pharmacokinetics and tolerability of oral cysteamine in adults with cystic fibrosis. Clin Drug Invest doi: 10.1007/s40261-016-0405-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zemanick ET, Emerson J, Thompson V, McNamara S, Morgan W, Gibson RL, Rosenfeld M, EPIC Study Group. 2015. Clinical outcomes after initial pseudomonas acquisition in cystic fibrosis. Pediatr Pulmonol 50:42–48. doi: 10.1002/ppul.23036. [DOI] [PubMed] [Google Scholar]
- 30.Mayer-Hamblett N, Rosenfeld M, Gibson RL, Ramsey BW, Kulasekara HD, Retsch-Bogart GZ, Morgan W, Wolter DJ, Pope CE, Houston LS, Kulasekara BR, Khan U, Burns JL, Miller SI, Hoffman LR. 2014. Pseudomonas aeruginosa in vitro phenotypes distinguish cystic fibrosis infection stages and outcomes. Am J Respir Crit Care Med 190:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.