Abstract
Community-acquired pneumonia is a common disease with considerable morbidity and mortality, for which Streptococcus pneumoniae is accepted as a leading cause. Although β-lactam-plus-macrolide combination therapy for this disease is recommended in several guidelines, the clinical efficacy of this strategy against pneumococcal pneumonia remains controversial. In this study, we examined the effects of β-lactam-plus-macrolide combination therapy on lethal mouse pneumococcal pneumonia and explored the mechanisms of action in vitro and in vivo. We investigated survival, lung bacterial burden, and cellular host responses in bronchoalveolar lavage fluids obtained from mice infected with pneumonia and treated with ceftriaxone, azithromycin, or both in combination. Although in vitro synergy was not observed, significant survival benefits were demonstrated with combination treatment. Lung neutrophil influx was significantly lower in the ceftriaxone-plus-azithromycin-treated group than in the ceftriaxone-treated group, whereas no differences in the lung bacterial burden were observed on day 3 between the ceftriaxone-plus-azithromycin-treated group and the ceftriaxone-treated group. Notably, the analysis of cell surface markers in the ceftriaxone-plus-azithromycin combination group exhibited upregulation of presumed immune checkpoint ligand CD86 and major histocompatibility complex class II in neutrophils and CD11b-positive CD11c-positive (CD11b+ CD11c+) macrophages and dendritic cells, as well as downregulation of immune checkpoint receptors cytotoxic-T lymphocyte-associated antigen 4 and programmed death 1 in T helper and T regulatory cells. Our data demonstrate that the survival benefits of ceftriaxone-plus-azithromycin therapy occur through modulation of immune checkpoints in mouse pneumococcal pneumonia. In addition, immune checkpoint molecules may be a novel target class for future macrolide research.
INTRODUCTION
Community-acquired pneumonia (CAP), a common but potentially serious illness, is associated with morbidity and mortality (1, 2). Streptococcus pneumoniae is commonly associated with CAP (2–4). However, despite recent progress in the molecular understanding of CAP pathogenesis, CAP remains a serious health concern (2).
The use of several antibiotics in CAP antimicrobial therapeutic regimens has been discussed and implemented. In particular, for hospitalized patients with S. pneumoniae-related CAP, combination therapy with β-lactams plus macrolides is the preferred choice in several guidelines (3–5). Although accumulating clinical evidence has shown the efficacy of combination therapies incorporating macrolides, the efficacy of combination therapies with quinolones or tetracyclines has not been demonstrated (6–9). A few available clinical studies have demonstrated contrasting results (2). No experimental evidence has shown the superiority of β-lactam-plus-macrolide combination therapy against lethal pneumococcal pneumonia. Thus, the efficacy of macrolide combination therapy is still controversial for severe CAP patients.
Macrolides are protein synthesis inhibitors that are active against various microorganisms, including Gram-positive cocci, anaerobic bacteria, and atypical pathogens. Certain macrolide antibiotics, such as erythromycin, clarithromycin, and azithromycin, have been reported to possess immunomodulatory potential beyond their direct antibacterial potential. The established efficacy of macrolides in patients with diffuse panbronchiolitis and cystic fibrosis, even in those infected or colonized with Pseudomonas aeruginosa, is the best illustration of such potential (10–12). Moreover, the application of macrolide therapy is being expanded to various diseases, such as chronic obstructive pulmonary disease and asthma (1, 8). Recently, immune checkpoint ligands (e.g., CD86 and major histocompatibility complex class II [MHC-II]) and receptors (e.g., cytotoxic-T lymphocyte-associated antigen 4 [CTLA-4] and programmed death 1 [PD-1]) have gained attention and have provided new insights into our understanding of the pathogenesis of various diseases, such as cancer (13, 14), autoimmune diseases (15), and sepsis. These factors are believed to be associated with an imbalance of inflammation and immune reactions, which may be responsible for determining severity and prognosis in several life-threatening conditions (16, 17). However, to our knowledge, no reports have examined the correlation between macrolide combination effects and immune checkpoint systems in S. pneumoniae pneumonia.
In this study, we evaluated the benefits of combination therapy using ceftriaxone, a β-lactam, and azithromycin in a mouse model of lethal pneumococcal pneumonia; these were characterized in terms of survival, bacterial burden, accumulation of inflammatory cells, and immune checkpoint ligands and receptors.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free 6- to 7-week-old female CBA/JN mice (Charles River Laboratories Japan, Inc., Yokohama, Japan) (18, 19) were housed under specific-pathogen-free conditions in the Laboratory Animal Research Center of Toho University School of Medicine until the day of sacrifice. All experiments were conducted according to our institution's ethical guidelines for animal experimentation. Animal protocols were performed with the approval of the Institutional Animal Care and Use Committee (approval number 14-52-220).
Bacteria.
We used a clinical isolate of Streptococcus pneumoniae strain 741 (serotype 19F) that was stocked in the Department of Microbiology and Infectious Diseases (Toho University School of Medicine, Tokyo, Japan). This strain of bacteria was used in our previous mouse pneumonia model (18).
Antimicrobial agents.
Ceftriaxone sodium hydrate was purchased from Chugai Pharmaceutical Co., Ltd. (Tokyo, Japan). Azithromycin hydrate was purchased from Pfizer Japan Inc. (Tokyo, Japan).
Antimicrobial susceptibility test and checkerboard assay.
An antimicrobial susceptibility test of ceftriaxone or azithromycin was determined by the broth microdilution method according to the recommendations of the Clinical and Laboratory Standards Institute (CLSI) (20). To investigate the combination effects of ceftriaxone and azithromycin, checkerboard assays were performed using a 96-well microplate with cation-adjusted Mueller-Hinton broth including 5% lysed horse blood that contained two antimicrobial agents in 2-fold dilutions dispensed in a checkerboard fashion. When the bacterial suspension was inoculated into the 96-well microplate, the final bacterial concentration was approximately 5 × 105 CFU/ml. We determined the MIC of each antimicrobial agent alone and in combination at 20 h after inoculation at 35°C. Then, we calculated the fractional inhibitory concentration index (FICI). The results were interpreted as follows: an FICI of ≤0.5 indicated synergy, an FICI of >0.5 and ≤4 indicated no interaction, and an FICI of >4 indicated antagonism (21). The S. pneumoniae strain ATCC 49619 was used as a quality control strain.
Experimental mouse model of lethal pneumococcal pneumonia.
S. pneumoniae strain 741 was incubated on Mueller-Hinton agar (Becton, Dickinson [BD] & Co., Sparks, MD, USA) supplemented with 5% defibrinated horse blood at 35°C for 14 h. The culture was then scraped from the agar and suspended in brain heart infusion broth (BD) that was supplemented with 0.5% yeast extract (BD) and cultured at 35°C for 5 h at log phase. CBA/JN mice were challenged with approximately 107 CFU of S. pneumoniae strain 741 (18). Mice were anesthetized intramuscularly with 50 mg/kg of body weight ketamine (Daiichi Sankyo Co., Ltd., Tokyo, Japan) and 10 mg/kg xylazine (Bayer Yakuhin, Ltd., Osaka, Japan). A 30-μl sample of bacterial suspension was administered intranasally.
Treatment protocol.
Mice infected with S. pneumoniae were randomly assigned to receive one of the following four treatments: ceftriaxone at 20 mg/kg (22) plus azithromycin at 50, 75, or 100 mg/kg; ceftriaxone at 20 mg/kg; azithromycin at 50, 75, or 100 mg/kg; and control (diluents, saline). A higher dose of azithromycin is required in mice than in humans because of their faster liver metabolism (23, 24), which results in a shorter half-life in mice. Therefore, azithromycin doses of 50, 75, and 100 mg/kg were chosen for the mouse survival study. Treatments were applied three times every 24 h beginning at 6 h after infection. Ceftriaxone was administered intraperitoneally. Azithromycin was administered subcutaneously.
Survival study.
Ten mice were included in each treatment group. Mouse survival was evaluated every 24 h for a total of 14 days after infection.
Bacteriological examination of the lung.
At day 1 and day 3 after infection, the right lungs (n = 5 for each group) were homogenized in 1 ml phosphate-buffered saline (PBS) containing a protease inhibitor cocktail (cOmplete; Roche Diagnostics GmbH, Mannheim, Germany) using a tissue homogenizer (IKA Japan K.K., Osaka, Japan). To count the bacterial burden, 10 μl of each homogenate was inoculated onto Mueller-Hinton agar that was supplemented with 5% defibrinated horse blood after serial 1:10 dilutions, followed by inoculation at 35°C for 24 h.
Bronchoalveolar lavage fluid analysis by flow cytometry.
At day 3 after infection, bronchoalveolar lavage was performed three times sequentially with 1 ml PBS each time. Leukocytes collected from the lavage fluid of each mouse were washed with the cells that were kept on ice. Leukocyte numbers were counted with a hemocytometer, and the frequencies of neutrophils and macrophages were analyzed by flow cytometry. Cell suspensions with stain buffer (PBS plus 2% bovine serum albumin) were incubated with an anti-Fc receptor-blocking antibody (purified anti-mouse CD16/32 antibody, clone 93) from BioLegend (San Diego, CA, USA) for 15 min on ice to reduce nonspecific antibody binding. Cells were then washed with stain buffer and surface stained for 30 min on ice using each experimental design combination of PerCP/Cy5.5 anti-mouse CD11b antibody (clone M1/70), FITC anti-mouse Ly6G antibody (clone 1A8), PE/Cy7 anti-mouse F4/80 antibody (clone BM8), PE rat IgG2a, κ isotype ctrl antibody (clone RTK2758), APC rat IgG2b, κ isotype ctrl antibody (clone RTK4530), APC/Cy7 anti-mouse CD3ε antibody (clone 145-2C11), PerCP anti-mouse CD4 antibody (clone RM4-5), FITC anti-mouse CD25 antibody (clone PC61), APC anti-mouse CD28 antibody (clone E18), PE anti-mouse CD152 (known as CTLA-4) antibody (clone UC10-4B9), APC mouse IgG2b, κ isotype ctrl antibody (clone MPC-11), PE Armenian hamster IgG isotype ctrl antibody (clone HTK888), and PE/Cy7 Armenian hamster IgG isotype ctrl antibody (clone HTK888) from BioLegend; APC/Cy7 anti-mouse CD11c (clone N418), PE anti-mouse CD86 (B7-2) (clone GL-1), and APC anti-mouse MHC class II (I-A/I-E) (clone M5/114.15.2) from Tonbo Biosciences (San Diego, CA, USA); or PE/Cy7 anti-mouse CD279 (PD-1) antibody (clone J43) from eBioscience, Inc. (San Diego, CA, USA). Isotype-matched controls and single-conjugate controls were always included. Cells were washed with stain buffer and fixed with 4% paraformaldehyde in PBS for 15 min. Cells were then washed and stored at 4°C until analysis by flow cytometry. Flow cytometry was performed on a BD FACSCanto II (BD Biosciences) and analyzed using FlowJo software (version 7.6.5; Tree Star, Ashland, OR, USA). Compensation was performed using cells stained with each labeled antibody individually.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism 5 software (GraphPad, Inc., La Jolla, CA, USA). Survival rates were presented using the Kaplan-Meier method, and survival analyses were tested by log-rank tests. Data are presented as means ± standard errors of the mean (SEM). Significance was tested by one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison post hoc test. A P value of <0.05 was considered statistically significant.
RESULTS
Effects of combinations of ceftriaxone and azithromycin using the in vitro checkerboard assay.
To determine whether ceftriaxone-plus-azithromycin combination therapy had synergistic effect, we examined 2 strains of S. pneumoniae (741 and ATCC 49619) in a checkerboard method as described in the Materials and Methods. As shown in Table 1, the MIC of azithromycin to strain 741 was 2 μg/ml (resistant according to the CLSI), whereas both strains were designated sensitive to ceftriaxone. From the checkerboard MIC data, the FICIs for strains 741 and ATCC 49619 were calculated as 1 and 0.75, respectively, which were defined as showing “no interaction.”
TABLE 1.
In vitro antimicrobial activities of ceftriaxone, azithromycin, or their combination against S. pneumoniae strains 741 and ATCC 49619
| Strain | MIC (μg/ml) |
FICIa | ||
|---|---|---|---|---|
| Ceftriaxone | Azithromycin | Ceftriaxone-azithromycin combination (ceftriaxone MIC/azithromycin MIC) | ||
| 741 | 0.5 | 2 | 0.25/1 | 1 |
| ATCC 49619b | 0.062 | 0.125 | 0.015/0.062 | 0.75 |
The results were interpreted as follows: FICI ≤ 0.5, synergy; 0.5 < FICI ≤ 4, no interaction; and FICI > 4, antagonism.
S. pneumoniae strain ATCC 49619 was used as a quality control strain.
Effects of combinations of ceftriaxone and azithromycin on the survival of mice with lethal pneumococcal pneumonia.
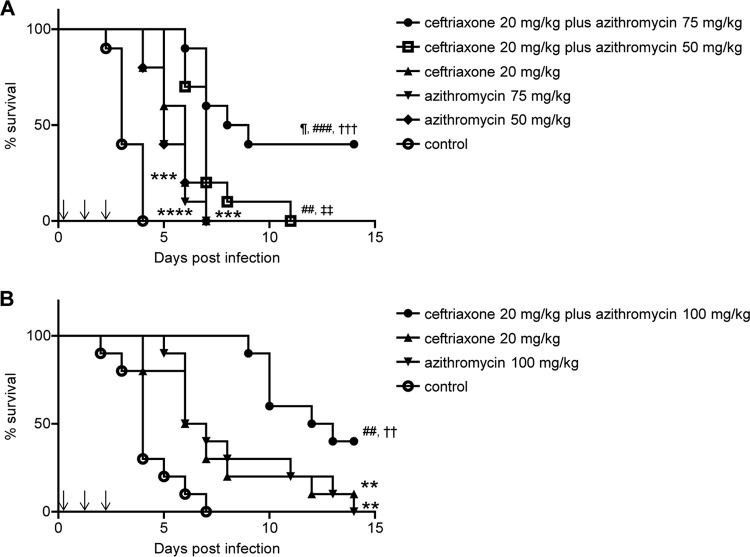
To investigate whether survival can be significantly improved by ceftriaxone-plus-azithromycin combination therapy, mouse survival was observed every 24 h for a total of 14 days after the intranasal bacterial challenge. Antibiotic treatments were started 6 h after infection and administered once a day for 3 days as described in the Materials and Methods. As shown in Fig. 1A, all control mice (but few in the treatment group) died by day 4. Single individual treatments with ceftriaxone at 20 mg/kg or azithromycin at 50 or 75 mg/kg significantly prolonged survival; however, no mice were alive after day 8 postinfection. Moreover, the survival of mice treated with a combination therapy of ceftriaxone at 20 mg/kg plus azithromycin at 50 or 75 mg/kg improved significantly compared with that of mice treated with monotherapy consisting of ceftriaxone at 20 mg/kg or azithromycin at 50 or 75 mg/kg. In particular, the survival of mice treated with a combination therapy of ceftriaxone at 20 mg/kg plus azithromycin at 75 mg/kg improved significantly compared with that of mice treated with a combination therapy of ceftriaxone at 20 mg/kg plus azithromycin at 50 mg/kg. In Fig. 1B, the results show that mice given 20 mg/kg ceftriaxone or 100 mg/kg azithromycin survived significantly longer than did control mice. Furthermore, mice given combination therapy with ceftriaxone at 20 mg/kg plus azithromycin at 100 mg/kg survived significantly longer than mice given monotherapy with ceftriaxone at 20 mg/kg or azithromycin at 100 mg/kg. Subsequently, we investigated how ceftriaxone-plus-azithromycin combination therapy worked in this mouse model, using ceftriaxone at 20 mg/kg, azithromycin at 75 mg/kg, or their combination.
FIG 1.
Effects of ceftriaxone-plus-azithromycin combination therapy on the survival of mice with pneumococcal pneumonia. Treatments (down arrows) were applied three times every 24 h beginning at 6 h after infection. Mouse survival was evaluated every 24 h for a total of 14 days after infection. (A) Mice infected by S. pneumoniae strain 741 were divided into six treatment groups (n = 10 each). The results are displayed as Kaplan-Meier curves and were compared using log-rank tests. ¶, P < 0.05 compared with the ceftriaxone 20 mg/kg plus azithromycin 50 mg/kg combination group; ###, P < 0.001 compared with the ceftriaxone 20 mg/kg group; ##, P < 0.01 compared with the ceftriaxone 20 mg/kg group; †††, P < 0.001 compared with the azithromycin 75 mg/kg group; ‡‡, P < 0.01 compared with the azithromycin 50 mg/kg group; ***, P < 0.001 compared with the control group; ****, P < 0.0001 compared with the control group. (B) Mice infected by S. pneumoniae strain 741 were divided into four treatment groups (n = 10 each). ##, P < 0.01 compared with the ceftriaxone 20 mg/kg group; ††, P < 0.01 compared with the azithromycin 100 mg/kg group; **, P < 0.01 compared with the control group.
Bacterial burden in the lungs of mice treated with ceftriaxone, azithromycin, or their combination.
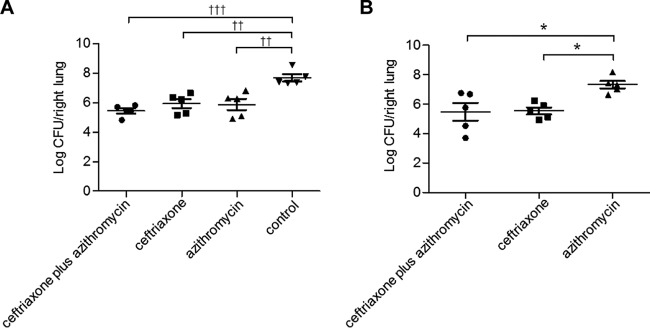
We examined the bacterial burden in the lungs of mice at day 1 and 3 after infection (Fig. 2). The three antibiotic treatment groups (ceftriaxone, azithromycin, and ceftriaxone plus azithromycin) had significantly reduced bacterial numbers in the lungs compared to that of the nontreatment group, although there were no differences among these three treatment groups on day 1 (Fig. 2A). On day 3, we observed a significant reduction in bacterial abundance in the lungs of ceftriaxone- and ceftriaxone-plus-azithromycin-treated mice compared to that of the azithromycin-treated group, whereas no difference was observed between the ceftriaxone and the ceftriaxone-plus-azithromycin groups (Fig. 2B).
FIG 2.
Effects of ceftriaxone-plus-azithromycin combination therapy on bacterial burden in the lungs of mice with pneumococcal pneumonia. The bacterial burden of the right lung was assessed among treatments of ceftriaxone plus azithromycin, ceftriaxone, azithromycin, or control at day 1 (A) and day 3 (B) after infection. Treatments were applied three times every 24 h beginning at 6 h after infection. Data are presented as means ± SEM (n = 5) and were statistically analyzed using one-way ANOVA followed by a Turkey's post hoc test. ††, P < 0.01 compared with treatment of the control group on day 1; †††, P < 0.001 compared with treatment of the control group on day 1; *, P < 0.05 compared with treatment of the azithromycin group on day 3.
Cell types and populations in bronchoalveolar lavage fluids of mice treated with ceftriaxone, azithromycin, or their combination.
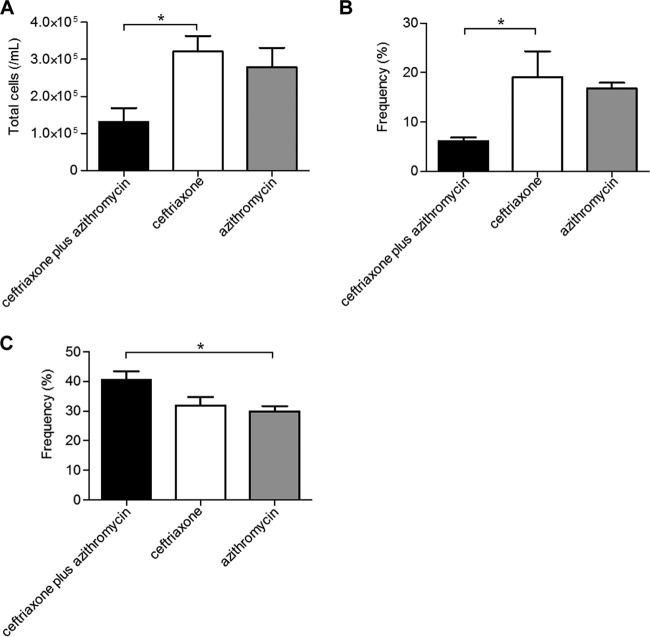
Next, we evaluated the cell types and populations in bronchoalveolar lavage fluids using a hemocytometer and flow cytometry at day 3 postinfection. Table 2 shows the specific antibodies used for the identification of neutrophils, macrophages, dendritic cells, T helper cells, and T regulatory cells as described previously (25–30). The ceftriaxone-plus-azithromycin-treated group demonstrated a lower number of total cells compared to the ceftriaxone- or azithromycin-treated groups, although statistical significance was only observed between the ceftriaxone and combination groups (Fig. 3A). A lower frequency of neutrophils was demonstrated in the ceftriaxone-plus-azithromycin combination group, although, as before, a significant difference was only observed between the ceftriaxone-treated and combination-treated groups (Fig. 3B). In contrast, a higher frequency of macrophages was demonstrated in the ceftriaxone-plus-azithromycin-treated group, although a significant difference was only observed between the azithromycin-treated group and the combination-treated groups (Fig. 3C).
TABLE 2.
Identification of specific cell types from bronchoalveolar lavage fluids
| Cell types | Properties |
|---|---|
| Neutrophils | F4/80− CD11b+ Ly6G+ |
| Macrophages | F4/80+ CD11c+ |
| CD11b+ CD11c+ | F4/80+ CD11b+ CD11c+ |
| CD11b− CD11c+ (alveolar macrophages) | F4/80+ CD11b− CD11c+ |
| Dendritic cells | |
| CD11b+ CD11c+ | F4/80− Ly6G− CD11b+ CD11c+ |
| CD11b− CD11c+ | F4/80− Ly6G− CD11b− CD11c+ |
| T helper cells | CD3+ CD4+ |
| T regulatory cells | CD3+ CD4+ CD25+ |
FIG 3.
Effects of ceftriaxone-plus-azithromycin combination therapy on the influx of inflammatory cells into the lungs of mice with pneumococcal pneumonia. Total cells (A) and the frequency of neutrophils (B) and macrophages (C) were evaluated in bronchoalveolar lavage fluids using a hemocytometer and flow cytometer at day 3 postinfection after three applications of each treatment. Data are presented as means ± SEM (n = 4), which were calculated by combining data from three independent experiments, and were statistically analyzed using one-way ANOVA followed by a Turkey's post hoc test. *, P < 0.05 compared with treatment of the ceftriaxone plus azithromycin group.
Effects of antibiotic treatments on CD86 and MHC class II expression on the surfaces of inflammatory cells accumulated in the bronchoalveolar lavage fluids of mice with pneumonia.
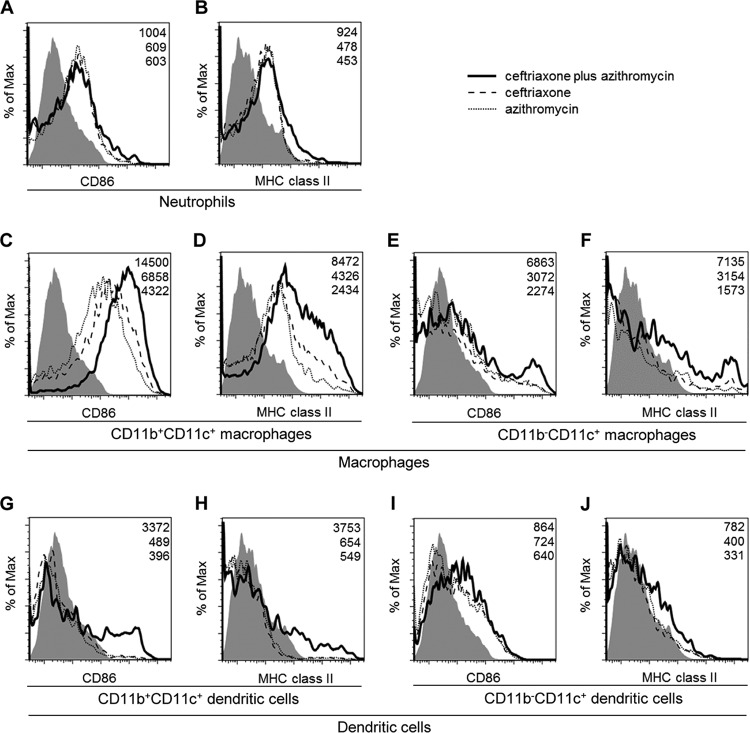
To investigate the impacts of the antibiotic treatments on the activation of inflammatory cells, such as neutrophils, macrophages, and dendritic cells, we examined the expression of the cell surface markers, CD86 and MHC class II, by flow cytometry. In neutrophils, slightly stronger expression levels of CD86 and MHC class II were shown in the ceftriaxone-plus-azithromycin-treated groups compared to those observed in the ceftriaxone- or azithromycin-treated groups, although there were no differences in the neutrophil expression of these factors between ceftriaxone- and azithromycin-treated mice (Fig. 4A and B). In contrast, when mice were treated with combination therapy, clearer upregulation of the expression levels of CD86 and MHC class II was demonstrated in macrophages (Fig. 4C to F) and in dendritic cells (Fig. 4G to J), especially for CD11b-positive CD11c-positive (CD11b+ CD11c+)-type cells (Fig. 4C, D, G, and H). These data demonstrated that the upregulated expression of CD86 and MHC class II in neutrophils, macrophages, and dendritic cells was apparent in the combination treatment group.
FIG 4.
Effects of ceftriaxone-plus-azithromycin combination therapy on the cell surface markers CD86 and major histocompatibility complex (MHC) class II on neutrophils, macrophages, and dendritic cells in mice with pneumococcal pneumonia. We performed bronchoalveolar lavages at day 3 postinfection after three applications of each treatment of ceftriaxone plus azithromycin, ceftriaxone, or azithromycin (n = 1 each). CD86 (A, C, E, G, and I) and MHC class II expression (B, D, F, H, and J) on neutrophils (A and B), CD11b+ CD11c+ macrophages (C and D), CD11b− CD11c+ macrophages (E and F), CD11b+ CD11c+ dendritic cells (G and H), and CD11b− CD11c+ dendritic cells (I and J) were determined by flow cytometry. Filled histograms show the respective isotype controls. Values in the upper right corner of each graph represent the specific mean fluorescence intensity (MFI) of treatments of ceftriaxone plus azithromycin (top), ceftriaxone (middle), or azithromycin (bottom). Data are representative of three independent experiments with similar results.
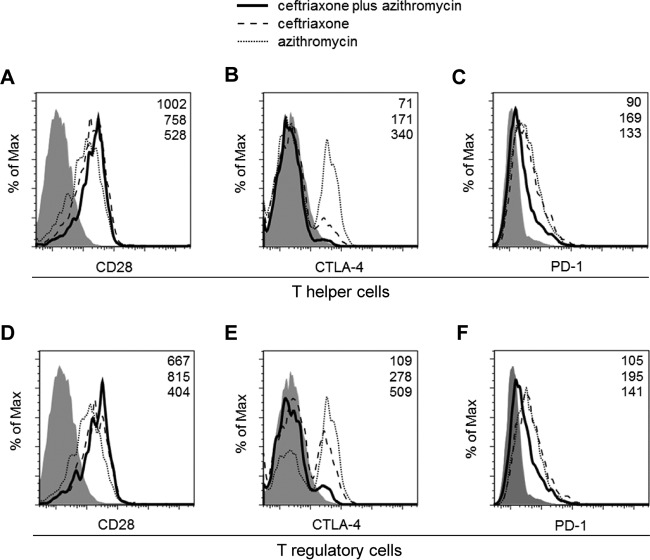
Effects of antibiotic treatments on the cell surface expression of immune checkpoint receptors on T helper and T regulatory cells.
To further examine the mechanisms of ceftriaxone-plus-azithromycin combination effects, we determined the expression of several immune checkpoint receptors, such as CD28, CTLA-4, and PD-1, on CD4-positive (CD4+) T helper cells and CD4+ CD25+ T regulatory cells. For CD28 expression, a slight increase in expression was observed in CD4+ T helper cells, but not in CD4+ CD25+ T regulatory cells, when mice were treated with a ceftriaxone-plus-azithromycin combination (Fig. 5A and D). In contrast, suppressed expression of CTLA-4 and PD-1 was seen in both cell-types from the mice treated with the ceftriaxone-plus-azithromycin combination (Fig. 5B, C, E, and F). Notably, the reduction of expression of CTLA-4 in the ceftriaxone-plus-azithromycin combination group was prominent, with less than 50% of the ceftriaxone monotherapy group expression levels observed in CD4+ T helper cells and CD4+ CD25+ T regulatory cells (Fig. 5B and E).
FIG 5.
Effects of ceftriaxone-plus-azithromycin combination therapy on the cell surface markers cytotoxic-T lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) on CD4+ T helper cells and CD4+ CD25+ T regulatory cells in mice with pneumococcal pneumonia. We performed bronchoalveolar lavages at day 3 postinfection after three treatment applications of ceftriaxone plus azithromycin, ceftriaxone, or azithromycin (n = 1 each). CD28 (A and D), CTLA-4 (B and E), and PD-1 (C and F) expression on CD4+ T helper cells (A, B, and C) and CD4+ CD25+ T regulatory cells (D, E, and F) were determined by flow cytometry. Filled histograms show the respective isotype controls. Values in the upper right corner of each graph represent the specific mean fluorescence intensity (MFI) of treatments of ceftriaxone plus azithromycin (top), ceftriaxone (middle), or azithromycin (bottom). Data are representative of three independent experiments with similar results.
DISCUSSION
The results of this study, to our knowledge, have shown for the first time a significant survival benefit of combination therapy using ceftriaxone plus azithromycin in a lethal model of pneumococcal pneumonia in immunocompetent mice.
Prior to performing animal model experiments, we examined the in vitro synergy effects of combination therapy in 2 strains of S. pneumoniae using the checkerboard method. As mentioned above, the results were defined as indicating “no interaction” in both strains. These results were well correlated with previous reports, in which the checkerboard method was applied along with time-kill analyses in multiple strains of S. pneumoniae with different susceptibilities to macrolides and β-lactams (31, 32). Consistent with our in vitro data, in the in vivo model, there were no significant differences in bacterial burdens in the lungs of mice on day 1 (ceftriaxone, azithromycin, and combination) and on day 3 (ceftriaxone and combination). These findings suggest that direct antimicrobial synergy in the combination group may not account for the survival benefit in the ceftriaxone-plus-azithromycin group. The present data suggest that the efficacy of combination therapy might involve modulation of the host immune/inflammation systems.
Despite their being no statistical difference in the lung bacterial burdens at the designated time points, significant differences were found in some of the examined markers, such as cell accumulation. Total cell numbers (especially neutrophils but not macrophages) were significantly lower in the ceftriaxone-plus-azithromycin combination group. These data suggest that ceftriaxone-plus-azithromycin may suppress robust, potentially excessive host inflammatory responses, which may in turn be associated with the improved survival observed following macrolide combination therapy.
In contrast to the inhibitory actions of combination therapy on the influx of neutrophils, an upregulation of CD86 and MHC class II molecules (i.e., presumed immune checkpoint ligands) was consistently observed in neutrophils, macrophages, and dendritic cells. Although upregulation of these markers was observed in several types of macrophages and dendritic cells (CD11b+ CD11c+, CD11b-negative CD11c-positive [CD11b− CD11c+]), the CD11b+ CD11c+ cell subset was shown to be the most susceptible to the effects of combination therapy under our experimental conditions. In the literature, mounting evidence has demonstrated the anti-infective and immunomodulatory roles of CD11b/CD11c-positive cells under a variety of conditions. Kirby et al. (33) reported an increase of a distinct subset of CD11bhi alveolar macrophages after pneumococcal challenge to the lungs. Furthermore, Poole and colleagues (34) demonstrated that CD11b+ CD11c+ macrophages in the lungs play a critical role in downregulating the inflammatory responses to organic dust extract. In addition, CD11b+ Ly6Chi F4/80+ macrophages expanded by galectin-9, a β-galactoside-binding lectin, were reported to possess immunosuppressive characteristics to ameliorate T-cell-mediated lung inflammation (35). CD11b+ CD11c+ MHC-II+ dendritic cells were also shown to be essential for the maintenance of inducible bronchus-associated lymphoid tissue, which supported T and B cell proliferation during the immune response in a mouse model of influenza infection (36). CCR2-mediated CD11b+ CD11c+ dendritic cells in the lungs were found to be important for the clearance of Cryptococcus neoformans (37).
In the present study, a lethal mouse model of pneumococcal pneumonia, which was likely complicated with bacteremia (19) and sepsis at the end stage of infection, was applied to evaluate the effects of ceftriaxone-plus-azithromycin combination therapy. The previous clinical data demonstrated that macrolide combination effects were more prominent in more severe cases of pneumonia (38, 39). Accordingly, the question of how macrolides affect and modulate sepsis and sepsis-related host responses reflects another important issue for investigation. Nearly four decades of investigating anti-inflammation strategies against sepsis, based on the concept that excessive inflammation might be the main cause for the adverse outcomes of sepsis, have yielded disappointing results. However, there is now evidence that the immunosuppressive state involves a misbalance between proinflammatory reactions and anti-inflammatory responses following the initial hyperinflammation state in sepsis, which may contribute to the high mortality of sepsis (16, 17, 40). Among several target molecules that have been proposed to overcome the immunosuppressive state in sepsis patients, most attention has been focused on immune checkpoint receptors such as PD-1 and CTLA-4 as promising molecules, based on several clinical and experimental findings.
In the present study, we observed downregulation of PD-1 and CTLA-4 but not of CD28 in T helper and T regulatory cells in the setting of ceftriaxone-plus-azithromycin combination therapy. This is the first report describing the effects of a macrolide on the coinhibitory molecules PD-1 and CTLA-4 in S. pneumoniae pneumonia. Risso et al. (41) reported the overexpression of CTLA-4 on CD4 T cells in bronchoalveolar lavage fluids from patients with infectious acute respiratory distress syndrome. Guignant and colleagues (42), in a prospective and observational study including 64 patients with septic shock, reported that PD-1 levels were correlated with increased mortality and immune dysfunctions in these patients. Brahmamdam et al. (43), using a novel therapeutic strategy, reported that an anti-PD-1 antibody administered 24 h after cecal ligation and puncture prevented the sepsis-induced depletion of lymphocytes and dendritic cells and improved survival. More recently, Chang and collaborators (40) reported that the blockade of PD-1 and CTLA-4 by the respective antibodies improved survival in a Candida-challenged sepsis mouse model. Ceftriaxone-plus-azithromycin combination therapy may involve an optimal modulation of CTLA-4 and PD-1 expression to restore immune functions and contribute to enhanced survival in a mouse model of lethal pneumococcal pneumonia.
Although the present data demonstrated significant survival benefits from combination therapy without the alteration of underlying bacterial burdens, it was difficult to clearly separate the macrolide combination effects on the host defense systems and the modulation of bacterial virulence without viability. Previous reports have demonstrated that macrolides suppress the virulence factors of S. pneumoniae, such as pneumolysin, regardless of the bacterial number (44). This reflects a limitation of animal infection models when applying live bacteria. Further studies, including virulence-factor-specific host responses and macrolide effects on immune checkpoint molecules, will be required to clarify the mechanisms of macrolide actions and to develop future strategies to treat life-threatening S. pneumoniae infections such as pneumonia and sepsis.
In conclusion, we provide the first evidence that the survival benefits of ceftriaxone-plus-azithromycin combination therapy occur through the modulation of the immune checkpoints in a mouse model of lethal pneumococcal pneumonia. The efficacy of combination therapy may involve not only modulation of host inflammation systems but also immune systems, such as the immune checkpoints. In addition, immune checkpoint molecules on inflammatory/immunological cells may serve as a novel potential target class for future macrolide research.
ACKNOWLEDGMENTS
The authors received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors in support of this work.
D.Y., J.K., and K.T. conceived and designed the study; D.Y and C.K. performed the experiments; D.Y., C.K., Y.I, K.U., K.H., J.K., and K.T. analyzed and interpreted the data; D.Y., J.K., and K.T. drafted the manuscript for important intellectual content; and D.Y., C.K., Y.I, K.U., K.H., J.K., and K.T. contributed to the final approval of the version submitted for publication.
We declare no conflict of interest.
REFERENCES
- 1.Kovaleva A, Remmelts HH, Rijkers GT, Hoepelman AI, Biesma DH, Oosterheert JJ. 2012. Immunomodulatory effects of macrolides during community-acquired pneumonia: a literature review. J Antimicrob Chemother 67:530–540. doi: 10.1093/jac/dkr520. [DOI] [PubMed] [Google Scholar]
- 2.Caballero J, Rello J. 2011. Combination antibiotic therapy for community-acquired pneumonia. Ann Intensive Care 1:48. doi: 10.1186/2110-5820-1-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG. 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44(Suppl):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyashita N, Matsushima T, Oka M, Japanese Respiratory Society. 2006. The JRS guidelines for the management of community-acquired pneumonia in adults: an update and new recommendations. Intern Med 45:419–428. doi: 10.2169/internalmedicine.45.1691. [DOI] [PubMed] [Google Scholar]
- 5.Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, Ortqvist A, Schaberg T, Torres A, van der Heijden G, Read R, Verheij TJ. 2011. Guidelines for the management of adult lower respiratory tract infections—summary. Clin Microbiol Infect 17(Suppl):S1–S24. http://dx.doi.org/10.1111/j.1469-0691.2011.03602.x. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigo C, McKeever TM, Woodhead M, Lim WS. 2013. Single versus combination antibiotic therapy in adults hospitalised with community acquired pneumonia. Thorax 68:493–495. doi: 10.1136/thoraxjnl-2012-202296. [DOI] [PubMed] [Google Scholar]
- 7.Metersky ML, Ma A, Houck PM, Bratzler DW. 2007. Antibiotics for bacteremic pneumonia: improved outcomes with macrolides but not fluoroquinolones. Chest 131:466–473. doi: 10.1378/chest.06-1426. [DOI] [PubMed] [Google Scholar]
- 8.Restrepo MI, Mortensen EM, Waterer GW, Wunderink RG, Coalson JJ, Anzueto A. 2009. Impact of macrolide therapy on mortality for patients with severe sepsis due to pneumonia. Eur Respir J 33:153–159. doi: 10.1183/09031936.00054108. [DOI] [PubMed] [Google Scholar]
- 9.Martin-Loeches I, Lisboa T, Rodriguez A, Putensen C, Annane D, Garnacho-Montero J, Restrepo MI, Rello J. 2010. Combination antibiotic therapy with macrolides improves survival in intubated patients with community-acquired pneumonia. Intensive Care Med 36:612–620. doi: 10.1007/s00134-009-1730-y. [DOI] [PubMed] [Google Scholar]
- 10.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, Coquillette S, Fieberg AY, Accurso FJ, Campbell PW III. 2003. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 290:1749–1756. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 11.Hansen CR, Pressler T, Koch C, Høiby N. 2005. Long-term azitromycin treatment of cystic fibrosis patients with chronic Pseudomonas aeruginosa infection; an observational cohort study. J Cyst Fibros 4:35–40. doi: 10.1016/j.jcf.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Fujii T, Kadota J, Kawakami K, Iida K, Shirai R, Kaseda M, Kawamoto S, Kohno S. 1995. Long term effect of erythromycin therapy in patients with chronic Pseudomonas aeruginosa infection. Thorax 50:1246–1252. doi: 10.1136/thx.50.12.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma P, Allison JP. 2015. The future of immune checkpoint therapy. Science 348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 14.Weber J. 2010. Immune checkpoint proteins: a new therapeutic paradigm for cancer–preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol 37:430–439. doi: 10.1053/j.seminoncol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Ceeraz S, Nowak EC, Noelle RJ. 2013. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol 34:556–563. doi: 10.1016/j.it.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frazier WJ, Hall MW. 2008. Immunoparalysis and adverse outcomes from critical illness. Pediatr Clin North Am 55:647–668. doi: 10.1016/j.pcl.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anas AA, Wiersinga WJ, de Vos AF, van der Poll T. 2010. Recent insights into the pathogenesis of bacterial sepsis. Neth J Med 68:147–152. [PubMed] [Google Scholar]
- 18.Tateda K, Takashima K, Miyazaki H, Matsumoto T, Hatori T, Yamaguchi K. 1996. Noncompromised penicillin-resistant pneumococcal pneumonia CBA/J mouse model and comparative efficacies of antibiotics in this model. Antimicrob Agents Chemother 40:1520–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takashima K, Tateda K, Matsumoto T, IIzawa Y, Nakao M, Yamaguchi K. 1997. Role of tumor necrosis factor alpha in pathogenesis of pneumococcal pneumonia in mice. Infect Immun 65:257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically—9th ed. CLSI document M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 21.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 22.Wang E, Bergeron Y, Bergeron MG. 2005. Ceftriaxone pharmacokinetics in interleukin-10-treated murine pneumococcal pneumonia. J Antimicrob Chemother 55:721–726. doi: 10.1093/jac/dki085. [DOI] [PubMed] [Google Scholar]
- 23.Conte JE Jr, Golden J, Duncan S, McKenna E, Lin E, Zurlinden E. 1996. Single-dose intrapulmonary pharmacokinetics of azithromycin, clarithromycin, ciprofloxacin, and cefuroxime in volunteer subjects. Antimicrob Agents Chemother 40:1617–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann N, Lee B, Hentzer M, Rasmussen TB, Song Z, Johansen HK, Givskov M, Høiby N. 2007. Azithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates chronic P. aeruginosa lung infection in Cftr(−/−) mice. Antimicrob Agents Chemother 51:3677–3687. doi: 10.1128/AAC.01011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaynagetdinov R, Sherrill TP, Kendall PL, Segal BH, Weller KP, Tighe RM, Blackwell TS. 2013. Identification of myeloid cell subsets in murine lungs using flow cytometry. Am J Respir Cell Mol Biol 49:180–189. doi: 10.1165/rcmb.2012-0366MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall JD, Woolard MD, Gunn BM, Craven RR, Taft-Benz S, Frelinger JA, Kawula TH. 2008. Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infect Immun 76:5843–5852. doi: 10.1128/IAI.01176-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. 2008. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol 83:64–70. [DOI] [PubMed] [Google Scholar]
- 28.Condon TV, Sawyer RT, Fenton MJ, Riches DW. 2011. Lung dendritic cells at the innate-adaptive immune interface. J Leukoc Biol 90:883–895. doi: 10.1189/jlb.0311134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catalfamo M, Tai X, Karpova T, McNally J, Henkart PA. 2008. TcR-induced regulated secretion leads to surface expression of CTLA-4 in CD4+ CD25+ T cells. Immunology 125:70–79. doi: 10.1111/j.1365-2567.2008.02822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y, Schneider H, Rudd CE. 2012. Murine regulatory T cells differ from conventional T cells in resisting the CTLA-4 reversal of TCR stop-signal. Blood 120:4560–4570. doi: 10.1182/blood-2012-04-421420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drago L, Nicola L, Rodighiero V, Larosa M, Mattina R, De Vecchi E. 2011. Comparative evaluation of synergy of combinations of beta-lactams with fluoroquinolones or a macrolide in Streptococcus pneumoniae. J Antimicrob Chemother 66:845–849. doi: 10.1093/jac/dkr016. [DOI] [PubMed] [Google Scholar]
- 32.Lin E, Stanek RJ, Mufson MA. 2003. Lack of synergy of erythromycin combined with penicillin or cefotaxime against Streptococcus pneumoniae in vitro. Antimicrob Agents Chemother 47:1151–1153. doi: 10.1128/AAC.47.3.1151-1153.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirby AC, Raynes JG, Kaye PM. 2006. CD11b regulates recruitment of alveolar macrophages but not pulmonary dendritic cells after pneumococcal challenge. J Infect Dis 193:205–213. doi: 10.1086/498874. [DOI] [PubMed] [Google Scholar]
- 34.Poole JA, Gleason AM, Bauer C, West WW, Alexis N, van Rooijen N, Reynolds SJ, Romberger DJ, Kielian TL. 2012. CD11c+/CD11b+ cells are critical for organic dust-elicited murine lung inflammation. Am J Respir Cell Mol Biol 47:652–659. doi: 10.1165/rcmb.2012-0095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arikawa T, Saita N, Oomizu S, Ueno M, Matsukawa A, Katoh S, Kojima K, Nagahara K, Miyake M, Yamauchi A, Kohrogi H, Hirashima M. 2010. Galectin-9 expands immunosuppressive macrophages to ameliorate T-cell-mediated lung inflammation. Eur J Immunol 40:548–558. doi: 10.1002/eji.200939886. [DOI] [PubMed] [Google Scholar]
- 36.GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D, Osterhaus AD, Hendriks R, Rimmelzwaan GF, Lambrecht BN. 2009. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med 206:2339–2349. doi: 10.1084/jem.20090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osterholzer JJ, Curtis JL, Polak T, Ames T, Chen GH, McDonald R, Huffnagle GB, Toews GB. 2008. CCR2 mediates conventional dendritic cell recruitment and formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. J Immunol 181:610–620. doi: 10.4049/jimmunol.181.1.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nie W, Li B, Xiu Q. 2014. Beta-lactam/macrolide dual therapy versus beta-lactam monotherapy for the treatment of community-acquired pneumonia in adults: a systematic review and meta-analysis. J Antimicrob Chemother 69:1441–1446. doi: 10.1093/jac/dku033. [DOI] [PubMed] [Google Scholar]
- 39.Lodise TP, Kwa A, Cosler L, Gupta R, Smith RP. 2007. Comparison of beta-lactam and macrolide combination therapy versus fluoroquinolone monotherapy in hospitalized Veterans Affairs patients with community-acquired pneumonia. Antimicrob Agents Chemother 51:3977–3982. doi: 10.1128/AAC.00006-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang KC, Burnham CA, Compton SM, Rasche DP, Mazuski RJ, McDonough JS, Unsinger J, Korman AJ, Green JM, Hotchkiss RS. 2013. Blockade of the negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit Care 17:R85. doi: 10.1186/cc12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Risso K, Kumar G, Ticchioni M, Sanfiorenzo C, Dellamonica J, Guillouet-de Salvador F, Bernardin G, Marquette CH, Roger PM. 2015. Early infectious acute respiratory distress syndrome is characterized by activation and proliferation of alveolar T-cells. Eur J Clin Microbiol Infect Dis 34:1111–1118. doi: 10.1007/s10096-015-2333-x. [DOI] [PubMed] [Google Scholar]
- 42.Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F, Malcus C, Cheron A, Allaouchiche B, Gueyffier F, Ayala A, Monneret G, Venet F. 2011. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care 15:R99. doi: 10.1186/cc10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. 2010. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol 88:233–240. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fukuda Y, Yanagihara K, Higashiyama Y, Miyazaki Y, Hirakata Y, Mukae H, Tomono K, Mizuta Y, Tsukamoto K, Kohno S. 2006. Effects of macrolides on pneumolysin of macrolide-resistant Streptococcus pneumoniae. Eur Respir J 27:1020–1025. [DOI] [PubMed] [Google Scholar]