Abstract
Drinking water has rarely been recognized as a source of antimicrobial resistance for humans, and only in low-income countries. Here, a sequence type 48 Escherichia coli isolate carrying the blaCTX-M-1 IncI1/ST3 plasmid was recovered from drinking water in France. This plasmid was similar to other blaCTX-M-1 IncI1/ST3 plasmids found previously in animals and humans. Our findings highlight the possible human transfer of extended-spectrum β-lactamase (ESBL) genes through drinking water in high-income countries.
TEXT
Extended-spectrum β-lactamases (ESBLs) are widespread enzymes that confer resistance to broad-spectrum cephalosporins. ESBL genes are mostly located on plasmids, which play key roles in horizontal transfer of the genes. Consumption of ESBL-contaminated foodstuff or contacts with ESBL-colonized/infected animals enhance the risk of human transfer of ESBL genes from nonhuman sources. ESBLs have also been found in soils and rivers polluted by human or animal waste, such as hospital sewage or runoff from land occupied by livestock. Drinking water is also a source of human contamination with waterborne pathogens and/or antimicrobial-resistant bacteria. However, reports of drinking water contaminated with ESBL-producing organisms are scarce (1–3).
Here, we describe an ESBL-positive Escherichia coli isolate (isolate 32420) recovered in 2011 from a 100-ml water sample collected after treatment from an old (>30-year-old) water supply located at the periphery of a city in northeastern France. Drinking water samples were collected from 28 water supplies throughout France between December 2011 and April 2012. Water supplies were selected on the basis of repeated quality failures, i.e., detection of coliforms, over the previous 3 years. Twenty-six supplies were small, serving fewer than 2,000 people, whereas two served around 10,000 people. Water treatment before distribution included disinfection with chlorine in the smallest water supplies or sand filtration, coagulation with aluminum, and a final chlorine dioxide treatment in larger supplies collecting water from rivers.
One-liter water samples were collected in sterile bottles containing sodium thiosulfate, at the point at which drinking water enters the distribution system or at a point of consumption. Samples were preserved in ice boxes and transported to the ANSES Nancy laboratory, where 100 ml was analyzed by membrane filtration and growth on selective media (4) to determine the presence of coliforms. E. coli isolates (n = 67) (confirmed by matrix-assisted laser desorption ionization–time of flight mass spectrometry [MALDI-TOF MS]) were detected in all samples, with counts ranging from 1 to 45 CFU/100 ml. None of the isolates was considered virulent, since stx1, stx2, and eae genes were absent. All isolates were tested for antimicrobial susceptibility to 32 antibiotics by disk diffusion, according to the Antibiogram Committee of the French Society for Microbiology guidelines (www.sfm-microbiologie.org), and 6 E. coli isolates from 6 different water supplies were determined to be resistant to at least one antimicrobial (Table 1). Among those isolates, one E. coli isolate that was resistant to ceftiofur, tetracyclines, and sulfonamides was studied further.
TABLE 1.
Antimicrobial-resistant E. coli isolates from the 28 water supplies
| Water supply system | Antibiotic resistance patterna |
|---|---|
| WS1 | AMX |
| WS3 | STR, TET, SUL |
| WS6 | STR, TET, NAL |
| WS7 | AMX, AMC, CEF, TET, SUL |
| WS13 | SUL |
| WS20 | ESBL phenotype, TET, SUL |
AMX, amoxicillin; AMC, amoxicillin-clavulanic acid; CEF, cephalothin; STR, streptomycin; TET, tetracyclines; SUL, sulfonamides; NAL, nalidixic acid.
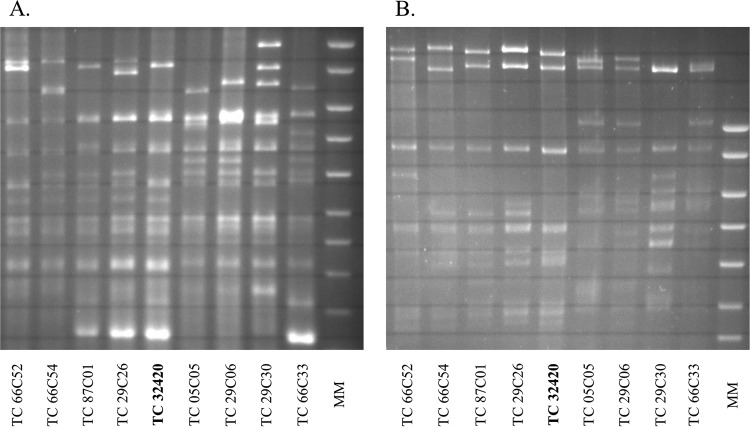
PCR and sequencing showed that E. coli isolate 32420 harbored the blaCTX-M-1 gene. Transferability of the ESBL-carrying plasmid was proved, since CTX-M-1-positive transconjugants presenting no additional resistance to non-β-lactam antibiotics were obtained after broth mating on Mueller-Hinton agar supplemented with rifampin (250 μg/ml) and cefotaxime (4 μg/ml). The blaCTX-M-1 gene was carried by a 100-kb IncI1/ST3 plasmid, as detected with S1 nuclease treatment of DNA followed by pulsed-field gel electrophoresis (PFGE), Southern blot hybridizations with adequate digoxigenin (DIG)-labeled probes (Roche Applied Science, Mannheim, Germany), PCR-based replicon typing (Diatheva, Fano, Italy), and plasmid multilocus sequence typing (MLST) (5). The restriction fragment length polymorphism (RFLP) profile of the IncI1/ST3 plasmid after digestion with PstI or EcoRI was comparable but nonidentical to those of previously published blaCTX-M-1 IncI/ST3 plasmids of animal and human origin (Fig. 1) (6). According to the protocol described on the MLST website (http://mlst.warwick.ac.uk/mlst/dbs/Ecoli), E. coli isolate 32420 was of sequence type 48 (ST48) (clonal complex 10 [CC10]), an ST that has been observed previously in both humans and animals (7, 8).
FIG 1.
RFLP analysis of E. coli plasmid DNA digested with PstI (A) or EcoRI (B). The E. coli isolated from drinking water (isolate 32420) was compared with E. coli plasmids originating from humans (6). MM, molecular mass markers.
Drinking water is a well-recognized source of direct human contamination with waterborne pathogens, but the risk of human transfer of resistance genes has rarely been documented. ESBL producers were detected in packaged drinking water bags sold by street vendors in Africa (9), in a public water supply in Bangladesh (3), and in drinking water in urban Nepal (1). In addition, NDM-1 carbapenemases were identified in tap water samples in India (2). However, these reports all came from countries with poor water supply systems and/or sanitation facilities, coupled to limited controls of antibiotic usage and residues. To the best of our knowledge, this is the first report of an ESBL producer in drinking water in a high-income country. Interestingly, isolate 32420 harbored a blaCTX-M-1 IncI1/ST3 plasmid, which was widely found in E. coli in food animals in France and neighboring countries (10, 11). Cattle farms are known to be located upstream of the sampling point for the old water supply, which collected water directly from the river. No absolute signature of the origin of ESBL plasmids exists, since such blaCTX-M-1 IncI1/ST3 plasmids are also sporadically found in humans (6), but an animal/environmental origin of this blaCTX-M-1 IncI1/ST3 plasmid is highly plausible.
E. coli and enterococci are used as indicators of fecal contamination in drinking water and should be totally absent from water, according to European legislation (12). In France, almost 3.3% of the population was served at least once in 2012 with drinking water that did not meet this criterion; 94% of the unsuitable drinking water involved small water supplies serving fewer than 2,000 people (13). Efforts are made continuously to improve water treatment and, as an example, the water supply from which the ESBL-producing E. coli strain was isolated has recently been fully renovated. Nevertheless, further investigations along the water distribution pipelines, including the use of ESBL-selective media, would make sense, as distribution systems exhibit favorable conditions for the formation of biofilms, which may hide internal reservoirs of ESBL producers. Consequently, although the isolate described here would probably not have been pathogenic to humans, our findings highlight the extent to which the growing enrichment of the environmental reservoir with ESBL genes may allow their colonization of remote niches, including in high-income countries.
ACKNOWLEDGMENTS
This work was supported by the Agency for Food, Environmental and Occupational Health and Safety.
We have no conflicts of interest to declare.
REFERENCES
- 1.Bhatta DR, Bangtrakulnonth A, Tishyadhigama P, Saroj SD, Bandekar JR, Hendriksen RS, Kapadnis BP. 2007. Serotyping, PCR, phage-typing and antibiotic sensitivity testing of Salmonella serovars isolated from urban drinking water supply systems of Nepal. Lett Appl Microbiol 44:588–594. doi: 10.1111/j.1472-765X.2007.02133.x. [DOI] [PubMed] [Google Scholar]
- 2.Rathinasabapathi P, Hiremath DS, Arunraj R, Parani M. 2015. Molecular detection of New Delhi metallo-beta-lactamase-1 (NDM-1) positive bacteria from environmental and drinking water samples by loop mediated isothermal amplification of blaNDM-1. Indian J Microbiol 55:400–405. doi: 10.1007/s12088-015-0540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talukdar PK, Rahman M, Rahman M, Nabi A, Islam Z, Hoque MM, Endtz HP, Islam MA. 2013. Antimicrobial resistance, virulence factors and genetic diversity of Escherichia coli isolates from household water supply in Dhaka, Bangladesh. PLoS One 8:e61090. doi: 10.1371/journal.pone.0061090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Organization for Standardization. 2000. Water quality: detection and enumeration of Escherichia coli and coliform bacteria. Part 1. Membrane filtration method. Method ISO 9308-1:2000. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 5.Garcia-Fernandez A, Chiaretto G, Bertini A, Villa L, Fortini D, Ricci A, Carattoli A. 2008. Multilocus sequence typing of IncI1 plasmids carrying extended-spectrum β-lactamases in Escherichia coli and Salmonella of human and animal origin. J Antimicrob Chemother 61:1229–1233. doi: 10.1093/jac/dkn131. [DOI] [PubMed] [Google Scholar]
- 6.Madec JY, Haenni M, Metayer V, Saras E, Nicolas-Chanoine MH. 2015. High prevalence of the animal-associated blaCTX-M-1 IncI1/ST3 plasmid in human Escherichia coli isolates. Antimicrob Agents Chemother 59:5860–5860. doi: 10.1128/AAC.00819-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smet A, Martel A, Persoons D, Dewulf J, Heyndrickx M, Claeys G, Lontie M, Van Meensel B, Herman L, Haesebrouck F, Butaye P. 2010. Characterization of extended-spectrum β-lactamases produced by Escherichia coli isolated from hospitalized and nonhospitalized patients: emergence of CTX-M-15-producing strains causing urinary tract infections. Microb Drug Resist 16:129–134. doi: 10.1089/mdr.2009.0132. [DOI] [PubMed] [Google Scholar]
- 8.Leverstein-van Hall MA, Dierikx CM, Cohen Stuart J, Voets GM, van den Munckhof MP, van Essen-Zandbergen A, Platteel T, Fluit AC, van de Sande-Bruinsma N, Scharinga J, Bonten MJ, Mevius DJ. 2011. Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin Microbiol Infect 17:873–880. doi: 10.1111/j.1469-0691.2011.03497.x. [DOI] [PubMed] [Google Scholar]
- 9.De Boeck H, Miwanda B, Lunguya-Metila O, Muyembe-Tamfum JJ, Stobberingh E, Glupczynski Y, Jacobs J. 2012. ESBL-positive Enterobacteria isolates in drinking water. Emerg Infect Dis 18:1019–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahmen S, Haenni M, Madec JY. 2012. IncI1/ST3 plasmids contribute to the dissemination of the blaCTX-M-1 gene in Escherichia coli from several animal species in France. J Antimicrob Chemother 67:3011–3012. doi: 10.1093/jac/dks308. [DOI] [PubMed] [Google Scholar]
- 11.Grami R, Mansour W, Dahmen S, Mehri W, Haenni M, Aouni M, Madec JY. 2013. The blaCTX-M-1 IncI1/ST3 plasmid is dominant in chickens and pets in Tunisia. J Antimicrob Chemother 68:2950–2952. doi: 10.1093/jac/dkt258. [DOI] [PubMed] [Google Scholar]
- 12.European Council. 1998. Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off J Eur Communities, L330, 32–54. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:1998:330:0032:0054:EN:PDF.
- 13.Direction Générale de la Santé et Agences Régionales de Santé. 2014. La qualité de l'eau du robinet en France: données 2012. Agences Régionales de Santé, Paris, France: http://social-sante.gouv.fr/IMG/pdf/rapport_qualite_eau_du_robinet_2012_dgs.pdf. [Google Scholar]