Abstract
Coagulase-negative staphylococci (CoNS) have become the leading cause of bloodstream infections (BSIs) in intensive care units (ICUs), particularly in premature neonates. Vancomycin-intermediate heteroresistant CoNS (hVICoNS) have been identified as sources of BSIs worldwide, and their potential to emerge as significant pathogens in the neonatal ICU (NICU) remains uncertain. This study describes the molecular epidemiology of an outbreak of vancomycin-heteroresistant (hV) Staphylococcus epidermidis central-line-associated BSI (CLABSI) in a single tertiary care NICU and compares it to a second tertiary care NICU that had not been associated with an outbreak. Between November 2009 and April 2014, 119 S. epidermidis CLABSIs were identified in two tertiary care NICUs in Quebec, Canada. Decreased vancomycin susceptibility was identified in about 88% of all collected strains using Etest methods. However, discrepancies were found according to the Etest and population analysis profiling–area under the concentration-time curve (PAP-AUC) methods used. All strains were susceptible to linezolid, and a few isolates were nonsusceptible to daptomycin. Great genetic diversity was observed within the collection, with 31 pulsed-field gel electrophoresis (PFGE) patterns identified. The outbreak strains were all determined to be heteroresistant to vancomycin and were polyclonal. The study identified two major clones, PFGE patterns E and G, which were found in both NICUs across the 5-year study period. This suggests the persistence of highly successful clones that are well adapted to the hospital environment. hV S. epidermidis seems more common than currently realized in the NICU, and certain hV S. epidermidis clones can become endemic to the NICU. The reservoirs for these clones remain unknown at this time, and identification of the reservoirs is needed to better understand the impact of hV S. epidermidis in the NICU and to inform infection prevention strategies. In addition, there is a need to investigate and validate hV determination protocols for different species of CoNS.
INTRODUCTION
Coagulase-negative staphylococci (CoNS) typically reside on healthy human skin and mucus membranes, rarely cause disease, and are most frequently encountered by clinicians as contaminants of microbiological cultures. However, CoNS have increasingly become recognized as health care-associated pathogens, causing infections in patients with predisposing factors, such as immunodeficiency and/or indwelling or implanted polymer bodies (1). CoNS have also emerged as a leading cause of central-line-associated bloodstream infections (CLABSI) in intensive care units (ICUs), with Staphylococcus epidermidis as the most common cause of CoNS infections (2–4), Patients in neonatal intensive care units (NICUs) are particularly at risk for health care-associated infections (HAI), given their immature immune systems, the acuity of care needed, and the frequency of invasive procedures performed (5, 6).
Pulsed-field gel electrophoresis (PFGE) has been the most widely used technique to genetically characterize S. epidermidis strains. The method is used for short-term epidemiological investigations and has been shown to be very sensitive in detecting genetic diversity among strains (7). Most recently, an improved multilocus sequence typing (MLST) scheme was developed to provide further information on the evolution, population structure, and long-term global epidemiology of S. epidermidis (8). With this method, Miragaia et al., Gordon et al., and Widerström et al. have determined that the global nosocomial S. epidermidis population is composed of nine clonal lineages. The most predominant of these clonal lineages is clonal complex 2 (CC2), which itself is composed of numerous sequence types (STs); ST2 is recognized as representative of hospital-acquired methicillin-resistant S. epidermidis strains (9–11).
About 90% of clinical CoNS isolates carry the mecA gene, which confers oxacillin (methicillin) resistance (12, 13). Therefore, vancomycin is often used as the first-line antimicrobial therapy. However, since the late 1980s, resistance to glycopeptides has been reported in CoNS (14–17). This resistance appears to be endogenous, does not seem to be associated with plasmids or transposons, and can be selected for in vitro by exposure to teicoplanin or vancomycin. Although the basis for this glycopeptide resistance is still unclear, it seems to be associated with ultrastructural changes (thickened cell wall) and a reduction in the rate of autolysis (17). Reduced susceptibility to vancomycin, a phenomenon dubbed heteroresistance, has been well described in Staphylococcus aureus (vancomycin-intermediate heteroresistant S. aureus [hVISA]) (16, 18). Vancomycin heteroresistance (hV), where a vancomycin-intermediate subpopulation of cells exists in an otherwise susceptible microbial population, has also been detected in clinical CoNS isolates (16, 19, 20).
Recently, one NICU in the province of Quebec has been struggling with an outbreak of CLABSI caused by strains of coagulase-negative Staphylococcus that seemed to have developed vancomycin-intermediate heteroresistance (hVICoNS). CLABSI rates increased from 5.09/1,000 catheter days in 2009 to 9.01 in 2011, reached a maximum of 11.3 in 2012, and returned to their baseline rate (5.52/1,000 catheter days) in 2013 (21). CoNS-associated CLABSI increased from 3 cases in 2009 to 14, 24, 19, and 17 cases per year for the following 4 years, respectively. All but 2 CoNS were S. epidermidis.
In this study, we analyzed the molecular epidemiology of S. epidermidis isolates that resulted in CLABSI from two tertiary care NICUs in Quebec: the first was the hospital with the outbreak described above, and the second was a hospital without a reported S. epidermidis CLABSI outbreak. The second hospital had a stable rate of CLABSI throughout the study period, with rates of 5.32/1,000 catheter days in 2009 and 6.39, 5.41, 2.65, and 4.35/1,000 catheter days for each of the following years, respectively (22). Additionally, we studied the levels of antibiotic resistance and clinical data to compare and identify differences between S. epidermidis isolates from the two hospitals.
MATERIALS AND METHODS
Study population and setting.
The Centre Hospitalier Universitaire Ste-Justine (STJ), a level III-IV NICU, is composed of 65 beds with approximately 1,100 admissions annually. STJ has a patient population born at the hospital and also serves as a reference center for other hospitals in the province of Quebec. The Montreal Children's Hospital (MCH), a level III NICU, is composed of 24 beds with approximately 450 admissions annually. MCH does not have in-house deliveries but is a reference center where newborns requiring tertiary care are admitted from other hospital centers in the province of Quebec. Both NICUs participate in the mandatory provincial surveillance program for CLABSI (SPIN-BACC) (23, 24). Additionally, both NICUs' microbiology laboratories freeze and systematically store all organisms isolated from sterile body sites.
All the patients admitted to one of the two tertiary care NICUs from 1 November 2009 through 31 March 2014 who developed a CLABSI were eligible for inclusion in the study. Patients were excluded if they had a CoNS CLABSI upon entry to the NICU. Additionally, cases of early-onset sepsis, occurring in the first 48 h of life, were excluded to increase the probability that the collected organisms were associated with the NICU. CoNS bloodstream isolates were identified retrospectively through clinical microbiological databases of blood cultures drawn from neonates of each hospital. Isolates were included in the study only if medical chart review and local infection control databases determined that they fulfilled CDC/National Healthcare Safety Network (NHSN) criteria (see “Definitions” below). Clinical data were collected retrospectively and included: date of birth, gender, birth weight, gestational age, dates of admission to and discharge from the NICU, date of sepsis onset, and all-cause mortality.
Definitions.
CoNS-positive blood cultures were defined using the CDC/NHSN surveillance definitions for specific types of infections criteria for laboratory-confirmed primary bloodstream infections (25). According to the criteria, the patient must either (i) have a recognized pathogen cultured from one or more blood cultures and the organism cultured from the blood must be unrelated to an infection at another site or (ii) have at least one of the following signs/symptoms: fever (>38°C core), hypothermia (<36°C core), apnea, or bradycardia; positive culture results that are unrelated to an infection at another site; and/or, if the organism is considered a common skin commensal (e.g., coagulase-negative staphylococcus), it must be cultured from two or more blood cultures drawn on the same or consecutive days at different sites or times. Additionally, for patients with more than one CLABSI, only the initial CLABSI was considered.
Strain identification.
Strains were identified with the Vitek-2 system (bioMérieux, Marcy l'Etoile, France) in both hospital laboratories, and if strain identification was not completed prior to collection for our project, sequencing of the tuf gene was done to identify Staphylococcus strains to the species level (26).
Antimicrobial susceptibility testing.
Resistance to vancomycin was evaluated according to the Clinical and Laboratory Standards Institute (CLSI) broth microdilution (BMD) reference method (susceptibility ≤ 4 mg/liter) (27). Collected isolates were also tested for vancomycin-intermediate heteroresistance with the GRD (glycopeptide resistance detection) and Macro Etests (bioMérieux, Marcy l'Etoile, France) according to CLSI and manufacturer recommendations (28, 29). The GRD Etest consists of a double-sided strip with vancomycin on one end and teicoplanin on the other, while the Macro Etest consists of separate vancomycin and teicoplanin strips. These Etests are more sensitive than the usual automated systems in detecting hV, as only a subpopulation of cells express vancomycin-intermediate heteroresistance (30). Isolates were considered hV if they were positive by either the GRD Etest or the Macro Etest. Furthermore, the Etest was used following the manufacturer's recommendations to evaluate the susceptibility of hVICoNS strains to alternative antibiotic therapies (daptomycin and linezolid). MIC interpretive criteria followed CLSI guidelines as follows: susceptible to daptomycin at ≤1 mg/liter and susceptible to linezolid at ≤4 mg/liter.
PAP-AUC testing.
Population analysis profiling–area under the concentration-time curve (PAP-AUC) testing was performed at the National Microbiology Laboratory in Winnipeg, Canada, following two different protocols. The first protocol (PAP-AUC 1) employed an adaptation of the method described by Wootton et al. (31) for the detection of vancomycin-intermediate heteroresistance in S. aureus. In brief, isolates of interest were stored at −80°C until they were ready to be plated onto plates containing tryptic soy agar (TSA) with 10% sheep blood. Following overnight incubation at 37°C in air, well-isolated colonies were inoculated into trypticase soy broth (TSB) for overnight incubation in air. The turbidity of the resulting bacterial suspension was adjusted to 2 McFarland standard using sterile 0.9% saline and serially diluted 10-fold in sterile 0.9% saline. The undiluted suspension and 103 and 106 dilutions were spiral plated using an Eddy Jet (IUL, SA, Barcelona, Spain) spiral plater onto blood-heart infusion agar plates infused with increasing concentrations of vancomycin (0, 0.5, 1, 2, 2.5, 3, 4, 6, and 8 mg/liter). The plates were incubated for 48 h in air, and colonies were manually counted using the grid system provided with the Eddy Jet system. AUC ratios were generated using GraphPad (La Jolla, CA) Prism version 5, comparing the tested isolates to ATCC strains 29213 and 700698 (Mu3) and 700699 (Mu50), as well as a vancomycin-intermediate heteroresistant Staphylococcus capitis strain isolated from a Manitoba, Canada, neonate. A positive PAP-AUC test was determined to be an AUC ratio of ≥0.9 when the isolate was compared to strain Mu3.
The vancomycin-intermediate heteroresistant phenotype has been shown to be unstable when isolates are serially passaged in the laboratory (32). We determined that the majority of isolates had been passaged a minimum of five times on non-antibiotic-containing media, which might have led to a loss of phenotype. To overcome this instability, a second protocol (PAP-AUC 2) was used, in which isolates were first plated on a TSA plate and, following overnight incubation, were then inoculated into TSB supplemented with 2 μg/ml vancomycin (instead of plain TSB). The remainder of the protocol was unchanged. To exclude the possibility that a single passage of S. epidermidis in the presence of vancomycin was able to induce hV in previously non-hV strains, a sample of non-vancomycin-intermediate heteroresistant strains of S. epidermidis (from MCH) were tested using this protocol.
PFGE.
Bacterial DNAs were digested with SmaI, and samples were run on a Chef-DR III or GenePath system (Bio-Rad, Mississauga, ON, Canada) (33). Banding patterns of S. epidermidis isolates were analyzed with BioNumerics software version 6.5 (Applied Maths, Austin, TX, USA). Similarity coefficients were obtained within BioNumerics by calculating Dice coefficients. Cluster analysis was done by the unweighted pair group method with arithmetic averages (UPGMA). Band position tolerances of 1% and optimization values of 0.8% were used for all analyses. Bands of ≥33 kb were included in the cluster analysis. PFGE patterns were considered similar using a similarity coefficient of 80% and visually according to the criteria of Tenover et al. (34). Letters identified the different PFGE patterns.
MLST.
MLST was performed according to the protocol of Thomas et al. (8). Analysis was performed within BioNumerics using the MLST plugin related to the MLST S. epidermidis database (http://sepidermidis.mlst.net) for allele and ST assignment. The eBURST method was used to infer the evolutionary relatedness of STs (35; http://eburst.mlst.net/). STs were included within the same group only if they shared identical alleles at six of the seven MLST loci. Additionally, with the eBURST algorithm, all members of a group were believed to have descended from the same founding genotype (the primary founder). The statistical confidences for the founders were assessed using 1,000 bootstrap resamplings.
Statistical analysis.
Differences in gestational age, average age at sepsis onset, and average length of stay were analyzed using the Mann-Whitney test. The differences in gender, birth weight category, and 28-day mortality were calculated using the χ2 test or Fisher's exact test. P values of ≤0.05 were considered statistically significant. All statistical tests were performed using STATA statistical software version 12 (STATA Corp., College Station, TX, USA). The correlation between typing methods and the correlation between vancomycin-intermediate heteroresistance determination methods were evaluated using the adjusted Rand index (ARI) and Wallace's coefficient (W) (36). The ARI represents the congruence between type assignments of different typing methods, taking into account chance agreement (37). Coefficients close to 0 indicate a lack of agreement among typing methods, and conversely, coefficients close to 1 indicate great agreement between typing methods. Meanwhile, the Wallace coefficient estimates that isolates typed the same using one method will also be predicted to be the same type by a second method. Here, a high W coefficient indicates that the type assignment given by the first method will likely be predicted to be the same by the second method. The ARI and W coefficients were calculated using tools available online (http://www.phyloviz.net/) (36, 38, 39).
RESULTS
Patient and strain characteristics.
A total of 133 CoNS isolates were collected during the study period: 87 isolates from 87 patients at STJ and 46 isolates from 35 patients at MCH. The greater number of isolates than patients at the MCH is due to three instances of polymicrobial infections, where two or more CoNS organisms were isolated for the same episode. Of the 133 presumed CoNS isolates collected, 1 S. hominis, 1 S. capitis, and 12 Staphylococcus warneri isolates were confirmed by tuf gene sequencing. The remaining 119 S. epidermidis isolates (79 from STJ and 40 from MCH) were used for further laboratory analysis and are the focus of this study.
Thirty-eight of 79 (48.1%) patients hospitalized at STJ were female, compared to 15 of 40 (35.4%) patients at MCH. Patients from STJ were younger than their MCH counterparts, with a mean gestational age of 27.5 weeks and a mean age at CoNS sepsis onset of 20.6 days (STJ) compared to a mean gestational age of 30.9 weeks and a mean age at CoNS sepsis onset of 72.5 days (MCH). Additionally, 41.8% of STJ patients had a birth weight of less than 750 g, while 32.5% of MCH patients weighed less than 750 g at birth. The average length of NICU stay was longer for patients at STJ, but not statistically significantly (P = 0.27; STJ, 87.5 days; MCH, 79.6 days). The 28-day mortality rates for the cohorts of patients from STJ and MCH were 16 deaths (20.2%) and 1 death (2.5%), respectively. The remaining demographic characteristics of the patients from STJ and MCH are summarized in Table 1.
TABLE 1.
Characteristics of patients with S. epidermidis isolates according to their hospitals of origin
| Patient characteristic | Value |
P value | |
|---|---|---|---|
| STJ (n = 79) | MCH (n = 40) | ||
| Female [n (%)] | 38 (48.1) | 15 (35.4) | 0.27 |
| Gestational age (wk) [mean (range)] | 27.5 (23.1–39.4) | 30.9 (23.9–39.4) | 0.0014 |
| Average age at sepsis onset (days) [mean (range)] | 20.6 (2–97) | 72.5 (4–217) | <0.0001 |
| Birth wt (g) category [no. (%)] | 0.007 | ||
| ≤750 | 33 (41.8) | 13 (32.5) | |
| 751–1,000 | 25 (31.6) | 6 (15) | |
| 1,001–1,500 | 12 (15.2) | 5 (12.5) | |
| 1,501–2,500 | 5 (6.3) | 8 (20) | |
| >2,500 | 4 (5.1) | 8 (20) | |
| Avg length of stay (days) [mean (range)] | 87.5 (0–257) | 79.6 (8–386) | 0.27 |
| 28-day mortality [n (%)] | 16 (20.2) | 1 (2.5) | 0.011 |
Antimicrobial resistance.
The antimicrobial susceptibility results are shown in Table 2. Five (6.3%) isolates from STJ were nonsusceptible to daptomycin, whereas all the isolates from MCH were susceptible. All the isolates from both hospitals were susceptible to linezolid. Among the isolates from MCH, 12 (30%) demonstrated reduced susceptibility to vancomycin (determined by broth microdilution), whereas all STJ isolates were susceptible. However, using the Macro and GRD Etests, all the isolates (100%) from STJ demonstrated vancomycin-intermediate heteroresistance, whereas 65% of the isolates from MCH were hV.
TABLE 2.
Antimicrobial susceptibility patterns of S. epidermidis isolates according to their hospitals of origin
| Antimicrobial agent | STJ (n = 79) |
MCH (n = 40) |
||||
|---|---|---|---|---|---|---|
| MIC50 (mg/liter) | MIC90 (mg/liter) | n (% resistant) | MIC50 (mg/liter) | MIC90 (mg/liter) | n (% resistant) | |
| Daptomycin | 0.75 | 1 | 5 (6.3) | 0.38 | 0.5 | 0 (0) |
| Linezolid | 1 | 1.5 | 0 (0) | 1 | 1.5 | 0 (0) |
| Vancomycin | 2 | 2 | 0 (0) | 2 | 4 | 12 (30)a |
| Vancomycin-intermediate heteroresistance | ||||||
| GRD Etest | 79 (100) | 24 (60) | ||||
| Macro Etest | 42 (53.2) | 13 (32.5) | ||||
| Totalb | 79 (100) | 26 (65) | ||||
Intermediate resistance, 8 to 16 mg/liter.
Isolates were considered vancomycin-intermediate heteroresistant if they were positive by either GRD Etest or Macro Etest.
PFGE.
Of the 119 S. epidermidis isolates, 6 were untypeable by the PFGE method. The remaining 113 isolates were grouped into 31 distinct PFGE patterns (Table 3; see Fig. S1 in the supplemental material). Twenty-one PFGE patterns were STJ specific, and of these, 11 PFGE patterns were represented by a single isolate (singletons). Five PFGE patterns were MCH specific, 4 of which were singletons. Finally, 4 PFGE patterns (AB, D, E, and G) harbored isolates from both hospitals. The PFGE pattern including the highest number of strains was PFGE pattern G (29 isolates [25.4%], 22 from STJ and 7 from MCH), followed by PFGE patterns E (28 isolates [24.6%], 8 from STJ and 20 from MCH) and Q (7 isolates [6.1%], all STJ).
TABLE 3.
Distribution of S. epidermidis PFGE patterns and MLSTs
| Parameter | Value for group: |
||
|---|---|---|---|
| 1 (common to both hospitals) | 2 (unique to STJ) | 3 (unique to MCH) | |
| No. of isolates | 65 | 47 | 7 |
| PFGE patterns (no. of isolates) | AB (5), D (3), E (28), G (29) | A (2), AC (2), AD (1), B (1), C (1), F (1), H (1), I (2), J (1), L (1), N (1), O (2), P (3), Q (7), R (2), S (5), T (3), U (1), V (1), Y (2), Z (1), untypeable (6) | AA (1), K (1), M (3), W (1), X (1) |
| MLST | ST2, ST5 | ST22, ST57, ST59, ST83, ST89, ST133, ST225, ST457 | ST14, ST54, ST218, ST297 |
Time distribution of PFGE patterns.
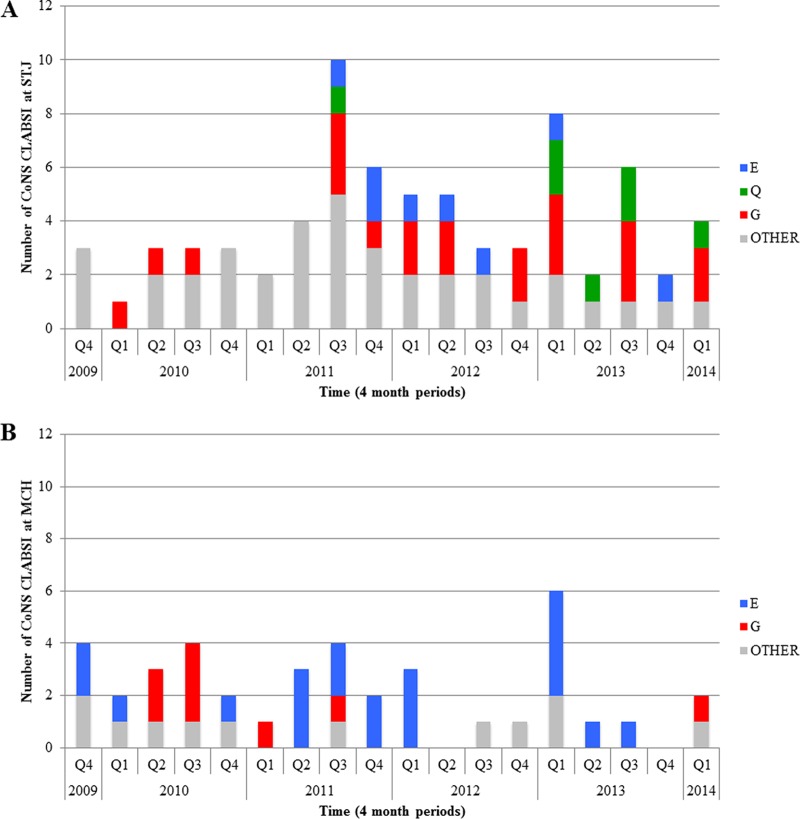
PFGE pattern G was the most predominant at STJ. The first pattern G clone from STJ was detected in the first quarter of 2010 (Q1 2010) and remained an etiology for CLABSI until the end of the study period (Fig. 1). PFGE pattern E from STJ was also a major clonal group, first appearing later, in Q3 2011, and spanned the study period until Q4 2013. Clones belonging to PFGE pattern Q were limited to STJ (the third predominant clone) and also appeared later, in Q3 2011. PFGE pattern Q strains became more frequent from 2013 to 2014. Conversely, PFGE pattern E from MCH was the predominant clone and was found from the start of the study period until Q3 2013. The other major clone at MCH, PFGE pattern G, was found from Q2 2010 until the end of the study period.
FIG 1.
PFGE pattern distribution of S. epidermidis infection from November 2009 to March 2014. Q1 to Q4 represent 4-month periods (quarters) within a year. Shown are cases from STJ (A) and from MCH (B).
MLST sequencing.
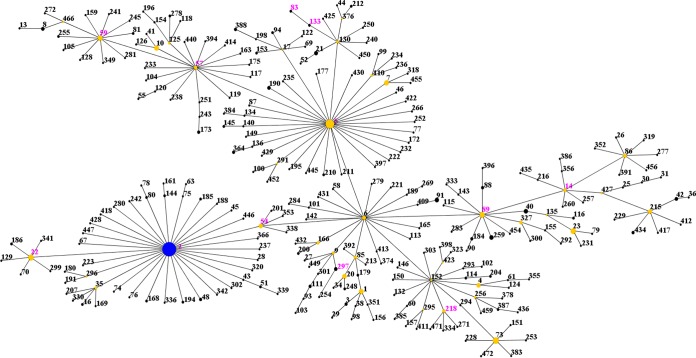
Thirty-nine isolates were typed by MLST (at least one per PFGE pattern and all the isolates that were untypeable by PFGE). In total, 14 different STs were identified, 10 (71.4%) of which were represented by a single isolate (Table 3; see Fig. S1 in the supplemental material). ST2 was the most common ST, comprising 53.8% (21 of 39) of the typed isolates and including all the untypeable isolates. There were 8 STs unique to STJ, 4 STs unique to MCH, and 2 STs that were common to both NICUs. All 14 of these STs had previously been recorded in the MLST database (as of January 2016). The eBURST algorithm clustered these STs into one major clonal complex (CC2) (Fig. 2). It was previously suggested that CC2 could be separated into clusters and subgroups (7). However, with the MLST database population, new subgroup founders were identified by the eBURST algorithm. Our isolates were classified into 11 different subgroups in CC2.
FIG 2.
eBURST analysis of S. epidermidis CC2 using all 448 STs available in the MLST database as of January 2016. Each ST is represented by a dot, and lines connect single-locus variants. The size of the dot is proportional to the number of isolates for each ST. The blue dot (ST2) represents the putative founder of CC2. The yellow dots represent putative subgroup founders. The pink STs represent the STs in our study.
Relationship of PFGE patterns to MLST.
To measure the concordance between PFGE and MLST (Table 3; see Fig. S1 in the supplemental material), the ARI and Wallace coefficients were calculated. The ARI was 0.054, indicating a very low congruence between PFGE and MLST. The concordance of type assignments between PFGE and MLST were also low (WPFGE→MLST = 0.567 [range, 0.345 to 0.789]; WMLST→PFGE = 0.028 [range, 0.000 to 0.101]). For instance, PFGE pattern E consisted of isolates typed as ST2 and ST54. Conversely, the strains typed as ST2 consisted of isolates belonging to 14 PFGE patterns, including E and G. Therefore, here, the ST is not predictive of the PFGE pattern and vice versa.
Relationship of PFGE patterns to antimicrobial resistance.
At MCH, 15 of 20 (75%) PFGE pattern E isolates were vancomycin-intermediate heteroresistant, as well as 2 of 8 (25%) PFGE pattern G isolates. While all the MCH isolates were daptomycin susceptible, there was a correlation between vancomycin-intermediate heteroresistance and increased daptomycin MICs. For instance, pattern G hV strains had higher daptomycin MICs than the vancomycin-susceptible strains (0.38 mg/liter versus 0.19 mg/liter; P = 0.036). This correlation of higher levels of vancomycin-intermediate heteroresistance and daptomycin nonsusceptibility was also found among the isolates from STJ. All the isolates from STJ were hV, and the daptomycin MIC50 and MIC90 for STJ were higher than those for MCH (Table 2). At the STJ, five of the six daptomycin-nonsusceptible strains were from PFGE pattern E.
PAP-AUC.
Thirty-six isolates were tested by PAP-AUC (at least one per PFGE pattern and all the isolates that were untypeable by PFGE among the hV isolates). In addition, 10 isolates from MCH that were not hV by Etests were tested (see Materials and Methods). Following the first protocol (PAP-AUC 1), none of the 36 isolates demonstrated vancomycin-intermediate heteroresistance. However, with the second protocol (PAP-AUC 2), 32 isolates (88.9%) showed vancomycin-intermediate heteroresistance. The 10 isolates that were hV negative by Etests were not shown to be hV with either PAP-AUC protocol (see Fig. S1 in the supplemental material).
DISCUSSION
In this study, we demonstrated the clonal dissemination and endemic persistence of two major S. epidermidis clonal groups within two NICUs in Quebec, Canada, over a 5-year period. From the data, S. epidermidis was the most frequently isolated organism in CLABSI cases. Using results from the GRD and Macro Etests, all the strains from the outbreak hospital (STJ) were hV, as were about 65% of the strains from MCH. Moreover, isolates with hV of the same PFGE pattern and ST were isolated from patients in the two NICUs. This indicates that hVICoNS can persist within hospitals and may be more common than previously thought. However, the clinical significance of hVICoNS remains unknown. In S. aureus, vancomycin resistance is associated with prolonged bacteremia, high-inoculum infections, and vancomycin therapeutic failure (40). To date, the clinical impact of hVICoNS in the NICU has yet to be addressed.
Vancomycin-intermediate heteroresistance determinations among isolated strains using the BMD and Etest methods were discordant (ARI and W coefficients, <0.11), although this is not uncommon. Etests are far more reliable than routine laboratory methods in detecting vancomycin-intermediate heteroresistance, with higher sensitivity and specificity (15, 30). Compared to BMD methods, Etests use higher volumes of inoculum, and plating allows visibility of microcolonies in the zones of inhibition (41). Here, we showed that MIC determination using the routine laboratory method does not foretell hV status and that surveillance of vancomycin MICs alone may be inappropriate to monitor hV.
Persistence of hVICoNS in the NICU has been reported in other studies, with prevalences ranging from 22.1 to 100% of collected CoNS isolates (15, 18, 42, 43). Additionally, the clonality of hVICoNS in the NICU has been previously described. For instance, Rasigade et al. reported the clonal spread of an S. capitis strain with reduced vancomycin susceptibility to various NICUs throughout France (42). Moreover, Villari et al. found four predominant S. epidermidis clones over a 3-year period in Italy (43). They emphasized the importance of cross-transmission and selective pressure from glycopeptide use in the high prevalence of hVICoNS in the NICU. These findings of successful hVICoNS clones suggest that reduced glycopeptide susceptibility may play a role in their prolonged persistence.
Due to the presence of vancomycin-intermediate heteroresistance among the collection of strains, we evaluated the strains' antimicrobial susceptibilities to known alternative therapies. Linezolid was given to 29 (33.3%) patients from STJ and was not administered to any patients from MCH. Despite prior linezolid treatment in some of the patients, all of the strains included in the study were susceptible to linezolid. Resistance could have been expected after administration of linezolid due to therapeutic pressure and selection of linezolid resistance subtypes, but this was not seen. The reported prevalence of linezolid resistance is low, with about 2% of clinical CoNS isolates exhibiting resistance globally (44). However, it has been found that some CoNS species acquire resistance after linezolid treatment much more rapidly than S. aureus (45). An outbreak of linezolid-resistant S. epidermidis has recently been reported in a tertiary care referral university hospital in Ireland. The outbreak was quickly contained through infection control practices and cessation of linezolid use (46). Although linezolid is an alternative to vancomycin, the potential for rapid acquisition of linezolid resistance in CoNS following treatment and trends toward increasing rates of resistance are of concern (45, 47, 48).
About 6.3% of strains from STJ were nonsusceptible to daptomycin. Interestingly, daptomycin was never used as a treatment in either hospital throughout the study period. Daptomycin resistance without prior treatment is not unusual. Several studies have highlighted the relationship between daptomycin resistance and vancomycin resistance/vancomycin-intermediate heteroresistance despite a lack of exposure to daptomycin in clinical isolates of S. aureus (49–51). These studies concluded that a thickened cell wall, which is a common feature and mechanism of resistance in clinical vancomycin-resistant/hV S. aureus strains, could inhibit daptomycin's activity on the cell membrane. Daptomycin is a bactericidal agent that acts by targeting and permeating the cell membrane, resulting in leakage of ions and ultimately cell death (52). A thickened cell wall could therefore make it difficult for daptomycin molecules to pass through the cell wall and reach their target. Nunes et al. used transmission electron microscopy to determine if the thickness of cell walls changed with varying levels of vancomycin susceptibility (53). Using derivative CoNS strains that grew on high concentrations of vancomycin or teicoplanin, they compared cell wall thicknesses and found that all derivative strains had thicker cell walls than the parental strains. Our study did not look into this mechanism of daptomycin resistance, and determining the cell wall thickness in hV strains would be important. Future studies are needed to fully understand daptomycin resistance among CoNS and would contribute greatly to the expanding knowledge of hVICoNS.
Great genetic diversity was observed within the collection of strains from the two NICUs. In particular, STJ had a higher degree of genetic diversity than MCH, with 21 versus 5 PFGE patterns specific to the two hospitals, respectively. The diversity found in each hospital is in line with the results of previous studies that investigated the molecular epidemiology of clinical S. epidermidis isolates (47, 54, 55). Further, molecular typing of the collected isolates using MLST demonstrated that ST2 was the predominant sequence type in this study, comprising more than 50% of all PFGE patterns, followed by ST5 (10.3%). All of the STs identified in our study belonged to CC2, the primary clonal complex found worldwide. Our findings are comparable to those of other studies of S. epidermidis in U.S. hospitals, which found ST2 and ST5 to be the most common sequence types in their collections (47, 56). PFGE patterns and STs were not correlated in our study; in particular, ST2 consisted of 14 different PFGE patterns. The study of Cherifi et al., who aimed to describe the molecular epidemiology of S. epidermidis in Belgian hospitals, as well as at the community level, found that ST2 was made up of only 2 PFGE patterns (54). The higher number in our study is somewhat surprising; nonetheless, ST2 has been identified in the large majority of clinical methicillin-resistant S. epidermidis strains (9, 10, 54).
Recently, two studies attempted to elucidate the population structure of S. epidermidis, using different clustering approaches than the BURST algorithm used in our study. The first study utilized a Bayesian-model-based clustering approach with MLST data, and the second study utilized a whole-genome-sequencing approach (57, 58). Thomas et al. (57) grouped the S. epidermidis population into 6 genetic clusters (GC), and Méric et al. (58) grouped S. epidermidis into 3 groups. In both cases, the clustering of S. epidermidis isolates was consistent with previously used methods. According to the scheme of Thomas et al. (57), our isolates clustered into GC1, GC5, and GC6. While GC1 and GC6 appear to have a more generalist lifestyle, GC5 appears to be suited to a more nosocomial lifestyle, which represents the majority of the MLST-typed isolates in our study (ST2 and ST22). Additionally, according to the scheme of Méric et al. (58), all of our isolates clustered into group A, within which there are a high number of recombination events. This could in part explain the great PFGE diversity observed in our study.
PFGE patterns E and G were found in both NICUs, suggesting that the same S. epidermidis clones could have successfully colonized both hospitals. The commonality of these clones to both NICUs is not due to transfers, as between-NICU transfers (patient, personnel, or equipment) did not occur during the study period. Additionally, PFGE pattern E did not appear at STJ until August 2011, while pattern E strains were found at MCH from the start of the study period in November 2009. Additionally, looking at antimicrobial resistance, the levels of daptomycin resistance from these strains at MCH around August 2011 were 0.25 mg/liter, while the first pattern E clones at STJ were nonsusceptible (1.5 mg/liter). This suggests that the PFGE pattern E clones may not have the same reservoir/origin in both institutions. Finally, PFGE patterns E, G, and Q belong to the most prevalent and successful strain type found worldwide (ST2) (9, 59). Therefore, it is not surprising to find that PFGE patterns E and G are ubiquitous in either of the NICUs in our study.
PFGE has been shown to be highly discriminatory and is useful for determining clonal relationships and short-term local epidemiology (7, 9). MLST is also discriminatory and is used for long-term global epidemiology (9). We used PFGE to infer clonality in the collection of strains and used MLST to give evolutionary context to the strains in terms of population structure within the hospitals and globally. Low ARI and W coefficients were calculated when correlating PFGE and MLST in our study, indicating that these typing methods were not congruent with one another. Miragaia et al. (7) found similar results, with ARI and W coefficients for PFGE and MLST of <0.75. They suggested that high rates of recombination among S. epidermidis were the reason for the lack of congruence (7, 9). Therefore, the use of both methods was needed to infer the extent and evolution of clonality within and between the two hospitals.
Controversy remains regarding the vancomycin-intermediate heteroresistance phenomenon in S. epidermidis, particularly in view of the methods for detection and the stability of the hV phenotype (32). PAP-AUC is generally recognized as the gold standard for hV determination (30, 31) and has been used in other studies to confirm hVICoNS (15, 18, 43, 60). However, the method is laborious and is not available in routine diagnostic microbiology laboratories. In addition, the interpretation criteria for the PAP-AUC test were developed and validated for S. aureus, not for CoNS (30, 31). We used two different protocols for PAP-AUC, taking into account the phenotype's possible instability. Our results show either that the strains harbored an unstable vancomycin-intermediate heteroresistance phenotype or that hV in CoNS may be inducible in the presence of vancomycin. Both of these hypotheses must be investigated further. The clinical impact of vancomycin-intermediate heteroresistance and its association with treatment failure could not be addressed in this study. Finally, specific testing protocols and interpretation criteria should be developed to accurately determine vancomycin-intermediate heteroresistance in CoNS, since using criteria developed for S. aureus may not be entirely suitable to confirm hV in CoNS strains.
Limitations were present in this study. We acknowledge that both the GRD Etest and the Macro Etest are not fully validated for VICoNS or hVICoNS, and thus, results showing that isolates are positive by Etest methods but negative in both broth microdilution and PAP-AUC tests have to be interpreted with caution. Further, the study was completed retrospectively and thus had to rely on information recorded in medical charts. Moreover, only the first positive blood culture from each patient was used to determine hV. Doing so may have disregarded the manifestation of hV in subsequent CLABSI cultures from MCH. While we used the CDC/NHSN definition to ensure our CoNS isolated from CLABSI cultures were true pathogens, it is possible that we included contaminated blood cultures. These false positives would overestimate the CLABSI prevalence in our study. Furthermore, the detection of two major clonal groups in our study could be due to a lack of discriminatory power of PFGE. However, we detected a large number of PFGE patterns in both NICUs, which makes this unlikely.
In conclusion, our study investigated a CLABSI outbreak caused by hV S. epidermidis in the NICU. The outbreak strains were all confirmed to be vancomycin-intermediate heteroresistant, based on routinely available methods or modified PAP-AUC protocols, and were polyclonal. However, as discrepancies were found between Etests and both PAP-AUC methods, there is a need to investigate and validate hV determination protocols for different species of CoNS. The presence of vancomycin-intermediate resistant subpopulations in the collection of isolates may be of concern, as it could lead to future vancomycin resistance. Consistent surveillance of vancomycin MICs is therefore crucial to avoid further resistance; however, MICs alone may be inappropriate to monitor hV. Finally, our results suggest the persistence of highly successful clones that are well adapted to the hospital environment. The reservoirs for these clones remain unknown at this time, and identification of the reservoirs is needed to better understand the impact of hVICoNS in the NICU and to inform infection prevention strategies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Guylaine Aubin, Agata Klebucki, and Karine Desjardins from the LSPQ for their invaluable laboratory support and guidance.
Funding Statement
The laboratory part of the study was funded by a Pfizer Canada grant. The funder had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00726-16.
REFERENCES
- 1.Rogers KL, Fey PD, Rupp ME. 2009. Coagulase-negative staphylococcal infections. Infect Dis Clin North Am 23:73–98. doi: 10.1016/j.idc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Gaynes RP, Edwards JR, Jarvis WR, Culver DH, Tolson JS, Martone WJ. 1996. Nosocomial infections among neonates in high-risk nurseries in the United States. National Nosocomial Infections Surveillance System. Pediatrics 98:357–361. [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, Oh W, Bauer CR, Korones SB, Shankaran S, Laptook AR, Stevenson DK, Papile LA, Poole WK. 2002. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 4.Widerström M, Wiström J, Sjöstedt A, Monsen T. 2012. Coagulase-negative staphylococci: update on the molecular epidemiology and clinical presentation, with a focus on Staphylococcus epidermidis and Staphylococcus saprophyticus. Eur J Clin Microbiol Infect Dis 31:7–20. doi: 10.1007/s10096-011-1270-6. [DOI] [PubMed] [Google Scholar]
- 5.Brodie SB, Sands KE, Gray JE, Parker RA, Goldmann DA, Davis RB, Richardson DK. 2000. Occurrence of nosocomial bloodstream infections in six neonatal intensive care units. Pediatr Infect Dis J 19:56–65. doi: 10.1097/00006454-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Schulman J, Stricof R, Stevens TP, Horgan M, Gase K, Holzman IR, Koppel RI, Nafday S, Gibbs K, Angert R. 2011. Statewide NICU central-line-associated bloodstream infection rates decline after bundles and checklists. Pediatrics 127:436–444. doi: 10.1542/peds.2010-2873. [DOI] [PubMed] [Google Scholar]
- 7.Miragaia M, Carrico J, Thomas J, Couto I, Enright M, De Lencastre H. 2008. Comparison of molecular typing methods for characterization of Staphylococcus epidermidis: proposal for clone definition. J Clin Microbiol 46:118–129. doi: 10.1128/JCM.01685-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas JC, Vargas MR, Miragaia M, Peacock SJ, Archer GL, Enright MC. 2007. Improved multilocus sequence typing scheme for Staphylococcus epidermidis. J Clin Microbiol 45:616–619. doi: 10.1128/JCM.01934-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miragaia M, Thomas J, Couto I, Enright M, De Lencastre H. 2007. Inferring a population structure for Staphylococcus epidermidis from multilocus sequence typing data. J Bacteriol 189:2540–2552. doi: 10.1128/JB.01484-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon RJ, Miragaia M, Weinberg AD, Lee CJ, Rolo J, Giacalone JC, Slaughter MS, Pappas P, Naka Y, Tector AJ. 2012. Staphylococcus epidermidis colonization is highly clonal across US cardiac centers. J Infect Dis 205:1391–1398. doi: 10.1093/infdis/jis218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Widerström M, McCullough CA, Coombs GW, Monsen T, Christiansen KJ. 2012. A multidrug-resistant Staphylococcus epidermidis clone (ST2) is an ongoing cause of hospital acquired infection in a Western Australian hospital. J Clin Microbiol 50:2147–2151. doi: 10.1128/JCM.06456-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Fukuchi Y, Kobayashi I. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 13.Venkatesh MP, Placencia F, Weisman LE. 2006. Coagulase-negative staphylococcal infections in the neonate and child: an update. Semin Pediatr Infect Dis 17:120–127. doi: 10.1053/j.spid.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Center KJ, Reboli AC, Hubler R, Rodgers GL, Long SS. 2003. Decreased vancomycin susceptibility of coagulase-negative staphylococci in a neonatal intensive care unit: evidence of spread of Staphylococcus warneri. J Clin Microbiol 41:4660–4665. doi: 10.1128/JCM.41.10.4660-4665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Mello D, Daley AJ, Rahman MS, Qu Y, Garland S, Pearce C, Deighton MA. 2008. Vancomycin heteroresistance in bloodstream isolates of Staphylococcus capitis. J Clin Microbiol 46:3124–3126. doi: 10.1128/JCM.00592-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrett DO, Jochimsen E, Murfitt K, Hill B, McAllister S, Nelson P, Spera RV, Sall RK, Tenover FC, Johnston J, Zimmer B, Jarvis WR. 1999. The emergence of decreased susceptibility to vancomycin in Staphylococcus epidermidis. Infect Control Hosp Epidemiol 20:167–170. doi: 10.1086/501605. [DOI] [PubMed] [Google Scholar]
- 17.Biavasco F, Vignaroli C, Varaldo P. 2000. Glycopeptide resistance in coagulase-negative staphylococci. Eur J Clin Microbiol Infect Dis 19:403–417. doi: 10.1007/s100960000299. [DOI] [PubMed] [Google Scholar]
- 18.Van Der Zwet WC, Debets-Ossenkopp YJ, Reinders E, Kapi M, Savelkoul PHM, Van Elburg RM, Hiramatsu K, Vandenbroucke-Grauls CMJE. 2002. Nosocomial spread of a Staphylococcus capitis strain with heteroresistance to vancomycin in a neonatal intensive care unit. J Clin Microbiol 40:2520–2525. doi: 10.1128/JCM.40.7.2520-2525.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sieradzki K, Villari P, Tomasz A. 1998. Decreased susceptibilities to teicoplanin and vancomycin among coagulase-negative methicillin-resistant clinical isolates of staphylococci. Antimicrob Agents Chemother 42:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duah M. 2010. Daptomycin for methicillin-resistant Staphylococcus epidermidis native-valve endocarditis: a case report. Ann Clin Microbiol Antimicrob 9:9. doi: 10.1186/1476-0711-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comité de surveillance provinciale des infections nosocomiales (SPIN). 2013. Bactériémies sur cathéters centraux aux soins intensifs. Résultats de surveillance 2012-2013. Institut National de Santé Publique du Québec, Québec, Canada. [Google Scholar]
- 22.Quach C, Milstone AM, Perpête C, Bonenfant M, Moore DL, Perreault T. 2014. Chlorhexidine bathing in a tertiary care neonatal intensive care unit: impact on central line-associated bloodstream infections. Infect Control Hosp Epidemiol 35:158–163. doi: 10.1086/674862. [DOI] [PubMed] [Google Scholar]
- 23.Blanchard AC, Fortin E, Rocher I, Moore DL, Frenette C, Tremblay C, Quach C. 2013. Central line-associated bloodstream infection in neonatal intensive care units. Infect Control Hosp Epidemiol 34:1167–1173. doi: 10.1086/673464. [DOI] [PubMed] [Google Scholar]
- 24.Fontela PS, Platt RW, Rocher I, Frenette C, Moore D, Fortin É, Buckeridge D, Pai M, Quach C. 2011. Surveillance Provinciale des Infections Nosocomiales (SPIN) Program: implementation of a mandatory surveillance program for central line-associated bloodstream infections. Am J Infect Control 39:329–335. doi: 10.1016/j.ajic.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Horan TC, Andrus M, Dudeck MA. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Lévesque S, Longtins Y, Domingo MC, Massé C, Bernatchez H, Gaudreau C, Tremblay C. 2016. Enteroccocus pallens as a potential novel human pathogen: three cases of spontaneous bacterial peritonitis. JMM Case Rep 3. doi: 10.1099/jmmcr.0.005024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI, Wayne, PA. [Google Scholar]
- 28.Yusof A, Engelhardt A, Karlsson Å, Bylund L, Vidh P, Mills K, Wootton M, Walsh TR. 2008. Evaluation of a new Etest vancomycin-teicoplanin strip for detection of glycopeptide-intermediate Staphylococcus aureus (GISA), in particular, heterogeneous GISA. J Clin Microbiol 46:3042–3047. doi: 10.1128/JCM.00265-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner J, Howe RA, Wootton M, Bowker KE, Holt HA, Salisbury V, Bennett PM, Walsh TR, MacGowan AP. 2001. The activity of vancomycin against heterogeneous vancomycin-intermediate methicillin-resistant Staphylococcus aureus explored using an in vitro pharmacokinetic model. J Antimicrob Chemother 48:727–730. doi: 10.1093/jac/48.5.727. [DOI] [PubMed] [Google Scholar]
- 30.Walsh TR, Bolmström A, Qwärnström A, Ho P, Wootton M, Howe RA, MacGowan AP, Diekema D. 2001. Evaluation of current methods for detection of staphylococci with reduced susceptibility to glycopeptides. J Clin Microbiol 39:2439–2444. doi: 10.1128/JCM.39.7.2439-2444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wootton M, Howe R, Hillman R, Walsh T, Bennett P, MacGowan A. 2001. A modified population analysis profile method to detect Staphylococcus aureus with decreased susceptibilities to vancomycin in a UK hospital. J Antimicrob Chemother 47:399–404. doi: 10.1093/jac/47.4.399. [DOI] [PubMed] [Google Scholar]
- 32.Plipat N, Livni G, Bertram H, Thomson RB. 2005. Unstable vancomycin heteroresistance is common among clinical isolates of methicillin-resistant Staphylococcus aureus. J Clin Microbiol 43:2494–2496. doi: 10.1128/JCM.43.5.2494-2496.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulvey M, Chui L, Ismail J, Louie L, Murphy C, Chang N, Alfa M. 2001. Development of a Canadian standardized protocol for subtyping methicillin-resistant Staphylococcus aureus using pulsed-field gel electrophoresis. J Clin Microbiol 39:3481–3485. doi: 10.1128/JCM.39.10.3481-3485.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol 186:1518–1530. doi: 10.1128/JB.186.5.1518-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carrico J, Silva-Costa C, Melo-Cristino J, Pinto F, De Lencastre H, Almeida J, Ramirez M. 2006. Illustration of a common framework for relating multiple typing methods by application to macrolide-resistant Streptococcus pyogenes. J Clin Microbiol 44:2524–2532. doi: 10.1128/JCM.02536-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hubert L, Arabie P. 1985. Comparing partitions. J Classif 2:193–218. doi: 10.1007/BF01908075. [DOI] [Google Scholar]
- 38.Pinto FR, Melo-Cristino J, Ramirez M. 2008. A confidence interval for the Wallace coefficient of concordance and its application to microbial typing methods. PLoS One 3:e3696. doi: 10.1371/journal.pone.0003696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Severiano A, Pinto FR, Ramirez M, Carriço JA. 2011. Adjusted Wallace coefficient as a measure of congruence between typing methods. J Clin Microbiol 49:3997–4000. doi: 10.1128/JCM.00624-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Hal SJ, Paterson DL. 2011. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob Agents Chemother 55:405–410. doi: 10.1128/AAC.01133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Halfawy OM, Valvano MA. 2015. Antimicrobial heteroresistance: an emerging field in need of clarity. Clin Microbiol Rev 28:191–207. doi: 10.1128/CMR.00058-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rasigade JP, Raulin O, Picaud JC, Tellini C, Bes M, Grando J, Ben Said M, Claris O, Etienne J, Tigaud S, Laurent F. 2012. Methicillin-resistant Staphylococcus capitis with reduced vancomycin susceptibility causes late-onset sepsis in intensive care neonates. PLoS One 7:e31548. doi: 10.1371/journal.pone.0031548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villari P, Sarnataro C, Iacuzio L. 2000. Molecular epidemiology of Staphylococcus epidermidis in a neonatal intensive care unit over a three-year period. J Clin Microbiol 38:1740–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu B, Kelesidis T, Tsiodras S, Hindler J, Humphries RM. 2013. The emerging problem of linezolid-resistant Staphylococcus. J Antimicrob Chemother 68:4–11. doi: 10.1093/jac/dks354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tewhey R, Gu B, Kelesidis T, Charlton C, Bobenchik A, Hindler J, Schork NJ, Humphries RM. 2014. Mechanisms of linezolid resistance among coagulase-negative staphylococci determined by whole-genome sequencing. mBio 5:e00894-14. doi: 10.1128/mBio.00894-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Connor C, Powell J, Finnegan C, O'Gorman A, Barrett S, Hopkins K, Pichon B, Hill R, Power L, Woodford N. 2015. Incidence, management and outcomes of the first cfr-mediated linezolid-resistant Staphylococcus epidermidis outbreak in a tertiary referral centre in the Republic of Ireland. J Hosp Infect 90:316–321. doi: 10.1016/j.jhin.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 47.Mendes RE, Deshpande LM, Costello A, Farrell DJ. 2012. Molecular epidemiology of Staphylococcus epidermidis clinical isolates from U.S. hospitals. Antimicrob Agents Chemother 56:4656–4661. doi: 10.1128/AAC.00279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farrell DJ, Mendes RE, Ross JE, Sader HS, Jones RN. 2011. LEADER Program results for 2009: an activity and spectrum analysis of linezolid using 6,414 clinical isolates from 56 medical centers in the United States. Antimicrob Agents Chemother 55:3684–3690. doi: 10.1128/AAC.01729-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cui L, Tominaga E, Neoh H-M, Hiramatsu K. 2006. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother 50:1079–1082. doi: 10.1128/AAC.50.3.1079-1082.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pillai SK, Gold HS, Sakoulas G, Wennersten C, Moellering RC, Eliopoulos GM. 2007. Daptomycin nonsusceptibility in Staphylococcus aureus with reduced vancomycin susceptibility is independent of alterations in MprF. Antimicrob Agents Chemother 51:2223–2225. doi: 10.1128/AAC.00202-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakoulas G, Alder J, Thauvin-Eliopoulos C, Moellering RC, Eliopoulos GM. 2006. Induction of daptomycin heterogeneous susceptibility in Staphylococcus aureus by exposure to vancomycin. Antimicrob Agents Chemother 50:1581–1585. doi: 10.1128/AAC.50.4.1581-1585.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bayer AS, Schneider T, Sahl HG. 2013. Mechanisms of daptomycin resistance in Staphylococcus aureus: role of the cell membrane and cell wall. Ann N Y Acad Sci 1277:139–158. doi: 10.1111/j.1749-6632.2012.06819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nunes APF, Teixeira LM, Iorio NLP, Bastos CCR, de Sousa Fonseca L, Souto-Padrón T, Dos Santos KRN. 2006. Heterogeneous resistance to vancomycin in Staphylococcus epidermidis, Staphylococcus haemolyticus and Staphylococcus warneri clinical strains: characterisation of glycopeptide susceptibility profiles and cell wall thickening. Int J Antimicrob Agents 27:307–315. doi: 10.1016/j.ijantimicag.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 54.Cherifi S, Byl B, Deplano A, Nonhoff C, Denis O, Hallin M. 2013. Comparative epidemiology of Staphylococcus epidermidis isolates from patients with catheter-related bacteremia and from healthy volunteers. J Clin Microbiol 51:1541–1547. doi: 10.1128/JCM.03378-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bogado I, Limansky A, Sutich E, Marchiaro P, Marzi M, Putero J, Viale A. 2002. Molecular characterization of methicillin-resistant coagulase-negative staphylococci from a neonatal intensive care unit. Infect Control Hosp Epidemiol 23:447–451. doi: 10.1086/502083. [DOI] [PubMed] [Google Scholar]
- 56.Wong A, Reddy SP, Smyth DS, Aguero-Rosenfeld ME, Sakoulas G, Robinson DA. 2010. Polyphyletic emergence of linezolid-resistant staphylococci in the United States. Antimicrob Agents Chemother 54:742–748. doi: 10.1128/AAC.00621-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas JC, Zhang L, Robinson DA. 2014. Differing lifestyles of Staphylococcus epidermidis as revealed through Bayesian clustering of multilocus sequence types. Infect Genet Evol 22:257–264. doi: 10.1016/j.meegid.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Méric G, Miragaia M, De Been M, Yahara K, Pascoe B, Mageiros L, Mikhail J, Harris LG, Wilkinson TS, Rolo J. 2015. Ecological overlap and horizontal gene transfer in Staphylococcus aureus and Staphylococcus epidermidis. Genome Biol Evol 7:1313–1328. doi: 10.1093/gbe/evv066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schoenfelder SM, Lange C, Eckart M, Hennig S, Kozytska S, Ziebuhr W. 2010. Success through diversity—how Staphylococcus epidermidis establishes as a nosocomial pathogen. Int J Med Microbiol 300:380–386. doi: 10.1016/j.ijmm.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 60.Satola SW, Lessa FC, Ray SM, Bulens SN, Lynfield R, Schaffner W, Dumyati G, Nadle J, Patel JB, Active Bacterial Core surveillance (ABCs) MRSA Investigators. 2011. Clinical and laboratory characteristics of invasive infections due to methicillin-resistant Staphylococcus aureus isolates demonstrating a vancomycin MIC of 2 micrograms per milliliter: lack of effect of heteroresistant vancomycin-intermediate S. aureus phenotype. J Clin Microbiol 49:1583–1587. doi: 10.1128/JCM.01719-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.