Abstract
Severely burned patients have altered drug pharmacokinetics (PKs), but it is unclear how different they are from those in other critically ill patient groups. The aim of the present study was to compare the population pharmacokinetics of micafungin in the plasma and burn eschar of severely burned patients with those of micafungin in the plasma and peritoneal fluid of postsurgical critically ill patients with intra-abdominal infection. Fifteen burn patients were compared with 10 patients with intra-abdominal infection; all patients were treated with 100 to 150 mg/day of micafungin. Micafungin concentrations in serial blood, peritoneal fluid, and burn tissue samples were determined and were subjected to a population pharmacokinetic analysis. The probability of target attainment was calculated using area under the concentration-time curve from 0 to 24 h/MIC cutoffs of 285 for Candida parapsilosis and 3,000 for non-parapsilosis Candida spp. by Monte Carlo simulations. Twenty-five patients (18 males; median age, 50 years; age range, 38 to 67 years; median total body surface area burned, 50%; range of total body surface area burned, 35 to 65%) were included. A three-compartment model described the data, and only the rate constant for the drug distribution from the tissue fluid to the central compartment was statistically significantly different between the burn and intra-abdominal infection patients (0.47 ± 0.47 versus 0.15 ± 0.06 h−1, respectively; P < 0.05). Most patients would achieve plasma PK/pharmacodynamic (PD) targets of 90% for non-parapsilosis Candida spp. and C. parapsilosis with MICs of 0.008 and 0.064 mg/liter, respectively, for doses of 100 mg daily and 150 mg daily. The PKs of micafungin were not significantly different between burn patients and intra-abdominal infection patients. After the first dose, micafungin at 100 mg/day achieved the PK/PD targets in plasma for MIC values of ≤0.008 mg/liter and ≤0.064 mg/liter for non-parapsilosis Candida spp. and Candida parapsilosis species, respectively.
INTRODUCTION
The management of patients with severe burn injuries requires critical care in specialized units with equipment, supplies, and personnel for intensive monitoring and life-sustaining organ support until the patients recover and the wounds are healed. Although improvements in supportive care have led to lower mortality rates, infections, especially wound fungal infections, continue to be a life-threatening complication (1, 2). Burn patients are a population at a significant risk of opportunistic infections (3), and burn wound infections are caused by fungi in 20 to 25% of the cases (4). Furthermore, in patients that require at least 3 weeks of critical care unit admission, Candida spp. become the most common cause of bloodstream infections (5). Among critically ill patients with severe burn injuries, infections caused by Candida spp. are increasingly common (6) and are associated with high rates of morbidity and mortality (7).
Due to the high burden of fungal infections, initiation of early and appropriate antifungal therapy that achieves optimal concentrations in plasma and at the infection site, such as the burn eschar, is essential to ensure clinical efficacy (8). In the selection of possible antifungal agents, the dose should be reviewed to ensure sufficient drug distribution to the infection site. Candida spp. can disseminate to pharmacologically protected body sites, such as the burn eschar, and an antifungal with the ability to penetrate these tissues should be used (9). Critically ill patients with severe burn injuries may present with various pathophysiologies (burns with various sizes and depths, sepsis, hypoalbuminemia, altered capillary permeability, altered renal function, various degrees of hydration, and the need for renal replacement therapies) that can significantly alter antifungal pharmacokinetics (PKs) (10). A high interindividual variability in the PKs of fluconazole, anidulafungin, caspofungin, and micafungin has been observed in this population (11, 12).
The echinocandins represent the newest class of antifungals used to treat Candida infections. Micafungin exhibits a concentration-dependent fungicidal activity (13) and has very low MICs that are similar for most Candida spp., typically ranging from 0.06 to 0.12 mg/liter (14). It has been approved for use in Europe and the United States for the treatment of invasive candidiasis, including candidemia (15). Only limited data on the PKs of antifungals in critically ill patients with burn injuries are available, and there are no specific dosage recommendations for this patient population (16–20). It is also unknown whether the PKs in burn patients are in fact different from those in other critically ill patient groups, which may mean that burn patients need their own dosing regimens.
In this context, new PK studies are needed to determine whether therapeutic concentrations in plasma and the burn eschar can be achieved with the recommended doses of micafungin to determine whether patients with severe burn injuries require doses different from those required by other critically ill patient groups.
The aim of this study was to compare the infection site population PKs of micafungin in critically ill patients with severe burn injuries with those in patients with intra-abdominal infections (IAIs). Furthermore, we aimed to use the final PK model to perform Monte Carlo dosing simulations to recommend optimized doses for these patients.
MATERIALS AND METHODS
Study design and population.
This was a prospective PK study in which critically ill adult patients with severe burn injuries were compared with patients with nosocomial peritonitis and a proven or suspected intra-abdominal fungal infection. The patients were admitted to the Burn Unit/Intensive Care Medicine Service, La Paz University Hospital/IdiPAZ Institute for Health Research (Madrid, Spain), and the Anesthesiology and Surgical Critical Care Department, Hospital del Mar (Barcelona, Spain), from April 2013 to January 2015.
The study protocol was approved by the Ethics Committee of the La Paz University Hospital (Madrid, Spain) and the Ethics Committee of the Hospital del Mar (Barcelona, Spain). Written informed consent was obtained from the patient (or his or her relatives, if the patient was unable to provide consent due to his or her critical condition) before study inclusion.
The inclusion criteria were an age of ≥18 years and a diagnosis of a severe burn injury or postsurgical nosocomial peritonitis due to anastomotic dehiscence and, in both cases, a clinical indication for micafungin therapy. The exclusion criteria were underweight (body mass index [BMI], <19 kg/m2) or morbid obesity (BMI, ≥40 kg/m2), previous treatment with micafungin, receipt of renal replacement therapy, a previous kidney transplant, or hepatic cirrhosis.
In all patients included, the following demographic and clinical data were recorded: age, gender, weight, BMI, sequential organ failure assessment (SOFA) score, percentage of total body surface area (TBSA) burned, full thickness (FT) burn, the abbreviated burn severity index (ABSI) (21), the Mannheim peritonitis index, data on treatment with micafungin, the serum creatinine (SCr) concentration at the first day of micafungin therapy, the number of days from admission to the start of micafungin, the risk for invasive candidiasis (Candida score), microbiological data, and crude mortality.
The potential relationship between the concentrations of micafungin in plasma, burn eschar tissue, and peritoneal fluid and different clinical and laboratory parameters was assessed. All patients with postsurgical intra-abdominal infection had a peritoneal catheter in situ to drain inflammatory exudate as part of standard therapy.
Micafungin dosing.
All patients were treated with 100 to 150 mg/day of micafungin (Astellas Pharma S.A., Spain) for a proven or suspected fungal infection. Micafungin was diluted in 100 ml isotonic saline solution and infused over 60 min.
Blood, peritoneal fluid, and burn tissue sample collection.
Blood samples were drawn on day 1 (after the first dose) and on day 3 or 4 of treatment (when the steady state had presumably been achieved) just before the initiation of micafungin (predose) and at different times (1, 3, 5, 8, 18, and 24 h) thereafter. In some burn patients, on day 5 of treatment, additional blood samples were obtained just before the initiation of micafungin treatment (predose) and at 1 h thereafter.
Fresh peritoneal fluid samples (1 ml) were drained by use of a vacuum suction system simultaneously with each blood sample, when possible. Tissue samples from the burn eschar were obtained on day 4 or 5 of micafungin treatment at different times after the start of the micafungin infusion: between 1 and 3 h in some patients and at 24 h in others. Tissue samples were excised from representative lesions in areas with third-degree burns.
Blood samples were collected in heparinized tubes and immediately centrifuged (at 3,000 × g for 10 min at 4°C), and burn eschar tissue and peritoneal fluid samples were collected in a tube without any additives. All samples were stored at −80°C until analysis.
Analytical method.
Total micafungin concentrations in plasma and tissues were determined by a high-performance liquid chromatography (HPLC) method (22) with minor modifications. Briefly, frozen plasma, tissue, or peritoneal fluid samples were thawed and protected from light. Protein was extracted from the blood and peritoneal fluid samples. After centrifugation at 4°C, the supernatant (50 μl) was directly injected into the HPLC column. Tissue specimens were defrosted, weighed, and homogenized with water (1:4, vol/wt) for 10 min using a high-speed tissue homogenizer. The homogenates were incubated for 10 min at 4°C and then centrifuged at 14,000 × g for 5 min.
A Waters Sunfire C18 analytical column (4.6 mm by 150 mm) was used, and the mobile phase was a mixture of 0.05 M ammonium phosphate buffer with acetonitrile. The injection volume of the samples was 50 μl. The UV absorbance for the detection of micafungin was set at 273 nm, and the chromatographic run time was 15 min. Quantification of the chromatogram was accomplished using the external standard method, and linear calibration curves were calculated from the peak areas of micafungin compared with the peak area of the external standard by spiking drug-free human serum and water with standard solutions of micafungin. The assay showed good linearity over the concentration range of 0.2 to 30 mg/liter for micafungin in plasma and 0.05 to 10 mg/liter for micafungin in water. The limits of detection and quantification were 0.05 mg/liter and 0.2 mg/liter, respectively, for plasma and 0.02 mg/liter and 0.1 mg/liter, respectively, for the other specimens (peritoneal fluid and burn tissue).
Population pharmacokinetic modeling.
Data from both burn and intra-abdominal infection patients were comodeled. Two- and three-compartment models were developed with the nonparametric adaptive grid (NPAG) algorithm within the Pmetrics package for R (Los Angeles, CA) (23, 24). Elimination from the central compartment and the intercompartmental distribution into the peritoneal fluid, burn tissue, and other compartments were modeled as first-order processes using differential equations. Estimates of assay error were included in the modeling process, and both gamma and lambda error models were tested. The area under the concentration-time curve from 0 to 24 h (AUC0–24) for plasma, peritoneal fluid, and burn tissue was calculated using the Pmetrics package.
Population pharmacokinetic covariate screening.
Covariate model building using various clinical and demographic descriptors, including sex, body weight, and the presence of burns, was performed in a stepwise fashion with forward inclusion based upon model selection criteria. If inclusion of the covariate resulted in a statistically significant improvement in the log likelihood values and/or improved the goodness-of-fit plots, then it was supported for inclusion.
Model diagnostics.
Goodness of fit was assessed by linear regression (intercept, close to zero; slope, close to 1) of the observed versus population and individual predicted concentrations and calculation of the coefficient of determination of the linear regression and log-likelihood values. Evaluation of the predictive performance was based on the mean prediction error (bias) and the mean bias-adjusted squared prediction error (imprecision) of the population and individual prediction models for the plasma, peritoneal fluid, and burn tissue compartments. A visual predictive check (VPC) using 1,000 simulations was used for further model evaluation. A statistically significant improvement in the log likelihood value (P < 0.05) was required for a more complex model to be supported.
Monte Carlo simulations of plasma concentrations and probability of target attainment.
Various Monte Carlo simulations of plasma micafungin concentrations for a hypothetical patient cohort (n = 1,000) were employed using the Pmetrics package to calculate the total AUC0–24/MIC for various MIC values (0.002 to 4 mg/liter). Intravenous doses of micafungin of 100 mg daily, 150 mg daily, and 200 mg daily for 0 to 24 h were simulated, with target micafungin exposures being defined according to total plasma AUC0–24/MIC targets of 3,000 for non-parapsilosis Candida spp. and 285 for Candida parapsilosis (25). A priori, a dosing regimen was considered successful if the probability of target attainment was >90%.
Statistical analysis.
Descriptive statistics included absolute and relative frequencies for categorical variables, means and 95% confidence intervals (CIs) for continuous quantitative variables, and medians and ranges for ordinal quantitative variables. For the analyses, a 2-sided P value of <0.05 was considered statistically significant. The Statistical Package for the Social Sciences (SPSS;, version 13.0) was used throughout.
RESULTS
During the study period, 15 critically ill patients with severe burn injuries were included, and these patients were compared with 10 postsurgical critically ill patients with intra-abdominal infections. Each patient had a proven or a suspected fungal infection. The demographic and clinical data for the burn patients and the patients with intra-abdominal infection are detailed in Table 1. The burn patients were younger and had a higher total body weight than patients with intra-abdominal infection. No differences in any other clinical characteristics were observed. In the group of burn patients, 13/15 (86.7%) presented a TBSA burned >30%. In all burn patients, the median time from the time of admission due to injury to the start of micafungin treatment was >48 h.
TABLE 1.
Clinical and demographic characteristics of burn patients and intra-abdominal infection patients
| Characteristic | Value(s) for: |
P value | |
|---|---|---|---|
| Burn patients (n = 15) | Intra-abdominal infection patients (n = 10) | ||
| Median (IQR) age (yr) | 43 (32.0–49.5) | 72 (54–77.8) | 0.015 |
| No. (%) male patients | 12 (80) | 6 (60) | NS |
| Median (IQR): | |||
| BW (kg) | 80.0 (75.0–87.5) | 65 (60.3–73.8) | 0.041 |
| BMI (kg/m2) | 26.1 (24.6–27.7) | 23.1 (21.5–24.0) | NS |
| SCr concnb (mg/dl) | 0.82 (0.62–1.06) | 0.96 (0.62–1.49) | NS |
| TBSA burned (%) | 50 (35.0–65.0) | ||
| FT burn (%) | 40 (31–57) | ||
| ABSI | 9.0 (8.0–10.5) | ||
| SOFA scoreb | 5.0 (3.0–6.0) | 5 (1.5–7.5) | NS |
| MPI | 26 (19–28.5) | ||
| Micafungin dose (mg/kg BW) | 1.3 (1.2–1.8) | 1.5 (1.3–1.7) | NS |
| No. of days from admission to start of micafungin therapy | 12 (10–15) | ||
| Candida score | 3 (2–4) | 4 (4–4) | NS |
| No. (%) of patients with: | |||
| Candidemia | 1 (6.7) | 4 (40) | NS |
| Crude mortality or nonsurvivors | 7 (46.7) | 2 (20) | NS |
F, female; M, male; BW, body weight; BMI, body mass index; SCr, serum creatinine; TBSA, total body surface area; FT, full thickness; ABSI, abbreviated burn severity index; SOFA, sequential organ failure assessment; MPI, Mannheim peritonitis index; IQR, interquartile range; NS, no significant difference.
At the beginning of micafungin therapy.
The median daily dose of micafungin normalized according to body weight was 1.25 mg/kg (interquartile range [IQR], 1.00 to 1.80 mg/kg) for the burn injury patients and 1.5 mg/kg (IQR, 1.4 to 1.7 mg/kg) for the patients with intra-abdominal infection. No differences in the micafungin dose normalized according to body weight, the severity status (measured by the SOFA score), or the serum creatinine concentration were observed between the burn and intra-abdominal infection patients (P = 0.461, 0.770, and 0.643, respectively). One (1/15, 6.7%) burn patient and 4 (4/10, 40%) patients with intra-abdominal infection were found by culture to harbor Candida albicans, and in all patients, the isolate was susceptible to micafungin (MIC value ≤ 0.016 mg/liter) (24). Micafungin was well tolerated by all patients, with no adverse effects being observed. The crude mortality rate was 36% (9/25 patients; 7 burn patients and 2 patients with intra-abdominal infections).
Micafungin concentrations.
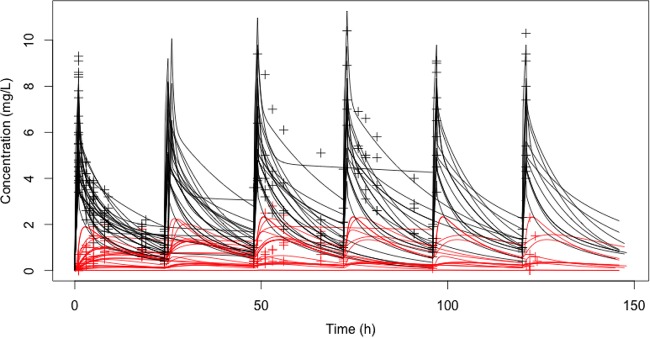
Figure 1 shows the micafungin concentrations observed in plasma and tissue for both patient groups. On day 1, the median AUC0–24 for plasma was not significantly different between the burn patients (48.3 mg · h/liter; IQR, 37.7 to 55.8 mg · h/liter) and the intra-abdominal infection patients (51.4 mg · h/liter; IQR, 44.6 to 56.4 mg · h/liter). The difference in the AUC0–24 for tissue fluid was numerically different between the groups but was not statistically significantly different: 3.8 mg · h/liter (IQR, 3.3 to 17.4 mg · h/liter) for burn tissue and 15.6 mg · h/liter (IQR, 11.8 to 17.2 mg · h/liter) for peritoneal fluid (P = 0.08). This corresponded to a median AUC0–24 for tissue fluid/AUC0–24 for plasma ratio of 0.15 (IQR, 0.06 to 0.38) for the burn patients and 0.29 (IQR, 0.24 to 0.37) for the intra-abdominal infection patients. Penetration into both tissue fluids was not statistically significantly different (P = 0.14).
FIG 1.
Observed micafungin concentrations in plasma (black symbols) and tissue fluid (red symbols). Posterior predictions of the concentration-time curve for plasma (black) and tissue fluid (red) are denoted with solid lines (n = 25 patients).
Micafungin concentrations in burn eschar.
The median concentration of micafungin in burn eschar tissue was 0.7 μg/g of tissue (IQR, 0.3 to 1.2 μg/g of tissue). In two patients, the concentration of micafungin in the burn eschar was below the limit of quantification. The concentrations of micafungin in burn eschar tissues were not linearly correlated with any of these patients' factors: micafungin daily dose adjusted according to body weight, the size of the burn (percentage of TBSA burned), or severity status (ABSI or SOFA score). A correlation between the concentrations of micafungin in plasma and those in burned tissues was also not found.
Pharmacokinetic model building.
The time course of the plasma and tissue fluid concentrations of micafungin was best described by a three-compartment linear model (Fig. 2). This model included a zero-order input of drug into the central compartment. No covariates were found to be statistically significant or improved the fit of the model.
FIG 2.
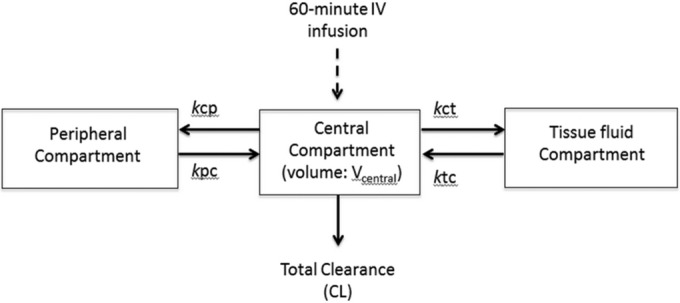
Structural pharmacokinetic model for micafungin in critically ill patients with intraperitoneal drains in situ. The model was linear and contained volume compartments for the central compartment (plasma; Vcentral), the peritoneal fluid compartment, and the peripheral compartment. Abbreviations: IV, intravenous; kcp, rate constant for the drug distribution from the central to the peripheral compartment; kpc, rate constant for the drug distribution from the peripheral to the central compartment; Vcentral, volume of distribution of the central compartment; kct, rate constant for the drug distribution from the central to the tissue fluid compartment; ktc, rate constant for the drug distribution from the tissue fluid to the central compartment; CL, clearance.
The mean ± standard deviation (SD) population PK parameter estimates from the final model are shown in Table 2. When the PK parameters between the burn and intra-abdominal infection patients were compared, no statistically significant differences were observed, except for the rate constant for the drug distribution from the tissue fluid to the central compartment (ktc), which was 3 times higher in burn patients than in the patients with intra-abdominal infection (median values, 0.47 h−1 and 0.15 h−1, respectively).
TABLE 2.
Parameter estimates for micafungin from the final three-compartment population PK modela
| Parameter | Mean ± SD value for: |
Coefficient of variation | Median | ||
|---|---|---|---|---|---|
| Burn patients (n = 15) | Intra-abdominal infection patients (n = 10) | All patients (n = 25) | |||
| CL (liters/h) | 1.61 ± 0.62 | 1.09 ± 0.72 | 1.38 ± 0.70 | 50.93 | 1.33 |
| Vcentral (liters) | 6.07 ± 2.77 | 5.85 ± 1.02 | 5.87 ± 2.44 | 41.55 | 5.21 |
| kct (h−1) | 0.59 ± 0.31 | 0.42 ± 0.35 | 0.43 ± 0.34 | 77.37 | 0.40 |
| ktc (h−1) | 0.47 ± 0.47 | 0.15 ± 0.06 | 0.32 ± 0.42 | 131.47 | 0.17 |
| kcp (h−1) | 16.87 ± 11.93 | 24.98 ± 8.03 | 19.93 ± 11.07 | 55.55 | 25.06 |
| kpc (h−1) | 9.93 ± 6.02 | 11.95 ± 4.02 | 9.90 ± 5.64 | 57.00 | 8.90 |
Data are presented as the mean ± SD for burn patients only, intra-abdominal infection patients only, and all patients combined and as the coefficient of variation and median for all patients. Boldface data indicate a statistically significant difference (P < 0.05) between the burn and intra-abdominal infection patients. CL, clearance; Vcentral, volume of distribution of the central compartment; kct, rate constant for the drug distribution from the central to tissue fluid compartment; ktc, rate constant for the drug distribution from the tissue fluid to the central compartment; kcp, rate constant for the drug distribution from the central to the peripheral compartment; kpc, rate constant for the drug distribution from the peripheral to the central compartment.
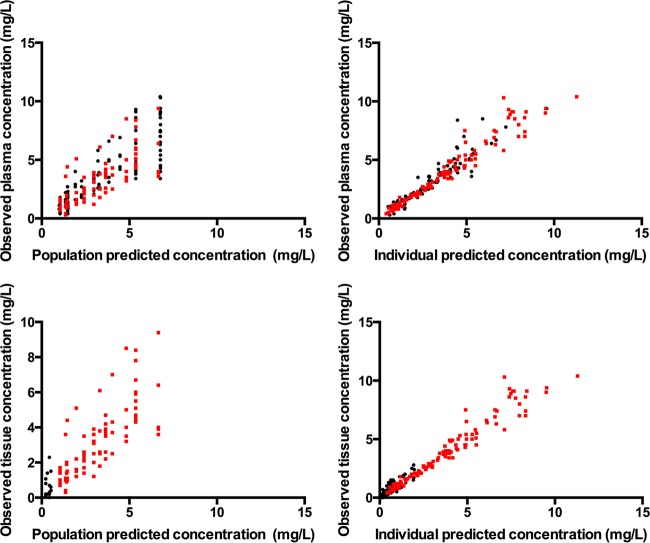
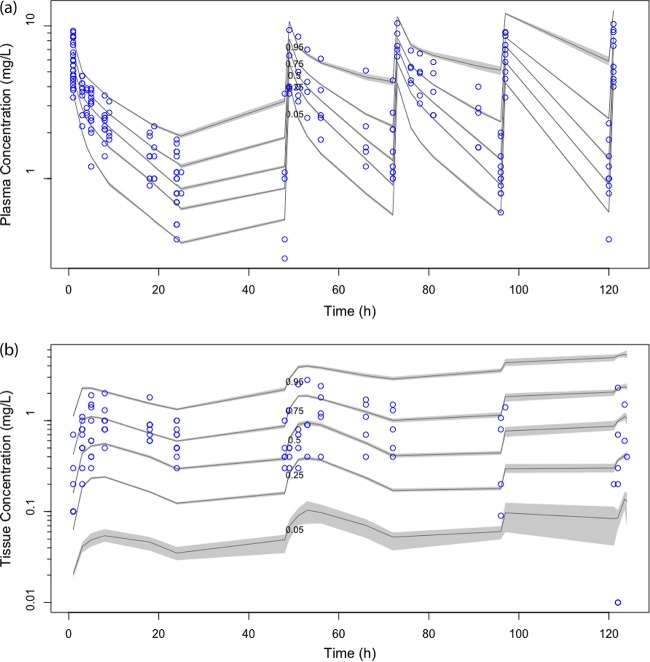
The diagnostic plots confirmed that the goodness of fit of the model was acceptable, as shown in Fig. 3 (observed versus population and individual predicted concentrations) and in Fig. 4a (VPC of plasma data) and Fig. 4b (VPC of tissue data). The final model was used for dosing simulations.
FIG 3.
Diagnostic plots for the final covariate model. (Top) Observed versus population predicted concentrations and individual predicted concentrations in plasma; (bottom) observed versus population predicted concentrations and individual predicted concentrations in tissue fluid. Red points, data for patients with intra-abdominal infections; black points, data for burn patients.
FIG 4.
Visual predictive check (n = 1,000 simulations) of plasma data (a) and tissue data (b) from the final covariate model showing that the population pharmacokinetic model has adequate performance; the raw data for individual patients are shown as dots, and the continuous lines represent the 5th, 25th, 50th, 75th, and 95th percentiles of the simulations.
Probability of target attainment.
The probabilities of target attainment for the simulated targets on day 1 (0 to 24 h) in plasma for a range of micafungin doses against the MIC distributions for Candida spp. and C. parapsilosis are shown in Tables 3 and 4, respectively. Only day 1 was simulated because no differences in PKs were observed between sampling occasions.
TABLE 3.
Probability of target attainment using a AUC0–24/MIC target of 3,000 for non-parapsilosis Candida spp. for different micafungin dosing regimens
| MIC (mg/liter) | Probability of target attainment for daily micafungin dose ofa: |
||
|---|---|---|---|
| 100 mg daily | 150 mg daily | 200 mg daily | |
| 0.001 | 0.999 | 0.999 | 0.999 |
| 0.002 | 0.998 | 0.998 | 0.999 |
| 0.004 | 0.994 | 0.998 | 0.998 |
| 0.008 | 0.975 | 0.992 | 0.994 |
| 0.016 | 0.58 | 0.899 | 0.975 |
| 0.032 | 0.037 | 0.253 | 0.58 |
| 0.064 | 0.002 | 0.009 | 0.037 |
| 0.125 | 0 | 0.001 | 0.002 |
| 0.25 | 0 | 0 | 0 |
The boldface data represent dosing regimens that achieved a >90% probability of target attainment.
TABLE 4.
Probability of target attainment using a AUC0–24/MIC target of 285 for Candida parapsilosis for different micafungin dosing regimens
| MIC (mg/liter) | Probability of target attainment for daily micafungin dose ofa: |
||
|---|---|---|---|
| 100 mg daily | 150 mg daily | 200 mg daily | |
| 0.001 | 1 | 1 | 1 |
| 0.002 | 1 | 1 | 1 |
| 0.004 | 1 | 1 | 1 |
| 0.008 | 0.999 | 0.999 | 1 |
| 0.016 | 0.998 | 0.999 | 0.999 |
| 0.032 | 0.997 | 0.998 | 0.998 |
| 0.064 | 0.99 | 0.994 | 0.997 |
| 0.125 | 0.845 | 0.975 | 0.99 |
| 0.25 | 0.169 | 0.589 | 0.845 |
| 0.5 | 0.005 | 0.038 | 0.169 |
| 1 | 0 | 0.002 | 0.005 |
| 2 | 0 | 0 | 0 |
The boldface data represent dosing regimens that achieved a >90% probability of target attainment.
In the simulations, the plasma PK/pharmacodynamic (PD) targets were achieved with a 90% probability for Candida spp. and C. parapsilosis with MICs of 0.008 and 0.064, respectively, for the 100-mg and 150-mg daily doses. The 200-mg daily dose achieved these PK/PD targets at MICs of 0.016 mg/liter and 0.125 mg/liter for Candida spp. and C. parapsilosis, respectively. It should be noted that these simulations did not discriminate between the burn and peritonitis patients because the covariate analysis did not identify significant differences between these two populations.
DISCUSSION
This is the first study of micafungin population PKs in plasma and burn eschar tissue in critically ill patients with severe burn injuries. We elected to compare the PKs of micafungin in patients with burns to those in patients with intra-abdominal infections because, although many clinicians would consider them to be different critically ill subpopulations, it is still recommended that both groups receive the same drug doses. Therefore, we hypothesized that if we determined any significant differences in PKs between these groups, it may lead to different dosing recommendations for the two subpopulations. When the PK parameter estimates for the burn patients were compared to those for the intra-abdominal infection patients, no statistically significant differences were observed between the groups, except for the rate constant for the drug distribution from the tissue fluid to central compartment (ktc) (0.47 ± 0.47 versus 0.15 ± 0.06 h−1, respectively; P < 0.05). Therefore, the use of different dosing regimens for the individual groups is not supported by these data. We observed that the standard dose of micafungin, 100 mg/day, achieves optimal PK/PD targets in plasma for MIC values of ≤0.008 mg/liter and ≤0.064 mg/liter for non-parapsilosis Candida spp. and Candida parapsilosis, respectively. By increasing the dose to 200 mg/day, the optimal PK/PD targets in plasma could be achieved for MIC cutoff values that are twofold higher (0.016 mg/liter and 0.125 mg/liter, respectively).
In this study, no differences in the PKs of micafungin were observed between the two populations of critically ill patients studied, even though the burn patients had a greater body weight than those with intra-abdominal infection. The plasma exposure to micafungin in our whole cohort (median AUC0–24 for plasma on day 1, 48.3 mg · h/liter [IQR, 37.7 to 55.8 mg · h/liter] and 51.4 mg · h/liter [IQR, 44.6 to 56.4 mg · h/liter] in burn patients and patients with intra-abdominal infections, respectively) was similar to that achieved in patients with invasive candidiasis and candidemia (mean ± SD AUC0–24, 56.6 ± 30.1 mg · h/liter) (25) but slightly lower than that reported in other critically ill patients (median AUC0–24, 78.6 mg · h/liter; IQR, 65.3 to 94.1 mg · h/liter) (11). Regarding the PK parameters, the estimated clearance for all patients (burn and intra-abdominal infection patients) was similar to that reported in patients with invasive candidiasis and candidemia (1.44 ± 0.73 liters/h) (26) and also in other critically ill patients (1.3 ± 1.1 to 1.5 liters/h) (12).
Information describing the PKs of antifungals in critically ill patients is limited, especially for echinocandins, which is of concern, given that it has been widely described how critical illness may significantly alter the PKs of many agents and compromise their efficacy (12, 27, 28). A prospective, multicenter study of the PKs of different antifungals that enrolled patients from 68 intensive care units across Europe reported a considerable interindividual variability in the PKs of caspofungin and anidulafungin, and the authors suggested the need to optimize the doses to reduce this variability (11). Unfortunately, patients treated with micafungin were not included in that study.
In the critically ill subpopulation of severely burned patients, data on the PKs of echinocandins are even scarcer (16, 18, 20). To date, we are aware of only 3 reports of the PKs of echinocandins in patients with severe burn injuries (16, 20, 29). Sasaki et al. performed a study of the PKs of 200 to 300 mg/day of micafungin in 6 patients with severe burns and reported plasma concentrations much higher than those observed in our study (16). However, that study administered a dose that was 2 to 3 times higher than the dose administered to our patients. Furthermore, the amount of time that elapsed between the start of micafungin therapy after injury was shorter (mean times, 7.7 days in the previous study [16] compared to 16.2 days in the present study). Indeed, it may be that the hypermetabolic phase (beyond 48 h after the burn injury), which can affect the intensity of physiological changes over different days, contributed to the altered and highly variable PKs (11).
This lower level of exposure to micafungin observed in our patients has also been reported for other antifungals in burn patients (17, 29, 30). The PKs of caspofungin were assessed in two burn patients, and the exposure was similar to that observed in healthy volunteers in one patient, but it was 50% lower in the other (29). Unfortunately, the concentrations in burn eschar tissue were not reported in that study. Another report described the PKs of fluconazole in nine patients with extensive burns injuries and demonstrated that the patients had higher clearances and volumes of distribution than healthy subjects (17).
An important limitation of our study might prevent a more accurate determination of the micafungin concentrations in burned tissues. That is, the burn eschar tissue homogenate combines intra- and extracellular material, and given that echinocandins do not penetrate intracellularly well, the current results may underestimate the interstitial fluid concentrations of micafungin. Additionally, tissue samples from eschars had different degrees of burns and were obtained at different times after the start of the micafungin infusion, which may have contributed to the variability of the observed concentrations.
Micafungin showed a low level of penetration into the eschars, with a median AUC0–24 for tissue fluid/AUC0–24 for plasma ratio of 0.15 (IQR, 0.06 to 0.38). To our knowledge, only one previous paper has reported the penetration of micafungin into burn tissues, but it was performed with only 3 patients and reported a very high interindividual variability in the peak concentration of micafungin in burn eschar (mean ± SD value after repeated administration, 6.65 ± 7.22 μg/ml) (20). However, in the previous study, the concentration of micafungin was expressed in different units (micrograms per milliliter instead of micrograms per gram of tissue), which makes any comparison with our results difficult.
Ensuring effective dosing likely maximizes the likelihood of clinical cure of invasive fungal infections in critically ill patients (31). AUC/MIC ratios of 3,000 and 285 for micafungin have been associated with positive therapeutic outcomes in a population pharmacokinetic/pharmacodynamic model of patients with invasive candidiasis or candidemia caused by C. albicans and C. parapsilosis, respectively (25). Micafungin at a dose of 100 to 150 mg would achieve a PK/PD target in plasma for non-parapsilosis Candida spp. and Candida parapsilosis species with MIC values of ≤0.008 mg/liter and ≤0.064 mg/liter, respectively. By increasing the dose to 200 mg/day, these targets would be achieved for the current EUCAST susceptibility breakpoint for C. albicans (0.016 mg/liter) but not for C. glabrata (0.03 mg/liter) and C. parapsilosis (2 mg/liter) (32).
This study has other limitations that we would like to declare. First, this was a small study which may not have been able to describe the true PK variability in this population. Nevertheless, this is the first population PK study of antifungals in severely burned patients. Second, we compared the exposure to micafungin between two tissue fluids with different characteristics, such as inflammatory status and protein concentrations, what could probably have influenced the degree of penetration of this antifungal. Finally, we could not correlate PK/PD target attainment with the patients' clinical response due to the low number of patients with a microbiologically confirmed infection.
In conclusion, this is the first population study of the PKs of micafungin in plasma and burn eschar tissue in critically ill patients with severe burn injuries, which were compared with the PKs of micafungin in patients with intra-abdominal infections. The PKs of micafungin in this subpopulation did not seem to differ from those in other critically ill patients, such as those with intra-abdominal infection. In burn patients, the level of penetration of micafungin into the burn eschar that was observed was low and showed a high level of interindividual variability. We found that micafungin at 100 to 150 mg/day achieved suboptimal exposure in plasma for the treatment of fungal infections caused by Candida strains by use of the current susceptibility breakpoints (1).
ACKNOWLEDGMENTS
J.A.R. received salary funding from the National Health and Medical Research Council of Australia (APP1048652). We acknowledge funding from the Australian National Health and Medical Research Council for a Centre of Research Excellence (APP1099452). This work was also supported in part by a grant from Astellas Pharma S.A. (Madrid, Spain).
A.G.-D.-L. has received fees from Astellas Pharma for participation in an expert meeting. A.A. has received travel grants from Astellas Pharma (Madrid, Spain) for ECCMID 2013 and Spanish National Mycology Congress attendance. S.L. has received travel grants from Astellas Pharma (Madrid, Spain) for ECCMID 2014 attendance. None of the other authors has a conflict to declare.
Funding Statement
This work was supported in part by a grant from Astellas Pharma S.A. (Madrid, Spain).
REFERENCES
- 1.Kasten KR, Makley AT, Kagan RJ. 2011. Update on the critical care management of severe burns. J Intensive Care Med 26:223–236. doi: 10.1177/0885066610390869. [DOI] [PubMed] [Google Scholar]
- 2.Horvath EE, Murray CK, Vaughan GM, Chung KK, Hospenthal DR, Wade CE, Holcomb JB, Wolf SE, Mason AD Jr, Cancio LC. 2007. Fungal wound infection (not colonization) is independently associated with mortality in burn patients. Ann Surg 245:978–985. doi: 10.1097/01.sla.0000256914.16754.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballard J, Edelman L, Saffle J, Sheridan R, Kagan R, Bracco D, Cancio L, Cairns B, Baker R, Fillari P, Wibbenmeyer L, Voight D, Palmieri T, Greenhalgh D, Kemalyan N, Caruso D, Multicenter Trials Group American Burn Association. 2008. Positive fungal cultures in burn patients: a multicenter review. J Burn Care Res 29:213–221. doi: 10.1097/BCR.0b013e31815f6ecb. [DOI] [PubMed] [Google Scholar]
- 4.Capoor MR, Gupta S, Sarabahi S, Mishra A, Tiwari VK, Aggarwal P. 2012. Epidemiological and clinico-mycological profile of fungal wound infection from largest burn centre in Asia. Mycoses 55:181–188. doi: 10.1111/j.1439-0507.2011.02065.x. [DOI] [PubMed] [Google Scholar]
- 5.Patel BM, Paratz JD, Mallet A, Lipman J, Rudd M, Muller MJ, Paterson DL, Roberts JA. 2012. Characteristics of bloodstream infections in burn injury patients: an 11-year retrospective study. Burns 38:685–690. doi: 10.1016/j.burns.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Lipman J, Saadia R. 1997. Fungal infections in critically ill patients. BMJ 315:266–267. doi: 10.1136/bmj.315.7103.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore EC, Padiglione AA, Wasiak J, Paul E, Cleland H. 2010. Candida in burns: risk factors and outcomes. J Burn Care Res 31:257–263. doi: 10.1097/BCR.0b013e3181d0f536. [DOI] [PubMed] [Google Scholar]
- 8.Felton T, Troke PF, Hope WW. 2014. Tissue penetration of antifungal agents. Clin Microbiol Rev 27:68–88. doi: 10.1128/CMR.00046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black KE, Baden LR. 2007. Fungal infections of the CNS: treatment strategies for the immunocompromised patient. CNS Drugs 21:293–318. doi: 10.2165/00023210-200721040-00004. [DOI] [PubMed] [Google Scholar]
- 10.Weinbren MJ. 1999. Pharmacokinetics of antibiotics in burn patients. J Antimicrob Chemother 44:319–327. doi: 10.1093/jac/44.3.319. [DOI] [PubMed] [Google Scholar]
- 11.Sinnollareddy MG, Roberts JA, Lipman J, Akova M, Bassetti M, De Waele JJ, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Dimopoulos G, DALI Study Authors. 2015. Pharmacokinetic variability and exposures of fluconazole, anidulafungin, and caspofungin in intensive care unit patients: data from multinational Defining Antibiotic Levels in Intensive Care Unit (DALI) Patients study. Crit Care 19:33. doi: 10.1185/03007990902990817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lempers VJ, Schouten JA, Hunfeld NG, Colbers A, van Leeuwen HJ, Burger DM, Verweij PE, Pickkers P, Brüggemann RJ. 2015. Altered micafungin pharmacokinetics in intensive care unit patients. Antimicrob Agents Chemother 59:4403–4409. doi: 10.1128/AAC.00623-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandrasekar PH, Sobel JD. 2006. Micafungin: a new echinocandin. Clin Infect Dis 42:1771–1778. [DOI] [PubMed] [Google Scholar]
- 14.Lewis RE. 2009. Overview of the changing epidemiology of candidemia. Curr Med Res Opin 25:1732–1740. doi: 10.1185/03007990902990817. [DOI] [PubMed] [Google Scholar]
- 15.Pappas PG, Rotstein CM, Betts RF, Nucci M, Talwar D, De Waele JJ, Vazquez JA, Dupont BF, Horn DL, Ostrosky-Zeichner L, Reboli AC, Suh B, Digumarti R, Wu C, Kovanda LL, Arnold LJ, Buell DN. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis 45:883–893. doi: 10.1086/520980. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki J, Yamanouchi S, Kudo D, Endo T, Nomura R, Takuma K, Kushimoto S, Shinozawa Y, Kishino S, Hori S, Aikawa N. 2012. Micafungin concentrations in the plasma and burn eschar of severely burned patients. Antimicrob Agents Chemother 56:1113–1115. doi: 10.1128/AAC.05381-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boucher BA, King SR, Wandschneider HL, Hickerson WL, Hanes SD, Herring VL, Canada TW, Hess MM. 1998. Fluconazole pharmacokinetics in burn patients. Antimicrob Agents Chemother 42:930–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han S, Kim J, Yim H, Hur J, Song W, Lee J, Jeon S, Hong T, Woo H, Yim DS. 2013. Population pharmacokinetic analysis of fluconazole to predict therapeutic outcome in burn patients with Candida infection. Antimicrob Agents Chemother 57:1006–1011. doi: 10.1128/AAC.01372-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asensio MJ, Sánchez M, Galván B, Herrero E, Cachafeiro L, Agrifoglio A, Perales E, Luque S, García-de-Lorenzo A. 2015. Micafungin at a standard dosage of 100 mg/day achieves adequate plasma exposure in critically ill patients with severe burn injuries. Intensive Care Med 41:371–372. doi: 10.1007/s00134-014-3586-z. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki J, Yamanouchi S, Sato Y, Abe S, Shinozawa Y, Kishino S, Aikawa N, Hori S. 2014. Penetration of micafungin into the burn eschar in patients with severe burns. Eur J Drug Metab Pharmacokinet 39:93–97. doi: 10.1007/s13318-013-0146-9. [DOI] [PubMed] [Google Scholar]
- 21.Hörbrand F, Schrank C, Henckel-Donnersmarck G, Mühlbauer W. 2003. Integration of preexisting diseases and risk factors in the abbreviated burn severity index (ABSI). Anasthesiol Intensivmed Notfallmed Schmerzther 38:151–157. doi: 10.1055/s-2003-37773. [DOI] [PubMed] [Google Scholar]
- 22.Martens-Lobenhoffer J, Rupprecht V, Bode-Böger SM. 2011. Determination of micafungin and anidulafungin in human plasma: UV- or mass spectrometric quantification? J Chromatogr B Analyt Technol Biomed Life Sci 879:2051–2056. doi: 10.1016/j.jchromb.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 23.Tatarinova T, Neely M, Bartroff J, van Guilder M, Yamada W, Bayard D, Jelliffe R, Leary R, Chubatiuk A, Schumitzky A. 2013. Two general methods for population pharmacokinetic modeling: non-parametric adaptive grid and non-parametric Bayesian. J Pharmacokinet Pharmacodyn 40:189–199. doi: 10.1007/s10928-013-9302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andes D, Ambrose PG, Hammel JP, Van Wart SA, Iyer V, Reynolds DK, Buell DN, Kovanda LL, Bhavnani SM. 2011. Use of pharmacokinetic-pharmacodynamic analyses to optimize therapy with the systemic antifungal micafungin for invasive candidiasis or candidemia. Antimicrob Agents Chemother 55:2113–2121. doi: 10.1128/AAC.01430-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Undre N, Stevenson P, Baraldi E. 2012. Pharmacokinetics of micafungin in HIV positive patients with confirmed esophageal candidiasis. Eur J Drug Metab Pharmacokinet 37:31–38. doi: 10.1007/s13318-011-0063-8. [DOI] [PubMed] [Google Scholar]
- 27.Grau S, Luque S, Campillo N, Samsó E, Rodríguez U, García-Bernedo CA, Salas E, Sharma R, Hope WW, Roberts JA. 2015. Plasma and peritoneal fluid population pharmacokinetics of micafungin in post-surgical patients with severe peritonitis. J Antimicrob Chemother 70:2854–2861. doi: 10.1093/jac/dkv173. [DOI] [PubMed] [Google Scholar]
- 28.Maseda E, Grau S, Villagran MJ, Hernandez-Gancedo C, Lopez-Tofiño A, Roberts JA, Aguilar L, Luque S, Sevillano D, Gimenez MJ, Gilsanz F. 2014. Micafungin pharmacokinetic/pharmacodynamic adequacy for the treatment of invasive candidiasis in critically ill patients on continuous venovenous haemofiltration. J Antimicrob Chemother 69:1624–1632. doi: 10.1093/jac/dku013. [DOI] [PubMed] [Google Scholar]
- 29.Jullien V, Blanchet B, Benyamina M, Tod M, Vinsonneau C. 2012. Pharmacokinetics of caspofungin in two patients with burn injuries. Antimicrob Agents Chemother 56:4550–4551. doi: 10.1128/AAC.00039-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rayatt S, Wienbren M, Clarke J. 2000. Fluconazole use in burns patients. Burns 26:109–110. doi: 10.1016/S0305-4179(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 31.Smith JA, Kauffman CA. 2010. Recognition and prevention of nosocomial invasive fungal infections in the intensive care unit. Crit Care Med 38(8 Suppl):S380–S387. doi: 10.1097/CCM.0b013e3181e6cf25. [DOI] [PubMed] [Google Scholar]
- 32.EUCAST. 2013. Micafungin and Candida spp.: rationale for the EUCAST clinical breakpoints, version 1.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Micafungin_rationale_document_1_0_final.pdf.