Abstract
Tachyplesin I is a 17-amino-acid cationic antimicrobial peptide (AMP) with a typical cyclic antiparallel β-sheet structure that is a promising therapeutic for infections, tumors, and viruses. To date, no bacterial resistance to tachyplesin I has been reported. To explore the safety of tachyplesin I as an antibacterial drug for wide clinical application, we experimentally induced bacterial resistance to tachyplesin I by using two selection procedures and studied the preliminary resistance mechanisms. Aeromonas hydrophila XS91-4-1, Pseudomonas aeruginosa CGMCC1.2620, and Escherichia coli ATCC 25922 and F41 showed resistance to tachyplesin I under long-term selection pressure with continuously increasing concentrations of tachyplesin I. In addition, P. aeruginosa and E. coli exhibited resistance to tachyplesin I under UV mutagenesis selection conditions. Cell growth and colony morphology were slightly different between control strains and strains with induced resistance. Cross-resistance to tachyplesin I and antimicrobial agents (cefoperazone and amikacin) or other AMPs (pexiganan, tachyplesin III, and polyphemusin I) was observed in some resistant mutants. Previous studies showed that extracellular protease-mediated degradation of AMPs induced bacterial resistance to AMPs. Our results indicated that the resistance mechanism of P. aeruginosa was not entirely dependent on extracellular proteolytic degradation of tachyplesin I; however, tachyplesin I could induce increased proteolytic activity in P. aeruginosa. Most importantly, our findings raise serious concerns about the long-term risks associated with the development and clinical use of tachyplesin I.
INTRODUCTION
Antibiotic resistance in pathogenic bacteria and the emergence of superbacteria have attracted attention from health care workers worldwide. Antimicrobial peptides (AMPs) are promising candidates for development of novel alternative antibiotics. However, studies have indicated that some bacteria (especially human pathogens) are resistant to certain AMPs (1, 2), and bacterial resistance to cationic AMPs can arise through long and continual selection in the laboratory (3). Furthermore, some evidence demonstrates that bacteria can evolve resistance to AMPs after extended clinical application (4). Bacterial resistance to the AMPs nisin, pexiganan, and colistin has arisen through their clinical use. There is also evidence that pathogen resistance to AMPs may produce resistance to other AMPs with the same source and similar action mechanisms (5), or even to those with different sources and different action mechanisms (6, 7). Habets and Brockhurst previously reported that therapeutic use of pexiganan, representing a promising new class of AMPs, could drive the evolution of pathogens that are resistant to our own immunity peptides (6). Bacterial evolution of AMP resistance would greatly shorten and restrict the use of AMPs, so elucidation of the potential mechanisms of bacterial resistance to AMPs is required to inform the design of more effective drugs and to reduce the incidence of bacterial drug resistance.
Molecular mechanisms of bacterial resistance to AMPs have been studied mainly by whole-genome sequencing, transcriptome sequencing (RNA-seq), microarray analysis, two-dimensional protein gel electrophoresis, and gene knockout and overexpression studies. Previous studies indicated that Gram-negative pathogens exhibit several different AMP resistance mechanisms, including proteolytic degradation of AMPs, shielding of the bacterial surface, modification of the bacterial outer membrane, pumping of AMPs into or out of the cell, and downregulation of AMP expression (8, 9).
Tachyplesin I, a cationic AMP with a disulfide-stabilized β-sheet conformation, was originally isolated from hemocytes of marine horseshoe crabs in 1988 (10). With potent and broad-spectrum activity against both Gram-positive and Gram-negative bacteria, tachyplesin I was a promising candidate for development of anti-infection, antitumor, and antivirus drugs. Previous studies showed that tachyplesin I can kill bacteria by permeabilizing the bacterial membrane and acting on intracellular targets in bacteria to inhibit DNA, RNA, and protein synthesis and enzyme activity (11–13). Tachyplesin I acts similarly to magainin-2, MSI-78, and LL-37 by forming a toroidal transmembrane pore (14). Thus, tachyplesin I exhibits multiple effects and synergistic antibacterial mechanisms, and the development of X33-GAP-TPI yeast strains that express recombinant tachyplesin I at high levels has facilitated its use as a novel alternative antibiotic for wide use in pharmaceuticals and animal feed in the future (15, 16).
There are few reports on bacterial resistance to tachyplesin I. We previously reported that neither nonsuccessive induction nor short-term successive induction (fewer than 20 serial passages) could induce resistance to tachyplesin I in Escherichia coli ATCC 25922 and F41 or Staphylococcus aureus ATCC 25923 (17), but we are unaware of any other studies in this area. Our results indicated that bacterial resistance to tachyplesin I was not easy to induce through short-term exposure, consistent with a previous report examining other AMPs (18). However, whether bacteria can evolve resistance to tachyplesin I under long-term continuous selection conditions remains unknown. A previous report on the mechanism of bacterial resistance to tachyplesin I indicated only that the Dlt and MprF cell surface proteins on Gram-positive bacteria (S. aureus) might mediate tachyplesin I resistance (19). Although no tachyplesin I-resistant strains have appeared in clinical use, whether bacteria (especially pathogenic bacteria) can evolve resistance to tachyplesin I with long-term clinical use remains unclear. To prevent repetition of the mistakes made with antibiotic use and to avoid or delay the evolution of bacterial resistance to tachyplesin I, it is imperative to understand whether bacteria can produce resistance under long-term selection pressure and to elucidate the underlying resistance mechanisms.
The goal of this study was to determine if tachyplesin I can induce resistance in bacteria after long-term exposure to tachyplesin I or UV mutagenesis. We employed both selection methods for Aeromonas hydrophila XS91-4-1, Pseudomonas aeruginosa CGMCC1.2620, and E. coli ATCC 25922 and F41, monitored bacterial resistance, characterized the stability, cross-resistance, cost of resistance, and ultrastructure of mutant strains, and investigated the potential role of extracellular proteases in the resistance mechanism. Our results provide a theoretical basis for the wide application of tachyplesin I and a preliminary analysis of bacterial resistance mechanisms.
MATERIALS AND METHODS
Microorganisms, media, and growth conditions.
E. coli ATCC 25922 was provided by the Microbial Culture Collection Center of Guangdong (GIMCC), China. P. aeruginosa CGMCC1.2620 was provided by the China General Microbiological Culture Collection Center (CGMCC). A. hydrophila XS91-4-1 was provided by Aihua Li of the Institute of Aquatic Biology, Chinese Academy of Sciences, China. Other strains were from the laboratory of the College of Life Science and Engineering, Henan University of Urban Construction, China. A. hydrophila XS91-4-1 was cultured on soybean-casein digest agar medium (Trypticase soy agar [TSA]) and in Trypticase soy broth (TSB) at 28°C; the other bacteria were cultured in Mueller-Hinton broth (MHB) and on nutrient agar plates at 37°C.
Antibacterial agents.
The AMPs used in this study were tachyplesin I, tachyplesin III, polyphemusin I, and pexiganan. These AMPs (>95% purity) were all synthesized by Gil Biochemical Co., Ltd. (Shanghai, China), and the peptide sequences were as follows: tachyplesin I, NH2-K-W-C-F-R-V-C-Y-R-G-I-C-Y-R-R-C-R-CONH2, including two disulfide bonds (C-3—C-16 and C-7—C-12) (10); pexiganan, G-I-G-K-F-L-K-K-A-K-K-F-G-K-A-F-V-K-I-L-K-K-NH2 (20); tachyplesin III, K-W-C-F-R-V-C-Y-R-G-I-C-Y-R-K-C-R-NH2 (21); and polyphemusin I, R-R-W-C-F-R-V-C-Y-R-G-F-C-Y-R-K-C-R-NH2 (22). These AMPs were dissolved in sterile water to yield 10-mg/ml stock solutions, which were filter sterilized before use. Peptide solutions were prepared fresh on the day of the assay or stored at −20°C for a short period.
Antibacterial drug-sensitive papers were provided by Hangzhou Microbial Reagent Co., Ltd. (China).
MIC determination.
The MIC was determined using a standard broth microdilution method for AMPs as described previously by the Clinical and Laboratory Standards Institute (CLSI). The MIC was determined as the lowest concentration for which no visible growth was observed. Briefly, cultured cells in the log phase were diluted to 2 × 105 to 4 × 105 cells/ml. The inoculum (100 μl) was added to each well of 96-well plates. Peptide samples diluted with fresh broth (100 μl) were added to each well, and the plates were incubated at 37°C or 28°C for 20 h. Experiments were performed in triplicate.
In vitro resistance study. (i) Long-term exposure to tachyplesin I.
Bacteria were cultured in MHB with constant shaking at 160 rpm at 37°C (E. coli strains and P. aeruginosa) or 28°C (A. hydrophila). We transferred bacteria daily by inoculating 20 μl of stationary-phase culture into 2 ml of MHB. All cells were initially grown in medium without tachyplesin I for 5 transfers. At transfer 6, 20 μl of cell suspension was added to 2 ml of nutrient broth, with or without tachyplesin I at a final concentration of half the MIC, for 20 h with shaking at 160 rpm, for 15 transfers. The regrown bacteria were thereafter transferred to broth containing a double concentration of tachyplesin I every 10 transfers, or more frequently if the selection strains showed weak growth. The experiment was conducted for 69 to 96 serial transfers. Experiments were performed in duplicate.
Every time we increased the concentration of tachyplesin I, a sample of the induction generation was inoculated into 20% (vol/vol) glycerol and stored at −70°C. The MIC value of tachyplesin I for each induced bacterial culture was determined as described above.
(ii) UV mutagenesis selection.
Log-phase cells (107 cells/ml) were collected and added to sterile petri dishes with a magnetic stir bar. E. coli ATCC 25922 and P. aeruginosa CGMCC1.2620 cells were mutated for 60 s and 90 s, respectively, by use of a UV lamp (irradiation distance, 28 cm; 18 W). After UV treatment, the bacteria were plated on nutrient agarose plates with high concentrations of tachyplesin I and incubated at 37°C for 20 h. A single colony was selected and cultured in nutrient broth overnight at 37°C. The cultured cells were used to determine the MIC of tachyplesin I for each selection strain.
In vitro susceptibility testing.
We assayed the resistance induced by measuring the MIC of tachyplesin I for each selection strain as described above. Based on a previous report (23), we identified bacterial resistance to AMPs as a significant increase in the MIC for mutant strains compared to that for control strains.
Assay of the stability of resistance.
To test the stability of resistance, 10 μl of a resistant strain was cultured in 2 ml of nutrient broth medium without tachyplesin I for 5 or 10 continuous passages of 20 h each. The MIC value for each passage strain was determined.
Cross-resistance assay.
The antimicrobial susceptibilities of resistant bacteria to several antimicrobial agents were determined by the K-B disc diffusion method. The inhibitory zone diameter represents the bacterial susceptibility to the antimicrobial agent. Each experiment was performed in triplicate. The K-B disc diffusion method was performed according to the criteria for the sensitivity and drug resistance of antimicrobial agents established by CLSI guidelines.
The susceptibilities of resistant mutants to synthetic tachyplesin III, polyphemusin I, and pexiganan AMPs were determined by MIC assays. The MIC values of these peptides were determined as described above.
Cost of resistance.
To evaluate whether resistance altered the physiology of the isolates, we estimated the maximum absorbance, the maximum growth rate, and the lag phase for all isolates of resistant strains and control strains in unsupplemented MHB and in MHB containing tachyplesin I at half the MIC for control strains. We measured the optical density at 600 nm (OD600) on a Spectramax M2 model microplate reader (PerkinElmer Instruments Co., Ltd., Shanghai, China) every hour.
Electron microscopy.
Electron microscopy was performed on the evolved and control E. coli ATCC 25922 strains. The samples were prepared for transmission electron microscopy (TEM) as described previously (13). The bacteria were exposed to 20 μg/ml tachyplesin I for 60 min at 37°C.
Protease assay.
Extracellular protease activity of the bacteria was determined using an agar plate assay as previously described (24), with minimal modifications. The test agar contained 1% skim milk, 4% glucose, and 3.0% agar powder. Cultures were grown at 37°C for 12 or 24 h, and then 10 ml of culture was filtered through 0.22-μm-pore-size filters. Next, 60 μl filtrate was loaded into holes in the agar plates, and plates were incubated at 37°C for 20 to 24 h.
Zymographic assay.
Zymographic assays were performed as previously described (24), with minimal modifications. SDS-polyacrylamide gels (12%) were copolymerized with skim milk at a final concentration of 1%. After electrophoresis, SDS was removed by washing the gel twice with 50 mM Tris-HCl (pH 7.8) and 2.5% (vol/vol) Triton X-100 for 30 min, followed by overnight incubation at 37°C in a buffer containing 50 mM Tris-HCl, 5 mM CaCl2, pH 7.8, 0.1% (vol/vol) Triton X-100, and 0.02% NaN3. Gels were stained for 1 h at room temperature with 0.1% Coomassie brilliant blue R-250 in 10% acetic acid and then destained with 10% acetic acid until clear bands over a dark background were observed.
Degradation assay.
Peptide samples (40 μg/ml) in 20 mM Tris-HCl and 1 mM CaCl2 (pH 7.8) were mixed with an equal volume of culture filtrate. The culture filtrate samples were prepared using the methods described above. The mixtures were incubated at 37°C for 5 h, and degradation was analyzed as follows. (i) Degradation was analyzed by use of 16.5% Tricine-SDS-polyacrylamide gels. Based on the determined molecular masses of peptide degradation products, the protease cleavage sites in the peptides were determined. (ii) Degradation was analyzed by determining the antibacterial activity of tachyplesin I pretreated with culture filtrates of P. aeruginosa by using the broth microdilution method.
Protease expression assay.
After bacterial cultures (P. aeruginosa, A. hydrophila, or E. coli) were grown for 3 h, tachyplesin I was added to a final concentration of 20 μg/ml. After continued growth for 10 h or 15 h, the supernatants were collected and filtered, and proteolytic activity and protease expression were analyzed using the methods described above.
RESULTS
Selection of resistance to tachyplesin I.
To explore the development of resistance to tachyplesin I in vitro, two selection procedures were performed, as follows.
(i) Selection by exposure to increasing tachyplesin I concentrations.
Resistance to tachyplesin I developed after 69 serial transfers only in A. hydrophila XS91-4-1 under long-term selection by exposure to increasing concentrations of tachyplesin I. The other strains were successively exposed to increasing concentrations of tachyplesin I for no fewer than 80 serial transfers under the same conditions. There were marked differences in the MIC values for E. coli F41 and P. aeruginosa between the control induction strains and the corresponding treatment induction strains by transfer 80 (from 10 μg/ml to >160 μg/ml), whereas high resistance to tachyplesin I in E. coli ATCC 25922 was produced only after 85 serial transfers (Table 1). The results showed that tachyplesin I-resistant mutants of A. hydrophila, E. coli F41, E. coli ATCC 25922, and P. aeruginosa were produced after long-term continuous passages.
TABLE 1.
Induction of resistance to tachyplesin Ia
| Organism | Treatment group | No. of transfer generations | MIC range of tachyplesin I (μg/ml) | Tachyplesin I concn during induction (μg/ml) |
|---|---|---|---|---|
| E. coli ATCC 25922 | Original strain | 0 | 5 | 0 |
| Treatment group | 80 | 20–40 | 5–10 | |
| Treatment group | 85 | 160 | 5–10 | |
| Treatment group | 96 | >160 | 10 | |
| Control group | 96 | 10–20 | 0 | |
| E. coli F41 | Original strain | 0 | 5–10 | 0 |
| Treatment group | 72 | 20–40 | 5–10 | |
| Treatment group | 78 | 40–80 | 5–10 | |
| Treatment group | 80 | >160 | 5–10 | |
| Control group | 80 | 10–20 | 0 | |
| P. aeruginosa CGMCC1.2620 | Original strain | 0 | 10 | 0 |
| Treatment group | 60 | 40–80 | 5–20 | |
| Treatment group | 66 | 40 | 5–20 | |
| Treatment group | 80 | >160 | 5–20 | |
| Control group | 80 | 10–30 | 0 | |
| A. hydrophila XS91-4-1 | Original strain | 0 | 10–20 | 0 |
| Treatment group | 42 | 20 | 5–20 | |
| Treatment group | 48 | 40–80 | 5–20 | |
| Treatment group | 69 | >160 | 5–20 | |
| Control group | 42–69 | 20–40 | 0 |
Induction strains were successively cultured in MHB supplemented with tachyplesin I (5 to 20 μg/ml), whereas control strains were successively cultured in unsupplemented medium under the same conditions. The MIC was measured using the serial broth microdilution method. The MIC was considered the lowest drug concentration for which no visible growth was observed. Each determination was made using three independent replicates for each line. The strains with the most resistance were used as the evolved resistant strains in the following experiments.
(ii) UV mutagenesis selection.
E. coli ATCC 25922 and P. aeruginosa CGMCC1.2620 mutagenized strains grown at high tachyplesin I concentrations were randomly selected to determine the MIC of tachyplesin I. We isolated nine P. aeruginosa strains and one E. coli strain with resistance to tachyplesin I. The MIC values for these mutants were 4-fold higher than those for their original strains (Table 2).
TABLE 2.
Comparison of tachyplesin I MICs before and after bacterial UV induction
| Strain | MIC (μg/ml) |
|
|---|---|---|
| Original strain | UV mutagenesis straina | |
| E. coli ATCC 25922 | 5 | 20 |
| P. aeruginosa CGMCC1.2620 | 10 | ≥80 |
The method for MIC determination is described in Table 1. Nine P. aeruginosa tachyplesin I-resistant mutants obtained by UV mutagenesis were named the P. aeruginosa UV-1 to -9 resistant strains; one E. coli tachyplesin I-resistant mutant was named the UV-induced E. coli resistant strain.
Stability of mutants with induced resistance.
After five serial passages, the MIC values for the resistant strains were unchanged; however, after 10 passages, the MIC values for the resistant strains gradually decreased, except in the case of resistant A. hydrophila strains. The MIC values for the E. coli-96, P. aeruginosa-80, and F41-80 (naming based on the species or strain name and the transfer generation) resistant strains were from >160 µg/ml to 20 µg/ml after six to nine serial passages.
Cross-resistance assay.
Resistant strains were tested for cross-resistance to several antimicrobial agents by the disc diffusion method (Table 3) and to other AMPs by measurement of the MIC (Table 4). Antimicrobial susceptibilities to several antimicrobial agents differed somewhat based on the selection method. No cross-resistance to cefoperazone or amikacin was observed in the UV-mutagenized P. aeruginosa and E. coli resistant strains, whereas cross-resistance was observed in the corresponding strains with evolution-induced resistance. Cross-resistance to ofloxacin was observed only in the UV-mutagenized E. coli strain. However, compared to that of the P. aeruginosa-80 resistant strain, the antimicrobial susceptibilities of the P. aeruginosa UV-1 and UV-7 (naming based on the induction method and the assigned strain number) resistant strains to polymyxin B were lower.
TABLE 3.
Antimicrobial susceptibility determination for induction strains by the disc diffusion methoda
| Antimicrobial agent | Drug content of assay paper (/piece) |
E. coli-96 resistant strain |
E. coli-96 control strain |
F41-80 resistant strain |
F41-80 control strain |
A. hydrophila-69 resistant strain |
A. hydrophila-69 control strain |
P. aeruginosa-80 resistant strain |
P. aeruginosa-80 control strain |
P. aeruginosa original strain |
P. aeruginosa UV-1 resistant strain |
P. aeruginosa UV-3 resistant strain |
P. aeruginosa UV-7 resistant strain |
UV-induced E. coli resistant strain |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhibitory zone diam (mm) | Judgment result | Inhibitory zone diam (mm) | Judgment result | Inhibitory zone diam (mm) | Judgment result | Inhibitory zone diam (mm) | Judgment result | Inhibitory zone diam (mm) | Judgment result | Inhibitory zone diam (mm) | Judgment result | Inhibitory zone diam (mm) | Judgment result | Inhibitory zone diam (mm) | Judgment result | Inhibitory zone diam (mm) | Judgment result | Inhibitory zone diam (mm) | Judgment result | Inhibitory zone diam (mm) | Judgment result | Inhibitory zone diam (mm) | Judgment result | Inhibitory zone diam (mm) | Judgment result | ||
| Cefoperazone | 75 μg | 0 | R | 22.8 ± 1.2 | S | 0 | R | 22.3 ± 1.5 | S | 0 | R | 24.8 ± 0.6 | S | 0 | R | 20.5 ± 0.9 | S | 27.9 ± 0.5 | S | 23.1 ± 0.5 | S | 31.5 ± 0.5 | S | 20.5 ± 0.9 | S | 30 ± 1.2 | S |
| Polymyxin B | 300 IU | 14.9 ± 0.2 | S | 13.9 ± 1.1 | S | 17.8 ± 0.4 | S | 15.9 ± 0.4 | S | 14.9 ± 0.6 | S | 15.9 ± 0.4 | S | 13.9 ± 0.5 | S | 13 ± 0.7 | S | 14.3 ± 1.0 | S | 11 ± 0.4 | I | 16 ± 0.4 | S | 10 ± 0.5 | I | 13 ± 1.0 | S |
| Ofloxacin | 5 μg | 29.9 ± 0.7 | S | 32.2 ± 0.4 | S | 34.7 ± 1.0 | S | 34.1 ± 1.5 | S | 30.7 ± 0.5 | S | 31.9 ± 0.7 | S | 29.9 ± 2.4 | S | 28.8 ± 1.1 | S | 29.7 ± 0.4 | S | 29.2 ± 0.7 | S | 33 ± 2.0 | S | 40 ± 1.0 | S | 0 | R |
| Amikacin | 30 μg | 0 | R | 22.0 ± 0.6 | S | 0 | R | 20.2 ± 1.6 | S | 0 | R | 21.8 ± 2.0 | S | 0 | R | 22.2 ± 0.8 | S | 27.3 ± 0.6 | S | 26.1 ± 1.0 | S | 37 ± 1.0 | S | 32.5 ± 0.6 | S | 26 ± 1.0 | S |
Zone diameter data represent means ± standard deviations from three independent experiments performed in triplicate. R, resistant; S, susceptible; I, intermediate.
TABLE 4.
MICs of other AMPs for induction strains
| Strain | MIC (μg/ml)a |
||
|---|---|---|---|
| Pexiganan | Tachyplesin III | Polyphemusin I | |
| E. coli original strain | 10 | 5–10 | 10 |
| E. coli-96 resistant strain | 10 | 10 | 20 |
| E. coli-96 control strain | 10–20 | 10 | 10 |
| UV-induced E. coli resistant strain | 40 | 40 | 160 |
| E. coli F41 original strain | 5–10 | 10 | 10 |
| E. coli F41-80 resistant strain | 20–40 | >160 | >160 |
| E. coli F41-80 control strain | 10–20 | 10 | 10 |
| P. aeruginosa original strain | 10 | 5 | >160 |
| P. aeruginosa-80 resistant strain | 80–160 | 80 | >160 |
| P. aeruginosa-80 control strain | 20–40 | 20 | >160 |
| P. aeruginosa UV-3 resistant strain | 20 | >160 | >160 |
| P. aeruginosa UV-7 resistant strain | 160 | >160 | >160 |
| A. hydrophila-69 resistant strain | 80 | >160 | >160 |
| A. hydrophila-69 control strain | 20 | >160 | >160 |
Data in bold indicate a significant increase in the MIC value of other AMPs for resistant strains compared to those of the control induction strains.
High-level cross-resistance to pexiganan was observed in the P. aeruginosa-80 and UV-7 strains, the E. coli strains with UV-induced resistance, and the A. hydrophila-69 resistant strain. No cross-resistance to tachyplesin III or polyphemusin I was observed in E. coli ATCC 25922 before or after induction. High-level cross-resistance to tachyplesin III was observed in UV-mutagenized E. coli strains, the E. coli F41 resistant strain, and the P. aeruginosa resistant strains, whereas high-level cross-resistance to polyphemusin I was observed only in UV-mutagenized E. coli resistant strains and the E. coli F41 resistant strain. High-level cross-resistance between tachyplesin I and tachyplesin III was observed in E. coli F41 and P. aeruginosa strains. P. aeruginosa and A. hydrophila strains were insensitive to polyphemusin I before and after the induction.
Cost of resistance.
An important aspect of bacterial resistance to AMPs is whether the acquisition of resistance affects the growth potential of the bacterium. The acquisition of tachyplesin I resistance did not markedly alter either the maximum growth rate or the maximum population density reached in vitro in either the presence or absence of tachyplesin I. However, tachyplesin I resistance in E. coli F41 or P. aeruginosa CGMCC1.2620 was associated with a longer lag phase in the absence of tachyplesin I and a shorter lag phase in the presence of tachyplesin I than those with the corresponding control strains (Table 5). In contrast, the acquisition of tachyplesin I resistance in E. coli ATCC 25922 or A. hydrophila was associated with a shorter lag phase only in the presence of tachyplesin I compared with the corresponding control strains.
TABLE 5.
Growth of control and resistant strains in unsupplemented medium and in medium containing tachyplesin Ia
| Strain | Growth parameter | Value with indicated medium |
|||
|---|---|---|---|---|---|
| MHB |
MHB plus tachyplesin I |
||||
| Control strain | Resistant strain | Control strain | Resistant strain | ||
| E. coli ATCC 25922-96 | K | 0.995 | 0.998 | 1.391 | 1.15 |
| Vmax | 0.25 | 0.169 | 0.225 | 0.195 | |
| Lag time (h) | 1 | 2 | 21 | 9 | |
| E. coli F41-80 | K | 1.456 | 1.474 | 1.017 | |
| Vmax | 0.207 | 0.198 | 0.165 | ||
| Lag time (h) | 1 | 8 | 36 | 10 | |
| A. hydrophila XS91-4-1-69 | K | 1.337 | 1.411 | 1.216 | 1.225 |
| Vmax | 0.197 | 0.229 | 0.179 | 0.165 | |
| Lag time (h) | 1 | 2 | 16 | 8 | |
| P. aeruginosa CGMCC1.2620-80 | K | 1.422 | 1.436 | 1.123 | 1.094 |
| Vmax | 0.346 | 0.262 | 0.184 | 0.272 | |
| Lag time (h) | 2 | 7 | 19 | 10 | |
Strains were grown in unsupplemented medium for 26 h or in medium containing half the MIC of tachyplesin I for the corresponding control strains for 40 h. We measured the OD600 values every hour. K, maximum absorbance; Vmax, rate of increase in absorbance (h−1). Values indicate overall means from two replicates using all strains.
Ultrastructural changes.
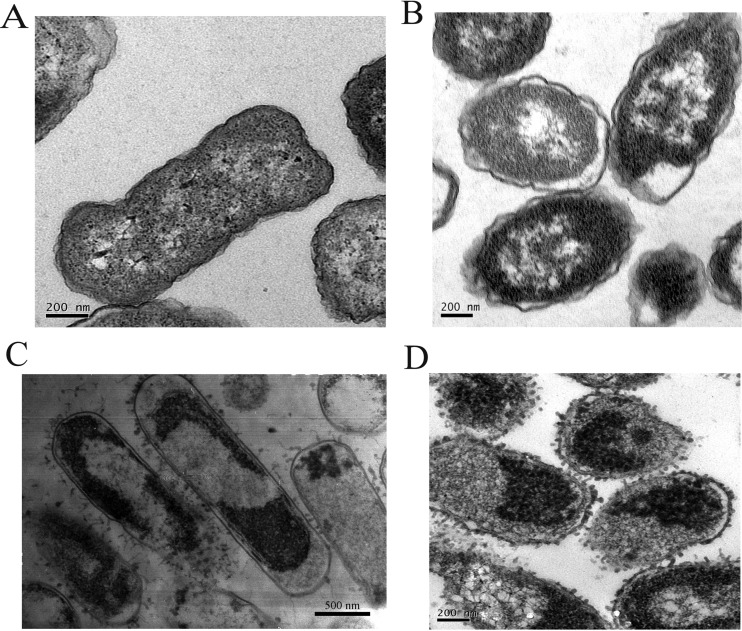
TEM was used to observe morphological and ultrastructural changes in E. coli ATCC 25922 before and after resistance induction. Significant differences in morphology were observed between the E. coli ATCC 25922 original and induction strains. The ultrastructural examination of the original strain revealed intact cell membranes without any noticeable damage and dense cellular cytoplasmic contents (Fig. 1A). In contrast, the E. coli ATCC 25922-96 resistant strain exhibited a cytoplasmic membrane structure that was markedly segregated and shrunken, many cytoplasmic vacuoles, and damage throughout the whole cell body (Fig. 1B). Thereafter, the morphological structures of both bacteria were observed after treatment with 20 μg/ml tachyplesin I for 60 min. Fibers extending from the cell surface, vacuum formation, cell deformation, and cytoplasmic membrane disruption were clearly observed in both the original strain and the E. coli ATCC 25922-96 resistant strain (Fig. 1C and D). The results show that tachyplesin I killed the original strain and the resistant strain of E. coli ATCC 25922 in similar ways.
FIG 1.
TEM photographs of the original and induction strains of E. coli ATCC 25922 treated with tachyplesin I. (A) E. coli ATCC 25922 original strain. (B) E. coli ATCC 25922-96 resistant strain. (C) E. coli ATCC 25922 treated with tachyplesin I (20 μg/ml, 60 min). (D) E. coli ATCC 25922-96 resistant strain treated with tachyplesin I (20 μg/ml, 60 min).
Extracellular protease activity and tachyplesin I-induced proteolytic activity in P. aeruginosa.
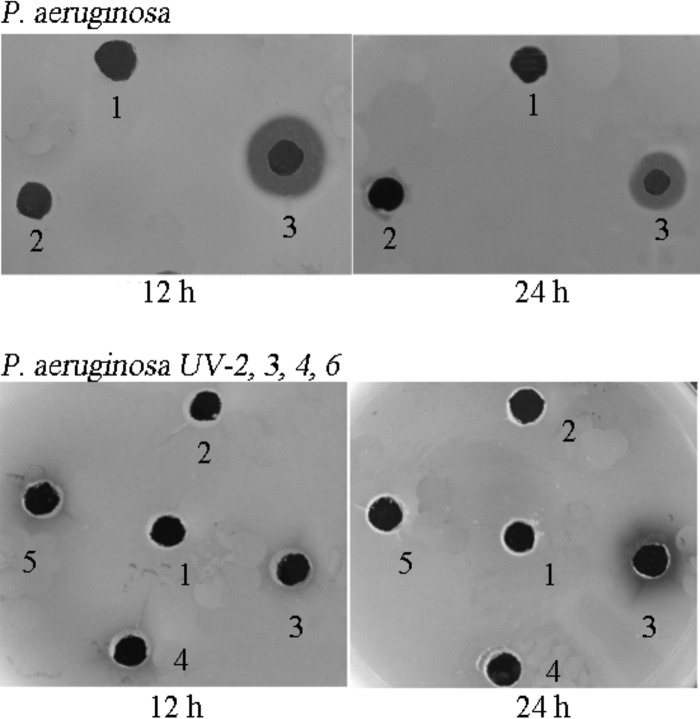
To explore the action of extracellular proteases on tachyplesin I and the effect on P. aeruginosa resistance to tachyplesin I, extracellular protease activity was determined by a skim milk agar plate assay (Fig. 2). Extracellular protease activity was greater in the P. aeruginosa-80 resistant strain than in the P. aeruginosa-80 control strain. The total extracellular proteolytic activities for the P. aeruginosa-80 strains differed somewhat between 12 h and 24 h. No obvious extracellular protease activity was observed for the E. coli F41-80 and E. coli-96 strains (data not shown) or the P. aeruginosa UV-2, UV-4, and UV-6 strains; only the P. aeruginosa UV-3 strain exhibited protease activity after 24 h.
FIG 2.
Extracellular protease activities determined by skim milk agar plate assay of E. coli and P. aeruginosa induction strains. Culture filtrates of 12- or 24-h bacterial cultures were pipetted into holes made in skim milk agar plates. Proteolysis can be seen by dark halos around the holes, which appear black. Hole numbers for P. aeruginosa: 1, blank; 2, P. aeruginosa-80 control strain; 3, P. aeruginosa-80 resistant strain. Hole numbers for UV-induced P. aeruginosa resistant strains: 1, blank; 2 to 5, P. aeruginosa UV-2, -3, -4, and -6 resistant strains.
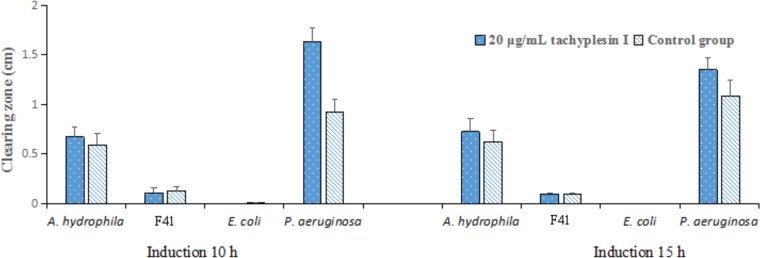
Thereafter, we observed increased proteolytic activity after addition of tachyplesin I to cultures of the original P. aeruginosa and A. hydrophila strains, which confirmed that upregulation of extracellular proteolytic activity might be an adaptive response of A. hydrophila or P. aeruginosa to the presence of tachyplesin. In contrast, no extracellular proteolytic activity expression was observed for E. coli (Fig. 3), suggesting different mechanisms of tachyplesin I-induced increases in proteolytic activity for different strains.
FIG 3.
Tachyplesin I induces increased extracellular proteolytic activity in bacteria. Concentrated culture filtrates of cultures grown with and without 20 μg/ml tachyplesin I for different times were pipetted into holes in skim milk agar plates and incubated. The assays were performed in triplicate. Clearing zone data represent clearing zone diameters minus the 0.2-cm hole diameter. All data represent means and standard deviations.
Zymographic assay.
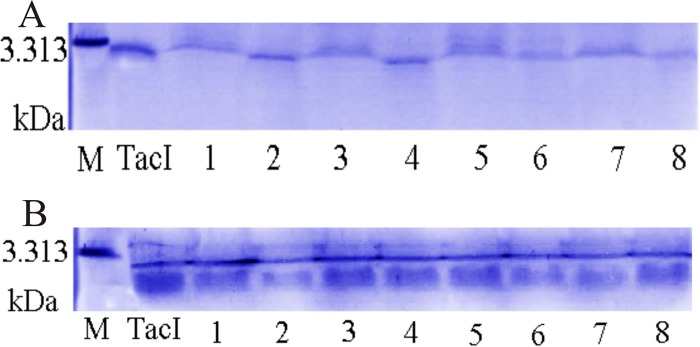
Zymographic analysis of the activities of extracellular proteases of the P. aeruginosa induction strains indicated that the difference was due mainly to the differential activity of >31-kDa proteases (Fig. 4A and B). The nature of these proteases was unknown.
FIG 4.
Zymographic analysis of extracellular proteases in P. aeruginosa induction strains. Culture filtrates (20 μl) were analyzed in SDS-PAGE gels copolymerized with skim milk. After electrophoresis, gels were washed, incubated, and counterstained. (A) Culture filtrates of P. aeruginosa-80 resistant and control strains after different times. Lanes: 1, control strain (12 h); 2, resistant strain (12 h); 3, control strain (24 h); 4, resistant strain (24 h). Images for 12 h and 24 h were taken from different areas of the same gel. (B) Culture filtrates of P. aeruginosa resistant strains obtained by UV mutagenesis. Lanes: 1 to 4, P. aeruginosa UV-2, -3, -4, and -6 resistant strains (12 h); 5 to 8, P. aeruginosa UV-2, -3, -4, and -6 resistant strains (24 h).
A P. aeruginosa mutant regulates degradation of tachyplesin I.
To analyze whether the differential activity of extracellular proteases observed in P. aeruginosa induction strains led to differential degradation, we incubated tachyplesin I with concentrated culture filtrates and analyzed degradation in SDS-polyacrylamide gels (Fig. 5A). The culture filtrates of the P. aeruginosa-80 resistant and control strains showed limited degradation of tachyplesin I, and there were no differences in tachyplesin I degradation between the P. aeruginosa-80 resistant and control strains. In addition, tachyplesin I was significantly degraded by the P. aeruginosa UV-3 mutant after 12 h and 24 h (Fig. 5B) and in the P. aeruginosa UV-4 mutant after 24 h. No significant degradation of tachyplesin I was observed for the P. aeruginosa UV-2 or UV-6 strain.
FIG 5.
Resistant strains of P. aeruginosa regulate degradation of tachyplesin I (TacI). Equal amounts of peptide were incubated with culture filtrates of the control strain and the P. aeruginosa-80 and UV-induced resistant strains for 5 h and then analyzed by SDS-PAGE. (A) Culture filtrates of P. aeruginosa-80 resistant and control strains regulate degradation of TacI for different times. Lanes: 1 and 2, control strain and control strain plus TacI (12 h); 3 and 4, resistant strain and resistant strain plus TacI (12 h); 5 and 6, control strain and control strain plus TacI (24 h); 7 and 8, resistant strain and resistant strain plus TacI (24 h). (B) Culture filtrates of UV-induced P. aeruginosa resistant strains regulate degradation of TacI for different times. Lanes: 1 to 4, UV-2 plus TacI, UV-3 plus TacI, UV-4 plus TacI, and UV-6 plus TacI (12 h); 5 to 8, UV-2 plus TacI, UV-3 plus TacI, UV-4 plus TacI, and UV-6 plus TacI (24 h).
Thereafter, we assayed the effect of tachyplesin I pretreatment with culture filtrates on antimicrobial activity. The results showed that the P. aeruginosa-80 resistant and control strains had no observable effect (Table 6), whereas the P. aeruginosa UV-2 to UV-4 and UV-6 strains reduced the antibacterial activity of tachyplesin I to some extent after 12 h (Table 7).
TABLE 6.
Effects of tachyplesin I treatment with culture filtrates of P. aeruginosa-80 induction strain for 5 h on inhibition rate
| Treatmenta | Inhibition rate (%) |
|---|---|
| Tac I | 97.21 |
| 12-h culture filtrates of P. aeruginosa-80 | |
| Tac I plus resistant strain | 98.97 |
| Tac I plus control strain | 95.91 |
| 24-h culture filtrates of P. aeruginosa-80 | |
| Tac I plus resistant strain | 98.97 |
| Tac I plus control strain | 95.73 |
Tac I, tachyplesin I.
TABLE 7.
Effects of tachyplesin I treatment with culture filtrates of P. aeruginosa UV induction strains for 5 h on inhibition rate
| Treatmenta | Inhibition rate (%) |
|---|---|
| Tac I | 89.79 |
| 12-h culture filtrates of P. aeruginosa strains | |
| Tac I plus UV-2 strain | 71.24 |
| Tac I plus UV-3 strain | 77.57 |
| Tac I plus UV-4 strain | 71.44 |
| Tac I plus UV-6 strain | 76.83 |
| 24-h culture filtrates of P. aeruginosa strains | |
| Tac I plus UV-2 strain | 68.16 |
| Tac I plus UV-3 strain | 84.84 |
| Tac I plus UV-4 strain | 84.86 |
| Tac I plus UV-6 strain | 82.66 |
Tac I, tachyplesin I.
DISCUSSION
The Gram-negative bacteria P. aeruginosa and E. coli are both important opportunistic pathogens of concern in the health care system, where they are frequent nosocomial pathogenic microorganisms. The pathogenic bacteria E. coli F41 and A. hydrophila XS91-4-1 were isolated from cases of porcine intestinal diarrhea and silver carp hemorrhagic septicemia, respectively. Understanding the different sources of tachyplesin I tolerance in the four Gram-negative bacteria studied herein has important implications for understanding possible AMP resistance mechanisms.
Our results demonstrate that tachyplesin I resistance can be induced in bacteria under conditions of long-term (69 to 85 serial passages) continuous increases in the tachyplesin I concentration. These results are consistent with previous reports that resistance may evolve from consistent long-term exposure to increasing levels of peptides (3) and synthetic AMP analogues, e.g., α-peptide/β-peptoid peptidomimetics (25). However, they contradict our previous report indicating no tachyplesin I-resistant mutants in bacteria exposed to fewer than 20 serial passages with higher-concentration tachyplesin I induction (17), possibly because of differences in induction time and induction method. The resistance observed using the long-term continuous induction method evolves more slowly than that obtained using the UV mutagenesis method. Bacterial resistance to tachyplesin I is slowly inducible and relatively stable.
For the first time, to our knowledge, we provide evidence that evolved resistance to a synthetic tachyplesin I peptide affects bacterial susceptibility to other AMPs and antibacterial agents, producing cross-resistance to the therapeutic peptide pexiganan and the closely related AMPs polyphemusin I and tachyplesin III. This finding raises serious concerns about the long-term risks associated with the development and use of tachyplesin I. However, the mechanism(s) for cross-resistance to the conventional antimicrobial agents cefoperazone and amikacin in evolved strains remains unclear. Based on previous studies, we speculate that tachyplesin I induced changes in aminoglycoside-modifying enzymes and β-lactamase function or regulation in resistant bacteria, which resulted in cross-resistance to amikacin and cefoperazone, respectively.
For resistant strains, we observed different growth features between the induction strains and the corresponding control strains in the same medium. For example, the disappearance of the metallic luster of the E. coli ATCC 25922 resistant strains grown on eosin methylene blue agar medium might be associated with changes in the charge of the cell membrane. The biological characteristics of P. aeruginosa resistant strains were also different, likely due to different metabolic and/or genetic changes in the resistant strains. Moreover, our resistant strains displayed a substantial cost of resistance, mainly in the form of a much longer lag phase in the absence of tachyplesin I for E. coli F41 and P. aeruginosa CGMCC1.2620, whereas no markedly longer lag phase was observed for E. coli ATCC 25922 and A. hydrophila. There were some differences among different resistant strains, which implies that the use of tachyplesin I might result in the spread and medical threat of partially resistant organisms. The results are consistent with previous reports that resistant bacteria took longer to start reproducing than control bacteria in the absence of medium, although their replication rate was unaffected once replication was initiated (3). However, Hein-Kristensen et al. reported that resistant strains obtained from different lineages have different growth rates (the same or significant decreases) in the absence of peptide compared to wild-type strains (25). In short, AMP resistance might compromise bacteria in other ways, for example, by reducing their growth rate or causing a longer lag phase.
Some bacteria evade host defense peptides by employing protease-mediated degradation. P. aeruginosa, Enterococcus faecalis, and S. aureus express extracellular proteases capable of degrading and inactivating LL-37 (24, 26). Some peptides, such as lactoferrin and HNP-1 and -2, are also degraded and inactivated in the presence of bacterial and host proteases (1). However, whether the mechanisms of resistance to tachyplesin I are related to degradation and inactivation by extracellular proteases is poorly understood. Our study showed that the tachyplesin I-resistant P. aeruginosa-80 strain exhibited increased levels of extracellular proteolytic activity; however, the increased activity of proteases did not increase tachyplesin I degradation or reduce the antimicrobial inhibition rate relative to that of control strains, which suggests that the P. aeruginosa-80 strain resistance to tachyplesin I is independent of extracellular proteolytic activity. In contrast, protease activity from the UV-3 mutant markedly degraded tachyplesin I and reduced the antimicrobial activity, suggesting that protease activity had a much greater influence on resistance against tachyplesin I than did the P. aeruginosa-80 evolved strain. Furthermore, the UV-2, -4, and -6 resistant mutants also reduce the antimicrobial activity of tachyplesin I relative to that for control strains, which implies that UV-induced mutants might secrete other material that inactivates tachyplesin I. This difference might arise from differential metabolic and/or genetic changes in the evolved-resistance strains and UV-induced mutants. P. aeruginosa resistance to tachyplesin I might involve other resistance mechanisms. Bacterial resistance to AMPs independently of protease production was also reported for Porphyromonas gingivalis. Some reports showed that Porphyromonas gingivalis was resistant to AMPs of human and nonhuman origins. Porphyromonas gingivalis is known to hydrolyze AMPs mainly by expressing robust proteolytic activity (27). However, Ouhara et al. suggested that Porphyromonas gingivalis resistance to d-enantiomer peptides was independent of its proteolytic capacity (27, 28). Thus, the mechanism of bacterial resistance to tachyplesin I requires further research.
In summary, we demonstrate that long-term continuous exposure to high concentrations of tachyplesin I can induce stably resistant Gram-negative bacterial strains with cross-resistance to other antimicrobial peptides and conventional antimicrobial agents. Furthermore, our results suggest the potential involvement of extracellular proteases in mediating this resistance but imply the likely existence of additional resistance mechanisms. We believe that this contribution is theoretically and practically relevant because AMPs are currently being developed for clinical use, and thus investigations of their safety and potential to induce bacterial resistance are necessary. Our results raise serious concerns about the long-term risks associated with the development and clinical use of tachyplesin I. Further experiments are under way to investigate the mechanism of bacterial resistance to tachyplesin I, and these studies may identify possible targets for new antimicrobial agents.
ACKNOWLEDGMENTS
All authors gave approval for the final version of the manuscript.
We declare no competing financial interests.
REFERENCES
- 1.Anaya-López JL, López-Meza JE, Ochoa-Zarzosa A. 2013. Bacterial resistance to cationic antimicrobial peptides. Crit Rev Microbiol 39:180–195. doi: 10.3109/1040841X.2012.699025. [DOI] [PubMed] [Google Scholar]
- 2.Cai Y, Chai D, Wang R, Liang B, Bai N. 2012. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother 67:1607–1615. doi: 10.1093/jac/dks084. [DOI] [PubMed] [Google Scholar]
- 3.Perron G, Zasloff M, Bell G. 2006. Experimental evolution of resistance to an antimicrobial peptide. Proc Biol Sci 273:251–256. doi: 10.1098/rspb.2005.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peschel A, Sahl HG. 2006. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol 4:529–536. doi: 10.1038/nrmicro1441. [DOI] [PubMed] [Google Scholar]
- 5.Mehla J, Sood SK. 2011. Substantiation in Enterococcus faecalis of dose-dependent resistance and cross-resistance to pore forming antimicrobial peptides by use of a polydiacetylene-based colorimetric assay. Appl Environ Microbiol 77:786–793. doi: 10.1128/AEM.01496-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Habets MG, Brockhurst MA. 2012. Therapeutic antimicrobial peptides may compromise natural immunity. Biol Lett 8:416–418. doi: 10.1098/rsbl.2011.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lofton H, Pranting M, Thulin E, Andersson DI. 2013. Mechanisms and fitness costs of resistance to antimicrobial peptides LL-37, CNY100HL and wheat germ histones. PLoS One 8:e68875. doi: 10.1371/journal.pone.0068875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gruenheid S, Moual H. 2012. Resistance to antimicrobial peptides in Gram-negative bacteria. FEMS Microbiol Lett 330:81–89. doi: 10.1111/j.1574-6968.2012.02528.x. [DOI] [PubMed] [Google Scholar]
- 9.Nuri R, Shprung T, Shai Y. 2015. Defensive remodeling: how bacterial surface properties and biofilm formation promote resistance to antimicrobial peptides. Biochim Biophys Acta 1848:3089–3100. doi: 10.1016/j.bbamem.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T, Furunaka H, Miyata T, Tokunaga Muta T, Iwanaga S, Shimonishi Y. 1988. Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus) isolation and chemical structure. J Biol Chem 263:16709–16713. [PubMed] [Google Scholar]
- 11.Hong J, Hu JY, Qu EJ, Shu J, Liu RF, Zhou Z, Guan YZ. 2013. Mechanisms of tachyplesin I against Escherichia coli. Microbiol China 40:1018–1026. [Google Scholar]
- 12.Kushibiki T, Kamiya M, Aizawa T, Kumaki Y, Kikukawa T, Mizuguchi M, Kawano K. 2014. Interaction between tachyplesin I, an antimicrobial peptide derived from horseshoe crab, and lipopolysaccharide. Biochim Biophys Acta 1844:527–534. doi: 10.1016/j.bbapap.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Hong J, Guan WT, Jin G, Zhao HY, Jiang XH, Dai JG. 2015. Mechanism of tachyplesin I injury to bacterial membranes and intracellular enzymes, determined by laser confocal scanning microscopy and flow cytometry. Microbiol Res 170:69–77. doi: 10.1016/j.micres.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Imura Y, Nishida M, Ogawa Y, Takakura Matsuzaki K. 2007. Action mechanism of tachyplesin I and effects of PEGylation. Biochim Biophys Acta 1768:1160–1169. doi: 10.1016/j.bbamem.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Dai JG, Xie HW, Jin G, Wang WG, Zhang Y, Guo Y. 2009. Preliminary study on high-level expression of tandem-arranged tachyplesin-encoding gene in Bacillus subtilis Wb800 and its antibacterial activity. Mar Biotechnol 11:109–117. doi: 10.1007/s10126-008-9125-6. [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Zhao HL, Feng X, Zhai RD, Zhu S, Du CT, Sun CJ, Lei LC. 2013. Expression of recombinant human lysozyme-tachyplesin I (hLYZ-TP I) in Pichia pastoris and analysis of antibacterial activity. Biomed Environ Sci 26:319–322. doi: 10.3967/0895-3988.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Hong J, Dai JG, Guan WT, Jin G, Huang ZL, Zhang LJ, Zhang Y. 2012. Tachyplesin I induce drug resistance in bacteria in vitro. J Anim Vet Adv 11:939–945. doi: 10.3923/javaa.2012.939.945. [DOI] [Google Scholar]
- 18.Hancock R, Chapple D. 1999. Peptide antibiotics. Antimicrob Agents Chemother 43:1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M, Lai YP, Villaruz AE, Cha DJ, Sturdevant DE, Otto M. 2007. Gram-positive three-component antimicrobial peptide-sensing system. Proc Natl Acad Sci U S A 104:9469–9474. doi: 10.1073/pnas.0702159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge YG, MacDonald D, Henry MM, Halt HI, Nelson KA, Lipsky BA, Zasloff MA, Holroyd KJ. 1999. In vitro susceptibility to pexiganan of bacteria isolated from infected diabetic foot ulcers. Diagn Microbiol Infect Dis 35:45–53. doi: 10.1016/S0732-8893(99)00056-5. [DOI] [PubMed] [Google Scholar]
- 21.Muta T, Fujimoto T, Nakajima H, Iwanaga S. 1990. Tachyplesins isolated from hemocytes of Southeast Asian horseshoe crabs (Carcinoscorpius rotundicauda and Tachypleus gigas): identification of a new tachyplesin, tachyplesin III, and a processing intermediate of its precursor. J Biochem 108:261–266. [DOI] [PubMed] [Google Scholar]
- 22.Miyata T, Tokunaga F, Yoneya T, Yoshikawa K, Iwanaga S, Niwa M, Shimonishi Y. 1989. Antimicrobial peptides, isolated from horseshoe crab hemocytes, tachyplesin II, and polyphemusins I and II: chemical structures and biological activity. J Biochem 106:663–668. [DOI] [PubMed] [Google Scholar]
- 23.Martinez J, Baquero F. 2000. Mutation frequencies and antibiotic resistance. Antimicrob Agents Chemother 44:1771–1777. doi: 10.1128/AAC.44.7.1771-1777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai YP, Villaruz AE, Li M, Cha DJ, Sturdevant DE, Otto M. 2007. The human anionic antimicrobial peptide dermcidin induces proteolytic defence mechanisms in staphylococci. Mol Microbiol 63:497–506. doi: 10.1111/j.1365-2958.2006.05540.x. [DOI] [PubMed] [Google Scholar]
- 25.Hein-Kristensen L, Franzyk H, Holch A, Gram L. 2013. Adaptive evolution of Escherichia coli to an a-peptide/β-peptoid peptidomimetic induces stable resistance. PLoS One 8:e73620. doi: 10.1371/journal.pone.0073620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sieprawska-Lupa M, Mydel P, Krawczyk K, Wójcik K, Puklo M, Lupa B, Suder P, Silberring J, Reed M, Pohl J, Shafer W, McAleese F, Foster T, Travis J, Potempa J. 2004. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob Agents Chemother 48:4673–4679. doi: 10.1128/AAC.48.12.4673-4679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouhara K, Komatsuzawa H, Yamada S, Shiba H, Fujiwara T, Ohara M, Sayama K, Hashimoto K, Kurihara H, Sugai M. 2005. Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides, β-defensins and LL37, produced by human epithelial cells. J Antimicrob Chemother 55:888–896. doi: 10.1093/jac/dki103. [DOI] [PubMed] [Google Scholar]
- 28.Bachrach G, Altman H, Kolenbrander PE, Chalmers NI, Gabai-Gutner M, Mor A, Friedman M, Steinberg D. 2008. Resistance of Porphyromonas gingivalis ATCC 33277 to direct killing by antimicrobial peptides is protease independent. Antimicrob Agents Chemother 52:638–642. doi: 10.1128/AAC.01271-07. [DOI] [PMC free article] [PubMed] [Google Scholar]