Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) causes large-scale epidemics of acute bacterial skin and skin structure infections (ABSSSI) within communities across the United States. Animal models that reproduce ABSSSI as they occur in humans are urgently needed to test new therapeutic strategies. Alpha-toxin plays a critical role in a variety of staphylococcal infection models in mice, but its role in the pathogenesis of ABSSSI remains to be elucidated in rabbits, which are similar to humans in their susceptibility to S. aureus superantigens and certain bicomponent pore-forming leukocidins. We report here a new rabbit model of ABSSSI and show that those infected with a mutant deficient in expression of alpha-toxin (Δhla) developed a small dermonecrotic lesion, whereas those infected with isogenic USA300 MRSA wild-type or complemented Δhla strains developed ABSSSI that mimic the severe infections that occur in humans, including the large central dermonecrotic core surrounded by erythema, induration, and marked subcutaneous hemorrhage. More importantly, immunoprophylaxis with MEDI4893*, an anti-alpha-toxin human monoclonal antibody, significantly reduced the severity of disease caused by a USA300 wild-type strain to that caused by the Δhla mutant, indicating that this toxin could be completely neutralized during infection. Thus, this study illustrates a potential high standard for the development of new immunotherapeutic agents in which a toxin-neutralizing antibody provides protection to the same degree achieved with a toxin gene knockout. When MEDI4893* was administered as adjunctive therapy with a subtherapeutic dose of linezolid, the combination was significantly more efficacious than either agent alone in reducing the severity of ABSSSI.
INTRODUCTION
Community-associated methicillin-resistant Staphylococcus aureus (MRSA), especially the USA300 clone, is causing disease epidemics within communities and hospitals across the United States. Skin and soft tissue infections account for >70% of the cases of disease (1–3). Due to the alarming spread of these difficult-to-treat infections and the emergence of antimicrobial resistance, the U.S. Food and Drug Administration issued a draft guidance in 2010 to facilitate the development of new drugs to treat acute bacterial skin and skin structure infections (ABSSSI) and provided specific recommendations for defining a sufficiently large lesion size that differentiates between major cutaneous abscesses and minor cutaneous abscesses (4). This distinction is important, because it is not possible to estimate reliably the quantitative effect of treatment with a new systemic drug for patients who have minor cutaneous abscesses, for which there is a 90% cure rate with surgical incision and drainage alone and no antibiotic treatment (5).
For preclinical drug development, there is an urgent need to develop animal models that reproduce the severe ABSSSI caused by USA300, so that new therapeutic strategies can be tested to predict their clinical efficacy in humans. Rabbit models have been used since the late 1800s for the study of staphylococcal infection because the course of disease in rabbits closely mimics that in humans (6). Rabbits exhibit susceptibilities to staphylococcal toxins and superantigens remarkably similar to those of humans, whereas mice, rats, and even nonhuman primates are resistant to many of these toxins (7–11). The pathogenic roles of many staphylococcal toxins, including alpha-toxin, have been validated in different rabbit infection models (6). However, previously described rabbit models of S. aureus skin infection recapitulated a less serious form of the disease (uncomplicated cutaneous abscess) that spontaneously resolved without treatment (12, 13).
In the study described here, we developed and validated a new rabbit model of ABSSSI that mimics severe USA300 infection in humans, including a large dermonecrotic ulceration accompanied by redness, edema, and induration. The hallmark dermonecrosis and ulceration from USA300 skin infections, which can often lead to a misdiagnosis of the skin lesion as a spider bite (1, 14), are recapitulated in our rabbit model. Using an animal species that is susceptible to many staphylococcal toxins, we validated the critical role of alpha-toxin in the pathogenesis of ABSSSI. We further demonstrated that MEDI4893*, a human anti-alpha-toxin monoclonal antibody (MAb), reduced the severity of USA300 ABSSSI to the same degree achieved by knocking out the alpha-toxin gene.
MATERIALS AND METHODS
Bacterial strains.
Community-associated MRSA clinical isolate SF8300 (pulsed-field type USA300-0114, multilocus sequence type 8) was isolated from a patient treated at San Francisco General Hospital (15). In-frame deletion of the alpha-toxin gene (Δhla) and its complementation (compΔhla) in the SF8300 wild-type (WT) strain were performed as described previously (16) using the pKOR1 allelic replacement mutagenesis system and the primers shown in Table S1 in the supplemental material. The linezolid MICs for the SF8300 wild-type, Δhla, and compΔhla strains were 0.5 μg/ml.
Growth curves of the bacterial strains were determined by diluting 1 ml of an overnight culture into 99 ml fresh tryptic soy broth (TSB) in a 1-liter flask and measuring the optical density at 600 nm (OD600) with shaking at 37°C.
Bacterial inoculum preparation.
Bacterial strains were cultured in TSB at 37°C with shaking for 6 h to exponential phase of growth (OD600 = 1.2 to 1.5; see Fig. S1 in the supplemental material), harvested by centrifugation, washed twice with phosphate-buffered saline (PBS), resuspended in PBS containing 10% glycerol to a concentration of 3 × 1010 to 4 × 1010 CFU/ml, aliquoted into individual cryovials, and immediately stored at −80°C. Frozen aliquots were thawed to room temperature immediately before each use and diluted with saline to 2.5 × 1010 to 3.0 × 1010 CFU/ml, and titers were determined by serial dilution and plating onto 5% sheep blood agar plates to determine the actual number of bacteria used for infection of rabbits.
Rabbit model of ABSSSI.
All rabbit studies were approved by the University of California San Francisco Institutional Animal Care and Use Committee (IACUC). New Zealand White rabbits (weight, 2.2 to 2.6 kg) were shaved on the right flank, disinfected with 70% ethanol, restrained in a cat sack restraint device (California Veterinary Supply), and infected by intradermal injection of 120 μl of the SF8300 wild-type or mutant strain into the right dorsal lumbar skin using a 1-ml insulin syringe (29 gauge by 1/2 in.). The dermonecrotic lesion was photographed with a standard ruler once daily for 7 days to document the progression of dermonecrotic ulceration. Because of their irregular shapes, the surface area for each dermonecrotic ulceration was quantified using the standard ruler in the photograph as a scale bar and converting the actual number of pixels within each lesion to millimeters squared using the widely used ImageJ software application (Image Processing and Analysis in Java, version 1.48v; National Institutes of Health) (17). Animals were euthanized on day 7 postinfection, and the dermonecrotic region was excised to obtain quantitative bacterial counts and to perform histopathological analysis.
For pathogenesis studies, 21 rabbits were randomized into 3 experimental groups that were challenged by intradermal injection with (i) the SF8300 WT, (ii) SF8300 Δhla, or (iii) SF8300 compΔhla.
For prophylaxis studies, 15 rabbits were randomized into 2 experimental groups that were passively immunized intravenously with either (i) 30 mg/kg of body weight MEDI4893*, a human anti-alpha-toxin MAb, or (ii) control IgG (c-IgG), a human IgG1 isotype-matched control (anti-HIV gp120) antibody (18–21). The rabbits were challenged 24 h later by intradermal injection with the SF8300 WT strain.
For the treatment studies, 47 rabbits were infected by intradermal injection of the SF8300 WT strain. Animals were randomized into 1 of 4 experimental groups: (i) 11 animals were treated intravenously with 30 mg/kg c-IgG at 2 h postinfection, (ii) 12 animals were treated intravenously with 30 mg/kg MEDI4893* at 2 h postinfection, (iii) 12 animals were treated subcutaneously with 25 mg/kg of linezolid at 2 h postinfection (redosed every 8 h, 4 doses total), and (iv) 12 animals were treated with a combination with 30 mg/kg MEDI4893* intravenously at 2 h postinfection and 25 mg/kg linezolid at 2 h postinfection (redosed every 8 h, 4 doses total).
Histopathology.
Lesions, including the skin and subcutaneous tissue at the site of inoculation, were harvested at day 7 postinfection and fixed in 10% neutral buffered formalin (VWR, Radnor, PA) for a minimum of 48 h. Fixed tissues were then processed according to standard methods as described previously (19) and stained with Gill's hematoxylin (Mercedes Medical, Sarasota, FL) and eosin (Surgipath, Richmond, IL) for histologic evaluation by a pathologist blind to the experimental conditions.
Serum concentrations of MEDI4893*.
Three rabbits were administered 30 mg/kg MEDI4893* intravenously. Blood for serum preparation was drawn at 1, 3, 8, 24, 48, 72, 96, 144, 192, 240, 288, and 312 h. The concentration of MEDI4893* was determined by an enzyme-linked immunosorbent assay. In brief, MaxiSorp plates (VWR International) were coated overnight at 4°C with 50 μl of 0.5 μg/ml sheep anti-human IgG (The Binding Site) in 0.2 M carbonate-bicarbonate buffer. Following three washes with PBS–0.1% Tween, the plates were blocked with PBS–5% bovine serum albumin for 1 h at room temperature. After three washes, the plates were incubated for 90 min at room temperature with serial dilutions of each serum sample (1:500 to 1:16,000 in 50 μl PBS). Anti-alpha-toxin MAb MEDI4893*was used as the standard (dilution range, 1,000 ng/ml to 0.98 ng/ml). The plates were then washed 3 times and incubated with horseradish peroxidase-conjugated goat anti-human IgG (Bethyl Laboratories, Montgomery, TX) at a 1:15,000 dilution in PBS. After 1 h of incubation at room temperature, the plates were washed and 100 μl of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (KPL, Gaithersburg, MD) was added. The reaction was stopped by addition of 100 μl of 0.2 M H2SO4, and the OD450 of the plate was read on a spectrophotometer (Molecular Devices, Sunnyvale, CA). Data were analyzed with Soft Max Pro software (Molecular Devices), and the serum MEDI4893* concentration at each time point was calculated as the mean for three serum dilutions in the linear range of the standard.
Alpha-toxin neutralization assay.
Rabbit serum collected at 24 h after intravenous dosing with 30 mg/kg MEDI4893* or 30 mg/kg c-IgG was tested for the titers for neutralization of alpha-toxin using rabbit red blood cells as previously described (20).
Serum concentrations of linezolid.
Three rabbits were subcutaneously administered 25 mg/kg linezolid (LGM Pharma, Boca Raton, FL), which was dissolved to 12 mg/ml in a solution of 5% (2-hydroxypropyl)-β-cyclodextrin (Sigma-Aldrich, St. Louis, MO). Blood for serum preparation was drawn at 0, 0.5, 1, 2, 3, 4, 6, and 8 h postdosing. Linezolid serum concentrations were determined as previously described using liquid chromatography-tandem mass spectrometry (LC-MS/MS) (21).
Statistical analysis.
GraphPad Prism (version 6.0) software was used for data analysis. For dermonecrosis data, in the event that statistical assumptions (independence, normality, homogeneity of variance) were met, one-way analysis of variance followed by Tukey's multiple-comparison test was applied to either raw or log10-transformed data. In the event that statistical assumptions were not met, a nonparametric Kruskal-Wallis test followed by Dunn's multiple-comparison test was applied to the raw dermonecrosis data. For data on the log10 number of CFU, the same statistical methodology described above was applied to account for multiple comparisons. Where appropriate, an unpaired t test with Welch's correction was used, after assessment of the group variance. Reported P values are for two-tailed tests. A P value of <0.05 was considered statistically significant.
RESULTS
Critical role of alpha-toxin in a rabbit model of ABSSSI.
To develop a new rabbit model of ABSSSI, we used SF8300, a minimally passaged USA300-0114 clinical strain isolated from a patient with a severe abscess lesion (15). We compared the subcutaneous and intradermal injection of increasing amounts of SF8300 into the dorsal lumbar skin of rabbits to produce severe dermonecrotic ulcerations that mimic USA300 ABSSSI as they occur in humans. Severe dermonecrosis was defined as having a minimum surface area of 300 mm2, which allowed a sufficient dynamic range to detect a significant reduction in pathology as a result of attenuation of the virulence in a mutant strain or an efficacious treatment modality. Severe dermonecrosis was reproducibly produced only by intradermal injection of 1 × 109 to 3 × 109 CFU of the SF8300 WT strain. Subcutaneous injection did not result in dermonecrosis but did result in the formation of a minor abscess that resolved spontaneously without treatment. The SF8300 WT strain injected intradermally did not appear to spread from the local dermonecrotic lesion to cause systemic infection, as evidenced by a lack of fever (defined as a daily rectal temperature not exceeding 103.5°F) and a lack of bacterial counts in the kidneys, the organ targeted by S. aureus during bloodstream infection (22).
We compared the virulence of the SF8300 WT, Δhla mutant, and compΔhla strains in a rabbit model of ABSSSI established by intradermal injection. We verified beforehand that the three isogenic strains did not exhibit any differences in in vitro growth rates (see Fig. S1 in the supplemental material). Rabbits infected with the Δhla mutant developed a circumscribed, small dermonecrotic lesion surrounded by healthy skin with minimal subcutaneous hemorrhage, whereas those infected with the SF8300 WT or compΔhla strain developed markedly larger areas of dermonecrosis with surrounding erythema and induration and evidence of marked subcutaneous hemorrhage (Fig. 1A; see also Fig. S2 and S3 in the supplemental material).
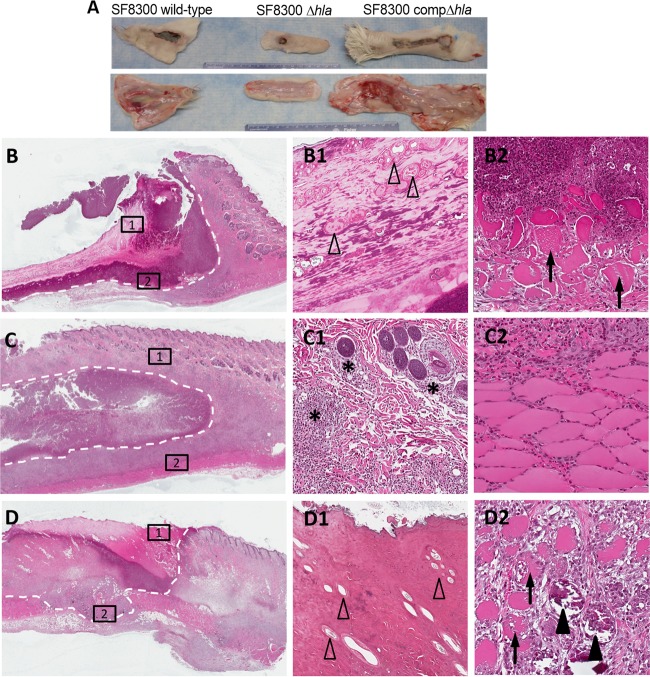
FIG 1.
Essential role of alpha-toxin in the rabbit model of skin and skin structure infection. (A) Representative gross pathology at 7 days postinfection of skin specimens excised from rabbits infected with the SF8300 WT, Δhla, and compΔhla strains. The gross sections show evidence of marked subcutaneous hemorrhage in rabbits infected with the SF8300 and compΔhla strains but not those infected with the Δhla mutant strain. (B to D2) Hematoxylin- and eosin-stained sections of skins excised from rabbits infected with the SF8300 WT (B, B1, B2), Δhla (C, C1, C2), and compΔhla (D, D1, D2) strains. (B, C, and D) Partial sections of skin lesions showing evidence of abscess formation and dermonecrotic ulceration (white dashed outlines; magnification, ×4). The squares labeled 1 and 2 are magnified in the corresponding panels on the right. Infection with the SF8300 WT (B1) and compΔhla (D1) strains resulted in ulceration with diffuse epidermal necrosis and a complete loss of adnexal structures (open arrowheads) with bacterial colonization and neutrophilic debris, whereas infection with SF8300 Δhla (C1) showed mild to moderate infiltration of viable hair follicles only by PMNs (asterisks; magnification, ×10). Myonecrosis with swelling and fragmentation of myocytes (arrows) was seen in rabbits infected with the SF8300 WT (B2) and SF8300 compΔhla (D2), as was myocyte mineralization (D2) (solid arrowhead; magnification, ×20). Inflammation extended to the muscle layer but did not cause myonecrosis with SF8300 Δhla infection (C2; magnification, ×20).
Consistent with the ABSSSI described in humans during the USA300 epidemic (1, 14), histopathological analysis performed on the skin excised from rabbits infected with the SF8300 WT or compΔhla strain demonstrated a disease severity significantly greater than that seen on skin excised from rabbits infected with the Δhla mutant, with consistent ulceration and necrosis of the epidermis and complete loss of all adnexal structures (Fig. 1, panels B to D2). Residual hair follicles bordering the ulcer were often necrotic and expanded by marked infiltrates of polymorphonuclear leukocyte (PMNs) (necrotizing folliculitis). Within the ulcerated lesion, there was a central necrotic core bordered by poorly to moderately organized layers of marked hemorrhage, heavy bands of degenerate PMNs admixed with necrotic cellular material, and clusters of bacterial colonies. The dermal collagen was diffusely necrotic and expanded by degenerate cellular debris, proteinaceous material (edema), and viable inflammatory infiltrates which extended into the adipose and subcutis muscle layer. Multifocal areas of myonecrosis and mineralization of necrotic myocytes were also frequently observed (Fig. 1B2 and D2).
FIG 2.
Dermonecrosis area and bacterial burden in rabbits infected with the SF8300 wild-type, Δhla, and compΔhla strains. Rabbits were challenged with 2.4 × 109 CFU of the SF8300 wild type, 2.4 × 109 CFU of the SF8300 Δhla strain, and 2.5 × 109 CFU of the SF8300 compΔhla strain. (A) Dermonecrosis area determined using ImageJ software on each day postinfection, with 7 rabbits per experimental group being tested. Error bars indicate SEMs. Pairwise comparisons of dermonecrosis area for the WT versus Δhla strains and for the compΔhla versus Δhla strain yielded multiplicity-adjusted P values of <0.01, whereas pairwise comparisons for the WT versus the compΔhla strain yielded multiplicity-adjusted P values of >0.05 (see Table S3 in the supplemental material). (B) Bacterial burden of dermonecrotic ulceration harvested from rabbits euthanized on day 7 postinfection. The log10 numbers of CFU per gram were not significantly different between the three experimental groups (all pairwise comparisons yielded multiplicity-adjusted P values of >0.05).
In contrast, gross lesion size and the histological depth of the affected skin were markedly reduced in the skin excised from rabbits infected with the Δhla mutant (Fig. 1C). Frequently, there was ulceration centrally with adjacent hyperplasia of the dermis and thickening of the epidermis. Hair follicles and associated adnexal structures exhibited mild to moderate PMN infiltration in the deeper layers overlying areas of abscessation or dermal inflammation. The underlying subcutis muscular layer remained intact with minimal necrosis or inflammation.
Quantitatively, rabbits infected with the SF8300 wild-type or compΔhla strain exhibited significantly (P < 0.01) larger areas of dermonecrosis (385 ± 63 and 385 ± 69 mm2, respectively) than rabbits infected with the Δhla mutant strain (63 ± 9 mm2) on day 7 postinfection (Fig. 2A; the results of statistical analyses are shown in Table S3 in the supplemental material). The bacterial counts in the dermonecrosis lesion were not statistically significantly different in animals infected with the SF8300 wild-type, Δhla, or compΔhla strain (Fig. 2B), suggesting that alpha-toxin production does not enhance bacterial survival. It further suggests that the dermonecrotic pathology was due to the direct toxic effects of alpha-toxin on skin pathology and was not due to the indirect effects of alpha-toxin promoting the survival of the bacteria, which then produced other skin-damaging virulence determinants.
Prophylaxis with a human anti-alpha-toxin MAb protected against ABSSSI.
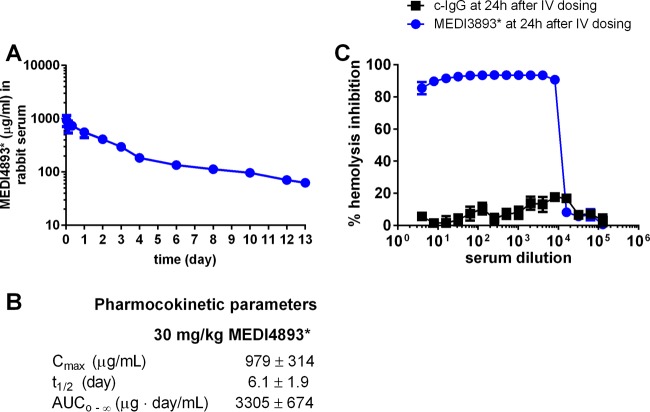
To determine whether immunoprophylaxis with an anti-alpha-toxin antibody offers protection in the rabbit model of ABSSSI, animals were randomized for intravenous administration of MEDI4893*, an anti-alpha-toxin human monoclonal IgG1, or a human isotype control IgG (c-IgG) 24 h before intradermal challenge with the SF8300 WT strain. Intravenous administration of 30 mg/kg MEDI4893* yielded an area under the concentration-time curve (AUC) from time zero to infinity of 3,305 ± 674 μg · day/ml (Fig. 3A and B), with the serum MEDI4893* concentrations being maintained above 100 μg/ml over 7 days and with high neutralization titers being produced in an alpha-toxin neutralization assay with rabbit red blood cells (Fig. 3C). Animals pretreated with MEDI4893* exhibited a significant reduction in dermonecrosis compared to those pretreated with c-IgG (31 ± 4 and 299 ± 69 mm2, respectively [P < 0.01] on day 7 postinfection; Fig. 4A and C; see also Table S4 in the supplemental material). Bacterial counts in the lesions of animals pretreated with MEDI4893* were also significantly reduced compared to those pretreated with c-IgG (P < 0.001) (Fig. 4B), although MEDI4893* did not affect the growth of the SF8300 WT strain in vitro (see Fig. S1 in the supplemental material).
FIG 3.
Pharmacokinetics of MEDI4893* in rabbits and alpha-toxin neutralization titers. (A) MEDI4893* concentration in 3 rabbit serum samples after intravenous dosing with 30 mg/kg. Error bars indicate SEMs. (B) Pharmacokinetics of MEDI4893* in 3 rabbits. Cmax, maximum concentration of drug in plasma; t1/2, half-life; AUC0–∞, area under the concentration-time curve from time zero to infinity. (C) Alpha-toxin neutralization titers of serum collected from rabbits at 24 h after dosing with 30 mg/kg MEDI4893* (4 rabbit serum samples) or 30 mg/kg c-IgG (2 rabbit serum samples). Error bars indicate SEMs. IV, intravenous.
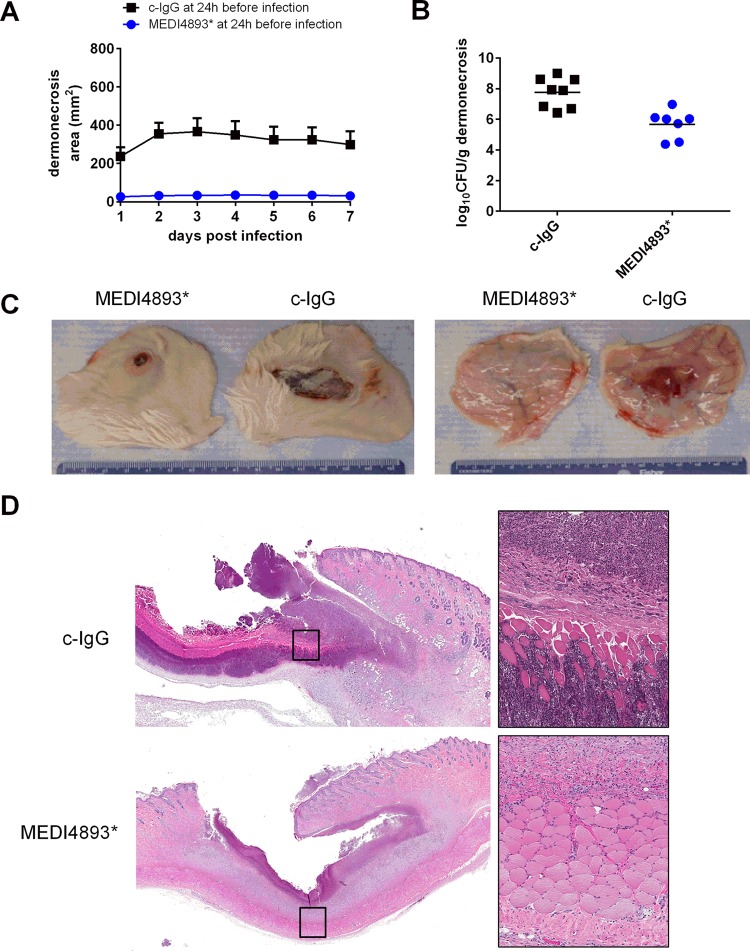
FIG 4.
Passive immune prophylaxis with MEDI4893*, an anti-alpha-toxin monoclonal antibody, protected against challenge with the SF8300 WT. Rabbits were administered 30 mg/kg MEDI4893* (7 rabbits) or 30 mg/kg c-IgG (8 rabbits) intravenously and then challenged 24 h later with 1.2 × 109 CFU of the SF8300 wild-type strain. (A) Dermonecrosis area on each day postinfection. Error bars indicate SEMs. Pairwise comparison of dermonecrosis area for animals pretreated with MEDI4893* versus those pretreated with c-IgG yielded P values of <0.01 (see Table S4 in the supplemental material). (B) Bacterial burden of dermonecrotic ulceration harvested from rabbits euthanized on day 7 postinfection. The log10 numbers of CFU per gram in the lesions of animals pretreated with MEDI4893* were significantly reduced compared to those in the lesions of animals pretreated with c-IgG (P < 0.001 by unpaired t test with Welch's correction). (C) Representative gross images of dermonecrotic ulceration from MEDI4893*- and c-IgG-pretreated animals taken on day 7 postinfection. (D) Histologically, animals treated with c-IgG (top left; magnification, 1×) had a significantly larger ulcerative lesion than animals treated with MEDI4893*, with a heavy accumulation of cellular debris, bacterial colonies, and necrosis of the muscle layer (top right, which is an image of the inset in the top left; magnification, 20×). In contrast, animals treated with MEDI4893* (bottom left; magnification, ×1) had a smaller epidermal ulcer than animals treated with c-IgG, with abscess formation, granulation tissue, and minimal inflammation of the subcutis muscle layer (bottom right, which is an image of the inset in the bottom left; magnification, ×20).
Histopathological analysis of skin lesions from animals pretreated with c-IgG showed a single large abscess surrounded by accumulations of viable and degenerate inflammatory infiltrates, sheets of bacterial colonies, and necrosis of the subjacent muscle layer (Fig. 4D). In contrast, skin lesions from animals pretreated with MEDI4893* were similar to those from animals infected with the Δhla mutant, showing multiple small to coalescing abscesses, dermal infiltration by inflammatory cells and fibroblasts, and minimal to no inflammation of the underlying subcutis muscle layer. Immunoprophylaxis with MEDI4893* greatly limited both the acute and progressive damage caused by inflammation in the skin, and animals demonstrated evidence of granulation tissue formation (healing) at day 7 postinfection.
MEDI4893* adjunctive therapy with linezolid significantly reduced disease severity.
Postinfection treatment of ABSSSI with antibiotics does not impact the presence or activity of preformed toxins, such as alpha-toxin. As such, we evaluated the efficacy of treatment with MEDI4893* alone or in combination with linezolid, an antibiotic approved for use for the treatment of ABSSSI (23). It is thought that the use of a protein synthesis inhibitor like linezolid may improve disease outcomes by inhibiting bacterial toxin synthesis (24–26). Previous experimental studies in rabbits have used linezolid at doses up to 75 mg/kg (27). Herein, we administered 25 mg/kg linezolid subcutaneously at 2, 10, 18, and 26 h postinfection, totaling just 4 doses over the 7-day study period. The rationale for this dosing regimen was based on pharmacokinetic parameters (see Table S2 in the supplemental material), which were designed to achieve subtherapeutic exposure compared to that achieved with the 600-mg human dose. Although the 25-mg/kg dose yielded a concentration in serum (16.0 μg/ml) higher than that observed with the human dose (12.9 μg/ml), a 3-fold reduction in the AUC and shortened half-life (rabbit, 0.64 h; human, 4.4 h) resulted in an AUC/MIC ratio of 13.15, well below the pharmacokinetic goal of a 24-h AUC/MIC of 80 to 120 (28). This subtherapeutic dosing regimen was not designed to achieve microbiological clearance but instead was designed to evaluate the potential adjunctive effect of MEDI4893* with linezolid.
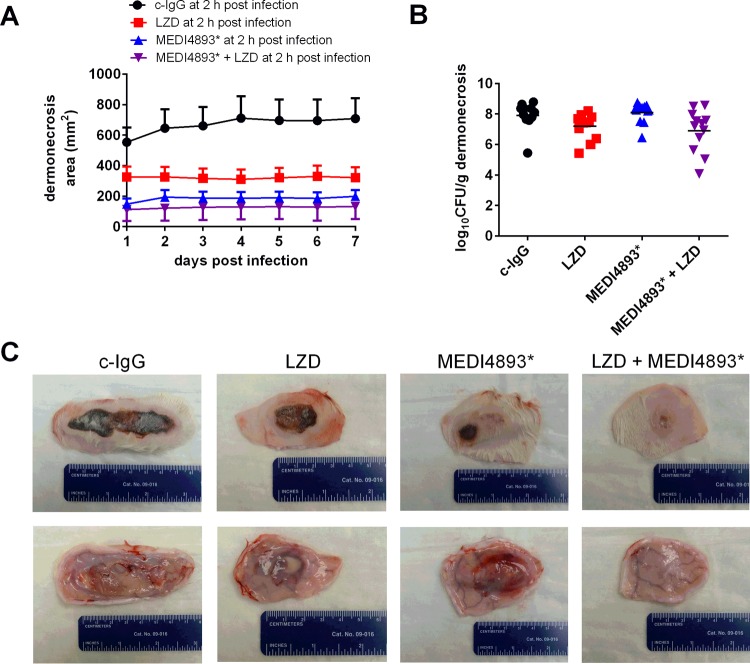
When administered at 2 h postinfection, MEDI4893*, linezolid, or a combination of both significantly reduced the dermonecrosis area compared to that achieved after administration of c-IgG (Fig. 5A and C; see also Table S5 in the supplemental material). However, the combination of MEDI4893* and linezolid resulted in a significant reduction in the dermonecrosis area compared to that achieved with linezolid alone (133 ± 24 versus 321 ± 68 mm2 [P = 0.036] on day 7 postinfection) (Fig. 5A; see also Table S5 in the supplemental material). Given the mechanism of action of linezolid (bacteriostatic) and MEDI4893* (alpha-toxin neutralization), there were only minimal between-group differences in bacterial burden in the skin (Fig. 5B).
FIG 5.
Protective efficacy of postexposure treatment with MEDI4893* and/or linezolid (LZD). After intradermal infection with 2.7 × 109 CFU of the SF8300 wild-type strain, rabbits were randomized into one of the following four treatment groups: (i) a single dose of 30 mg/kg c-IgG intravenously at 2 h postinfection (11 rabbits), (ii) a single dose of 30 mg/kg MEDI4893* intravenously at 2 h postinfection (12 rabbits), (iii) four doses of 25 mg/kg linezolid (LZD) subcutaneously at 2, 10, 18, and 26 h postinfection (12 rabbits), and (iv) a single dose of 30 mg/kg MEDI4893* intravenously at 2 h postinfection and four doses of 25 mg/kg linezolid subcutaneously at 2, 10, 18, and 25 h postinfection (12 rabbits). (A) Dermonecrosis area on each day postinfection. Error bars indicate SEMs. Pairwise comparisons of the daily dermonecrosis area for rabbits treated with c-IgG versus linezolid (except on days 1 and 2), c-IgG versus MEDI4893*, c-IgG versus linezolid-MEDI4893*, and linezolid versus linezolid-MEDI4893* yielded multiplicity-adjusted P values of <0.05, whereas pairwise comparisons of the daily dermonecrosis area for rabbits treated with linezolid versus MEDI4893* and MEDI4893* versus linezolid-MEDI4893* yielded P values of >0.05 (see Table S5 in the supplemental material). (B) Bacterial burden of dermonecrotic ulceration harvested from rabbits euthanized on day 7 postinfection. Pairwise comparisons of the log10 number of CFU per gram for rabbits treated with c-IgG versus LZD, c-IgG versus MEDI4893*, c-IgG versus linezolid-MEDI4893*, and linezolid versus linezolid-MEDI4893* yielded P values of >0.05, whereas pairwise comparisons of the log10 number of CFU per gram for rabbits treated with linezolid versus MEDI4893* and MEDI4893* versus linezolid-MEDI4893* yielded P values of 0.028 and 0.043, respectively. (C) Representative gross images of dermonecrotic ulceration taken on day 7 postinfection.
DISCUSSION
S. aureus is the predominant cause of ABSSSI in the United States, due in large part to the epidemic spread of community-associated MRSA clone USA300 (1). High rates of antimicrobial resistance are making it increasingly difficult to treat this and other life-threatening staphylococcal infections (29). Monoclonal antibody technology has enabled pathogen-specific targeting, which is less likely to elicit the development of bacterial resistance and which could be used to augment current antibiotic therapy to improve disease outcomes (30). Toward this end, relevant preclinical animal models of diseases mimicking S. aureus diseases in humans are urgently needed not only for evaluation of the therapeutic efficacy of MAbs in prophylaxis and therapy but also for the important first step of target identification and validation. In the study described here, we developed a rabbit model of ABSSSI that mimics some features of severe skin infections in humans, including histologic evidence of wound infection, ulceration, inflammation with necrosis of the epidermal and dermal layers, and abscessation comparable to that in human infections. In this model, the Δhla mutant was grossly attenuated in its capacity to cause dermonecrosis compared to the capacity of the SF8300 WT or compΔhla strain, indicating that alpha-toxin plays a key role in the pathogenesis of serious ABSSSI in the rabbit, a species similar to humans in its susceptibility not only to alpha-toxin but also to many other exotoxins. These data validate alpha-toxin as a viable immunotherapeutic target for this serious disease (Fig. 1 and 2). We reasoned that if an MAb could fully neutralize the alpha-toxin produced at the site of infection, then the resulting attenuated dermonecrotic ulceration should be similar to that produced by the Δhla mutant. Indeed, MEDI4893* treatment resulted in minimal dermonecrotic ulcerations and pathology that were remarkably similar to those caused by the Δhla mutant (compare Fig. 4C and 1A), indicating that the activity of this MAb was relatively complete in its neutralization of alpha-toxin. We further showed that when MEDI4893* was administered as adjunctive therapy with a subtherapeutic dose of linezolid, the combination was significantly more efficacious than linezolid alone in reducing the severity of dermonecrosis (Fig. 5).
While developing the rabbit model of ABSSSI, we found that the intradermal injection of SF8300 caused a severe acute inflammatory response with large central dermonecrotic ulcerations and subcutaneous hemorrhage, whereas subcutaneous injection caused only a minor skin abscess. The latter finding is similar to that obtained in the model of Kobayashi et al. (12), in that subcutaneous injection also resulted in an uncomplicated skin abscess with no dermonecrotic ulceration that spontaneously resolved without antibiotic treatment. The thickness of rabbit skin, which is similar to that of human skin (1.7 mm versus 1.5 mm) (31, 32), may explain why staphylococci are not able to break through the skin when injected subcutaneously and cause dermonecrosis. In contrast, subcutaneous injection of SF8300 and LAC/USA300 resulted in dermonecrotic ulceration in mice (21, 33, 34), but this may have been due to the thinner skin of this species (0.7 mm) (32). It should be noted that Li et al. also used the intradermal route with injection of 100 μl containing 5 × 108 CFU of the SF8300 WT strain, but severe dermonecrotic ulceration was not observed in their rabbit model (13). In our dose escalation study, we also found that intradermal injection of 5 × 108 CFU of the SF8300 WT strain was insufficient and that a higher dose of 2 × 109 to 3 × 109 CFU was required to produce severe dermonecrosis in rabbits.
Inasmuch as alpha-toxin has been shown to be an essential virulence determinant of ABSSSI, an anti-alpha-toxin MAb could be a good candidate for the prevention and treatment of this serious disease. Anti-alpha-toxin human MAb MEDI4893* has demonstrated protective efficacy for the prevention and treatment of dermonecrosis and pneumonia in mice (18–21). MEDI4893* recognizes a novel epitope in the rim domain of alpha-toxin and neutralizes it through a dual mechanism which sterically blocks binding of alpha-toxin to its cell receptor and prevents it from adopting a lytic heptameric transmembrane pore (35). In the rabbit model of ABSSSI, rabbits that received prophylaxis with MEDI4893* 24 h before infection exhibited a significant reduction in dermonecrotic ulceration compared to rabbits immunized with c-IgG, a human IgG1 isotype-matched control (P < 0.01). These results indicate that MEDI4893* can prevent disease not only in mice (18–21) but also in rabbits, a species that is susceptible to a greater array of S. aureus toxins (7–10, 36).
Linezolid, the first approved agent in the oxazolidinone class of antibiotics, is recommended as a second-line antibiotic for the treatment of complicated MRSA skin infections (37). Although linezolid and other protein synthesis inhibitors present the advantage of inhibiting the production of bacterial toxins, they do not affect preformed toxins. Similar to the results of previous studies in mice (21), adjunctive therapy with MEDI4893* and linezolid was superior to that with linezolid alone when they were administered at 2 h postinfection (P < 0.01; Fig. 5). Taken together, these results indicate that adjunctive therapy with an anti-alpha-toxin MAb and linezolid may be a rational therapeutic strategy to combat serious S. aureus skin infections.
A potential limitation of our study is that the therapeutic efficacy of MEDI4893*, with or without linezolid, was demonstrated when the therapeutic regimens were administered at 2 h postinfection, a relatively short treatment window. For the rabbit model of ABSSSI, we used a relatively high number of bacteria to achieve a reproducibly large dermonecrotic ulceration, which also resulted in a rapidly progressive infection with a treatment window shorter than that typically encountered in clinical situations. As such, these rabbit data cannot be used to extrapolate to a treatment window for anti-alpha-toxin MAb in humans. It is also of note that a single representative strain of the USA300 lineage was used in these studies, and it remains to be determined whether MEDI4893* protects against other strains in the rabbit model of ABSSSI.
In summary, using a USA300 clinical strain and its isogenic Δhla mutant, we have developed a rabbit model of ABSSSI mimicking these severe infections in humans and validated the essential role of alpha-toxin in the disease process. Prophylaxis with a human anti-alpha-toxin MAb, MEDI4893*, completely neutralized alpha-toxin-mediated pathogenesis, such that the resulting skin pathology was indistinguishable from that caused by the Δhla mutant strain. Treatment with MEDI4893* alone or as adjunctive therapy with linezolid also demonstrated significant protection.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by U.S. Public Health Service grant NIH R01 AI087674 and a MedImmune, LLC, grant.
The following authors are employed by MedImmune, LLC: Christine Tkaczyk, Lily Cheng, Sally Lee, Holly Koelkebeck, Jamese J. Hilliard, Xiang Qing Yu, Terrence O'Day, C. Kendall Stover, and Bret R. Sellman.
Patent 2014/0072,577 describing MEDI4893*, an anti-alpha-toxin human monoclonal antibody, used in this work has been filed by MedImmune, LLC.
Funding Statement
This work was supported by U.S. Public Health Service grant NIH R01 AI087674 and a MedImmune, LLC, grant.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00710-16.
REFERENCES
- 1.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 2.King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, Blumberg HM. 2006. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med 144:309–317. doi: 10.7326/0003-4819-144-5-200603070-00005. [DOI] [PubMed] [Google Scholar]
- 3.Diep BA, Chambers HF, Graber CJ, Szumowski JD, Miller LG, Han LL, Chen JH, Lin F, Lin J, Phan TH, Carleton HA, McDougal LK, Tenover FC, Cohen DE, Mayer KH, Sensabaugh GF, Perdreau-Remington F. 2008. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med 148:249–257. doi: 10.7326/0003-4819-148-4-200802190-00204. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration. Guidance for industry: acute bacterial skin and skin structure infections–developing antimicrobial drugs for treatment. U.S. Food and Drug Administration, Rockville, MD. http://www.fda.gov/downloads/Drugs//Guidances/ucm071185.pdf: Accessed 31 July 2015. [Google Scholar]
- 5.Rajendran PM, Young D, Maurer T, Chambers H, Perdreau-Remington F, Ro P, Harris H. 2007. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother 51:4044–4048. doi: 10.1128/AAC.00377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berube BJ, Bubeck Wardenburg J. 2013. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel) 5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spaan AN, Schiepers A, de Haas CJ, van Hooijdonk DD, Badiou C, Contamin H, Vandenesch F, Lina G, Gerard NP, Gerard C, van Kessel KP, Henry T, van Strijp JA. 2015. Differential interaction of the staphylococcal toxins Panton-Valentine leukocidin and gamma-hemolysin CB with human C5a receptors. J Immunol 195:1034–1043. doi: 10.4049/jimmunol.1500604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loffler B, Hussain M, Grundmeier M, Bruck M, Holzinger D, Varga G, Roth J, Kahl BC, Proctor RA, Peters G. 2010. Staphylococcus aureus Panton-Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog 6:e1000715. doi: 10.1371/journal.ppat.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diep BA, Chan L, Tattevin P, Kajikawa O, Martin TR, Basuino L, Mai TT, Marbach H, Braughton KR, Whitney AR, Gardner DJ, Fan X, Tseng CW, Liu GY, Badiou C, Etienne J, Lina G, Matthay MA, DeLeo FR, Chambers HF. 2010. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci U S A 107:5587–5592. doi: 10.1073/pnas.0912403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spaan AN, Henry T, van Rooijen WJ, Perret M, Badiou C, Aerts PC, Kemmink J, de Haas CJ, van Kessel KP, Vandenesch F, Lina G, van Strijp JA. 2013. The staphylococcal toxin Panton-Valentine leukocidin targets human C5a receptors. Cell Host Microbe 13:584–594. doi: 10.1016/j.chom.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Schlievert PM. 2009. Cytolysins, superantigens, and pneumonia due to community-associated methicillin-resistant Staphylococcus aureus. J Infect Dis 200:676–678. doi: 10.1086/605333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi SD, Malachowa N, Whitney AR, Braughton KR, Gardner DJ, Long D, Bubeck Wardenburg J, Schneewind O, Otto M, Deleo FR. 2011. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis 204:937–941. doi: 10.1093/infdis/jir441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Cheung GY, Hu J, Wang D, Joo HS, Deleo FR, Otto M. 2010. Comparative analysis of virulence and toxin expression of global community-associated methicillin-resistant Staphylococcus aureus strains. J Infect Dis 202:1866–1876. doi: 10.1086/657419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daum RS. 2007. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med 357:380–390. [DOI] [PubMed] [Google Scholar]
- 15.Diep BA, Carleton HA, Chang RF, Sensabaugh GF, Perdreau-Remington F. 2006. Roles of 34 virulence genes in the evolution of hospital- and community-associated strains of methicillin-resistant Staphylococcus aureus. J Infect Dis 193:1495–1503. doi: 10.1086/503777. [DOI] [PubMed] [Google Scholar]
- 16.Bae T, Schneewind O. 2006. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55:58–63. doi: 10.1016/j.plasmid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tkaczyk C, Hamilton MM, Datta V, Yang XP, Hilliard JJ, Stephens GL, Sadowska A, Hua L, O'Day T, Suzich J, Stover CK, Sellman BR. 2013. Staphylococcus aureus alpha toxin suppresses effective innate and adaptive immune responses in a murine dermonecrosis model. PLoS One 8:e75103. doi: 10.1371/journal.pone.0075103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hua L, Hilliard JJ, Shi Y, Tkaczyk C, Cheng LI, Yu X, Datta V, Ren S, Feng H, Zinsou R, Keller A, O'Day T, Du Q, Cheng L, Damschroder M, Robbie G, Suzich J, Stover CK, Sellman BR. 2014. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother 58:1108–1117. doi: 10.1128/AAC.02190-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tkaczyk C, Hua L, Varkey R, Shi Y, Dettinger L, Woods R, Barnes A, MacGill RS, Wilson S, Chowdhury P, Stover CK, Sellman BR. 2012. Identification of anti-alpha toxin monoclonal antibodies that reduce the severity of Staphylococcus aureus dermonecrosis and exhibit a correlation between affinity and potency. Clin Vaccine Immunol 19:377–385. doi: 10.1128/CVI.05589-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilliard JJ, Datta V, Tkaczyk C, Hamilton M, Sadowska A, Jones-Nelson O, O'Day T, Weiss WJ, Szarka S, Nguyen V, Prokai L, Suzich J, Stover CK, Sellman BR. 2015. Anti-alpha-toxin monoclonal antibody and antibiotic combination therapy improves disease outcome and accelerates healing in a Staphylococcus aureus dermonecrosis model. Antimicrob Agents Chemother 59:299–309. doi: 10.1128/AAC.03918-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diep BA, Palazzolo-Ballance AM, Tattevin P, Basuino L, Braughton KR, Whitney AR, Chen L, Kreiswirth BN, Otto M, DeLeo FR, Chambers HF. 2008. Contribution of Panton-Valentine leukocidin in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. PLoS One 3:e3198. doi: 10.1371/journal.pone.0003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Ryback MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 52:285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 24.Diep BA, Afasizheva A, Le HN, Kajikawa O, Matute-Bello G, Tkaczyk C, Sellman B, Badiou C, Lina G, Chambers HF. 2013. Effects of linezolid on suppressing in vivo production of staphylococcal toxins and improving survival outcomes in a rabbit model of methicillin-resistant Staphylococcus aureus necrotizing pneumonia. J Infect Dis 208:75–82. doi: 10.1093/infdis/jit129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens DL, Ma Y, Salmi DB, McIndoo E, Wallace RJ, Bryant AE. 2007. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J Infect Dis 195:202–211. doi: 10.1086/510396. [DOI] [PubMed] [Google Scholar]
- 26.Sharma-Kuinkel BK, Zhang Y, Yan Q, Ahn SH, Fowler VG Jr. 2013. Host gene expression profiling and in vivo cytokine studies to characterize the role of linezolid and vancomycin in methicillin-resistant Staphylococcus aureus (MRSA) murine sepsis model. PLoS One 8:e60463. doi: 10.1371/journal.pone.0060463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dailey CF, Pagano PJ, Buchanan LV, Paquette JA, Haas JV, Gibson JK. 2003. Efficacy of linezolid plus rifampin in an experimental model of methicillin-susceptible Staphylococcus aureus endocarditis. Antimicrob Agents Chemother 47:2655–2658. doi: 10.1128/AAC.47.8.2655-2658.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dryden MS. 2011. Linezolid pharmacokinetics and pharmacodynamics in clinical treatment. J Antimicrob Chemother 66(Suppl 4):iv7–iv15. doi: 10.1093/jac/dkr072. [DOI] [PubMed] [Google Scholar]
- 29.Chambers HF, Deleo FR. 2009. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fowler VG Jr, Proctor RA. 2014. Where does a Staphylococcus aureus vaccine stand? Clin Microbiol Infect 20(Suppl 5):S66–S75. doi: 10.1111/1469-0691.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oznurlu Y, Celik I, Sur E, Telatar T, Ozparlak H. 2009. Comparative skin histology of the White New Zealand and Angora rabbits: histometrical and immunohistochemical evaluations. J Anim Vet Adv 8:1694–1701. [Google Scholar]
- 32.Boudry I, Trescos Y, Vallet V, Cruz C, Lallement G. 2008. Methods and models for percutaneous absorption studies of organophosphates. Pathol Biol (Paris) 56:292–299. (In French.) doi: 10.1016/j.patbio.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 33.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, Otto M. 2007. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med 13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR. 2010. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis 202:1050–1058. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oganesyan V, Peng L, Damschroder MM, Cheng L, Sadowska A, Tkaczyk C, Sellman BR, Wu H, Dall'Acqua WF. 2014. Mechanisms of neutralization of a human anti-alpha-toxin antibody. J Biol Chem 289:29874–29880. doi: 10.1074/jbc.M114.601328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alonzo F III, Torres VJ. 2014. The bicomponent pore-forming leucocidins of Staphylococcus aureus. Microbiol Mol Biol Rev 78:199–230. doi: 10.1128/MMBR.00055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, Hirschmann JV, Kaplan SL, Montoya JG, Wade JC. 2014. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 59:e10–e52. doi: 10.1093/cid/ciu296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.