Abstract
The activity of ceftazidime-avibactam was compared with that of ceftazidime alone and meropenem against a collection of 190 Pseudomonas aeruginosa clinical isolates recovered from a multicenter study of bloodstream infections. The addition of avibactam increased ceftazidime susceptibility in the complete collection of strains (64.7% to 91.1%) and particularly among subsets of isolates showing AmpC hyperproduction (10.9% to 76.1%) or multidrug resistance (MDR) profiles (27% to 77.8%). The MICs of ceftazidime-avibactam, in contrast with those of ceftazidime or meropenem, remained at ≤4 μg/ml for a panel of 16 P. aeruginosa PAO1 isogenic mutants expressing multiple combinations of the most relevant β-lactam resistance mechanisms.
TEXT
Pseudomonas aeruginosa causes a wide range of severe infections and represents a therapeutic challenge due to its low intrinsic susceptibility to most antimicrobials and its extraordinary ability to develop resistance to nearly all available antibiotics through chromosomal mutations (1). Although the prevalence of acquired β-lactamases, particularly class B carbapenemases (metallo-β-lactamases [MBLs]), is increasing in certain areas, the overexpression of AmpC is still the most frequent and relevant resistance mechanism to penicillins and cephalosporins in P. aeruginosa, frequently leading to pan-β-lactam resistance profiles when combined with the inactivation of carbapenem porin OprD and/or the overexpression of diverse efflux pumps (2, 3).
Avibactam is a new broad-spectrum inhibitor of β-lactamases from classes A and C as well as some from class D, recently commercialized in combination with ceftazidime in the United States and Europe, with treatment indications for complicated urinary tract infections, complicated intra-abdominal infections, and hospital-acquired pneumonia (Europe) (4).
The objective of this study was to evaluate the activity of ceftazidime-avibactam, compared with that of ceftazidime alone and meropenem, against a collection of 190 P. aeruginosa clinical isolates recovered from a bloodstream infection multicenter study performed in Spain (5). Resistance mechanisms produced by this collection have been deeply characterized previously (5, 6). Additionally, a panel of 16 P. aeruginosa PAO1 isogenic mutants, expressing multiple combinations of the most relevant β-lactam resistance mechanisms, such as AmpC hyperproduction, OprD inactivation, and efflux pump overexpression, were tested. MICs were determined for ceftazidime alone or combined with avibactam (at a fixed concentration of 4 μg/ml) and for meropenem by broth microdilution using CLSI breakpoints (7). Recommendations by Magiorakos et al. were used for the definition of multidrug resistance (MDR) profiles (8).
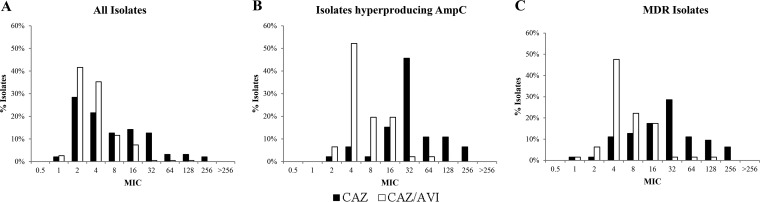
Consistent with previous reports (9, 10), the addition of avibactam significantly increased overall ceftazidime susceptibility in the collection of clinical strains from 64.7% to 91.1% (Table 1). Up to 74.6% of the isolates nonsusceptible to ceftazidime remained susceptible to ceftazidime-avibactam. Moreover, ceftazidime-avibactam overall susceptibility percentages were well above those of meropenem (77.4%). The effect of the addition of avibactam was even higher for the subset of isolates showing MDR profiles (27% to 77.8%) and AmpC hyperproduction (10.9% to 76.1%). The MIC distributions (Fig. 1) corroborated the significant increase in activity, with modal MICs of ceftazidime for MDR and AmpC-hyperproducing strains decreasing from 32 to 4 μg/ml with the addition of avibactam; the MIC50s and MIC90s (Table 1) also revealed an at least 4-fold higher potency of ceftazidime-avibactam compared to ceftazidime alone in these subsets of strains. Moreover, up to 74.1% of pan-β-lactam-resistant isolates were susceptible to ceftazidime-avibactam (Table 1).
TABLE 1.
MIC50/90 and susceptibility percentages for the entire collection of bloodstream isolates and subsets of isolates showing AmpC or efflux pump hyperproduction or MDR profiles
| MIC or susceptibility | All isolates (n = 190) |
AmpC-hyperproducing isolates (n = 46)a |
MexAB-hyperproducing isolates (n = 24)a |
MexXY-hyperproducing isolates (n = 25)a |
MDR isolates (n = 63) |
Pan-β-lactam-resistant isolates (n = 27)b |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAZ | CAZ-AVI | MER | CAZ | CAZ-AVI | MER | CAZ | CAZ-AVI | MER | CAZ | CAZ-AVI | MER | CAZ | CAZ-AVI | MER | CAZ-AVI | |
| MIC50 (μg/ml) | 4 | 4 | 1 | 32 | 4 | 8 | 8 | 8 | 8 | 8 | 4 | 8 | 32 | 4 | 8 | 8 |
| MIC90 (μg/ml) | 32 | 8 | 16 | 128 | 16 | 32 | 64 | 16 | 32 | 32 | 8 | 16 | 128 | 16 | 32 | 16 |
| % susceptiblec | 64.7 | 91.1 | 77.4 | 10.9 | 76.1 | 41.3 | 50.0 | 87.5 | 41.7 | 60.0 | 96.0 | 44.0 | 27.0 | 77.8 | 41.3 | 74.1 |
Previous definitions were used (5). Strains were considered positive for ampC or mexY overexpression when the corresponding mRNA level was at least 10-fold higher than that of PAO1. Strains were considered positive for mexB overexpression when the corresponding mRNA level was at least 3-fold higher than that of PAO1.
Pan-β-lactam-resistant isolates are defined as nonsusceptible to ceftazidime, cefepime, aztreonam, piperacillin-tazobactam, imipenem, and meropenem.
Breakpoints for ceftazidime (CAZ) susceptibility (S), ≤8 μg/ml; ceftazidime-avibactam (CAZ-AVI) S, ≤8/4 μg/ml; meropenem (MER) S, ≤4 μg/ml.
FIG 1.
(A) Ceftazidime (CAZ) and ceftazidime-avibactam (CAZ-AVI) MIC distributions for a collection of 190 P. aeruginosa bloodstream isolates recovered from a 10-hospital multicenter study performed in Spain. (B) CAZ and CAZ-AVI MIC distribution for the 46 isolates from the collection showing AmpC hyperproduction. (C) CAZ and CAZ-AVI MIC distribution for the 63 isolates from the collection showing an MDR profile.
Ceftazidime MIC50s were not much different in the presence of avibactam for the isolates overexpressing major efflux pumps (MexAB or MexXY), likely indicating that, as expected, avibactam does not provide protection against these resistance mechanisms. However, MIC90S and nonsusceptibility percentages were lower for ceftazidime-avibactam than for ceftazidime alone in these subsets of isolates, likely due to the coexpression of additional resistance mechanisms, particularly AmpC hyperproduction. On the other hand, the activity of the comparator meropenem was much lower than that of ceftazidime-avibactam among all subgroups of isolates (MDR, AmpC hyperproduction, and efflux pump overexpression), with susceptibility rates below 50% in all cases (Table 1).
Of the 17 (8.9%) isolates nonsusceptible to ceftazidime-avibactam (MICs >8 μg/ml), two of them produced the MBL VIM-2. These two isolates showed the highest ceftazidime-avibactam MIC values, 64 and 128 μg/ml, and were the only isolates found to produce an acquired β-lactamase in the complete collection of 190 isolates (5). The MICs for all other nonsusceptible strains ranged from 16 (14 isolates) to 32 (1 isolate) μg/ml. Thus, most nonsusceptible isolates remained within the CLSI ceftazidime-intermediate category (16 μg/ml). The analysis of resistance mechanisms (AmpC and efflux pumps) in this subset of isolates failed to detect specific differences with ceftazidime-avibactam-susceptible isolates, arguing in favor of the existence of yet-identified mechanisms modulating ceftazidime-avibactam susceptibility (11).
The activity of ceftazidime-avibactam, compared with that of ceftazidime alone and meropenem, was also evaluated in a collection of PAO1 isogenic mutants expressing multiple combinations of the most relevant β-lactam resistance mechanisms, including multiple levels of AmpC hyperproduction, mutation of nonessential penicillin-binding proteins (PBPs), inactivation of the porin OprD, and/or efflux pump overexpression. As shown in Table 2, MICs of ceftazidime-avibactam, in contrast with those of ceftazidime or meropenem, remained at ≤4 μg/ml in all cases. The potentiation of the activity of ceftazidime by avibactam was highest among isolates showing multiple combinations of mutations leading to very high-level AmpC production, such as the triple ampD mutant, the mutant defective in all 3 nonessential PBPs or the AmpD-PBP4 double mutant, for which the MIC of ceftazidime was reduced from 64 to 4 μg/ml. Thus, the results are similar to those documented for the novel combination ceftolozane-tazobactam (3, 12). It should be noted, however, that resistance to both novel combinations may emerge through the (infrequent) selection of different mutations leading to the modification of AmpC structure (13). MexAB-OprM overexpression determined a reduction (4-fold MIC increase) in ceftazidime susceptibility, which was not restored, as expected (14), by the addition of avibactam. However, the positive effect of avibactam on AmpC-hyperproducing strains was still seen even when they simultaneously overexpressed MexAB-OprM (see MexR and AmpD-MexR mutants in Table 2). On the other hand, consistent with previous data (2), the susceptibility of meropenem was highly compromised by combinations of OprD inactivation and AmpC or efflux pump (MexAB-OprM) hyperproduction.
TABLE 2.
MICs for CAZ, CAZ-AVI, and MER for PAO1 isogenic mutants expressing multiple combinations of most relevant β-lactam resistance mechanisms
| Strain | Phenotypea | Reference | MIC (μg/ml) |
||
|---|---|---|---|---|---|
| CAZ | CAZ-AVI | MER | |||
| PAO1 | Wild-type strain | 1 | 1 | 0.5 | |
| PAO ΔdacB | PAO1 PBP4 mutant (↑ampC [ca. 50-fold]) | 15 | 32 | 2 | 0.5 |
| PAO ΔdacC | PAO1 PBP5 mutant | 16 | 1 | 1 | 0.5 |
| PAO ΔdacB ΔdacC | PAO1 PBP4-PBP5 mutant (↑ampC [ca. 500-fold]) | 16 | 64 | 2 | 0.5 |
| PAO ΔdacB ΔpbpG ΔdacC | PAO1 PBP4-PBP5-PBP7 mutant (↑ampC [ca. 1,200-fold]) | 16 | 64 | 2 | 0.5 |
| PAO ΔampD | PAO1 AmpD mutant (↑ampC [ca. 50-fold]) | 17 | 16 | 2 | 1 |
| PAOΔDΔDh2ΔDh3 | PAO1 AmpD-AmpDh2-AmpDh3 mutant (↑ampC [ca. 1,000-fold]) | 17 | 64 | 4 | 1 |
| PAOD1 | OprD− spontaneous PAO1 mutant (W65X) | 12 | 1 | 1 | 2 |
| PAOD1 ΔampD | PAOD1 (OprD−) AmpD mutant (↑ampC [ca. 50-fold]) | 12 | 16 | 2 | 8 |
| PAOΔdB ΔampD | PAO1 PBP4 AmpD mutant (↑ampC [ca. 1,800-fold]) | 15 | 64 | 4 | 1 |
| PAOD1 ΔdacB | PAOD1 (OprD−) PBP4 mutant (↑ampC [ca. 50-fold]) | 12 | 32 | 2 | 2 |
| PAOΔMxR | PAO1 MexR mutant (↑mexB [ca. 10-fold]) | 18 | 4 | 4 | 2 |
| PAODΔMxR | PAOD1 (OprD−) MexR mutant (↑mexB [ca. 10-fold]) | 2 | 4 | 4 | 8 |
| PAOΔDΔMxR | PAO1 AmpD-MexR mutant (↑ampC [ca. 50-fold] + ↑mexB [ca. 10-fold]) | 2 | 32 | 4 | 4 |
| PAOΔNB | PAO1 NfxB mutant (↑mexD [ca. 150-fold]) | 19 | 1 | 1 | 0.25 |
| PAOΔMxZ | PAO1 MexZ mutant (↑mexY [ca. 15-fold]) | 20 | 1 | 1 | 0.5 |
| PAODΔMxZ | PAOD1 (OprD−) MexZ mutant (↑mexY [ca. 15-fold]) | This work | 1 | 1 | 2 |
Expression levels and oprD amino acid changes are in reference to PAO1.
Thus, ceftazidime-avibactam could be a new useful therapeutic option for the treatment of nosocomial infections by P. aeruginosa, including non-MBL-producing MDR strains.
Funding Statement
This study was supported by AstraZeneca Pharmaceutical Spain and by the Ministerio de Ciencia e Innovación of Spain, Instituto de Salud Carlos III through the Spanish Network for Research in Infectious Diseases (grants REIPI RD12/0015 and RD16/00016), cofinanced by European Regional Development Fund “A Way To Achieve Europe” ERDF.
REFERENCES
- 1.Lister PD, Wolter DJ, Hanson ND. 2009. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 22:582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riera E, Cabot G, Mulet X, García-Castillo M, del Campo R, Juan C, Cantón R, Oliver A. 2011. Pseudomonas aeruginosa carbapenem resistance mechanisms in Spain: impact on the activity of imipenem, meropenem and doripenem. J Antimicrob Chemother 66:2022–2027. doi: 10.1093/jac/dkr232. [DOI] [PubMed] [Google Scholar]
- 3.Moyá B, Beceiro A, Cabot G, Juan C, Zamorano L, Alberti S, Oliver A. 2012. Pan-β-lactam resistance development in Pseudomonas aeruginosa clinical strains: molecular mechanisms, penicillin-binding protein profiles, and binding affinities. Antimicrob Agents Chemother 56:4771–4778. doi: 10.1128/AAC.00680-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmeli Y, Armstrong J, Laud PJ, Newell P, Stone G, Wardman A, Gasink LB. 2016. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomized, pathogen-directed, phase 3 study. Lancet Infect Dis 16:661–673. doi: 10.1016/S1473-3099(16)30004-4. [DOI] [PubMed] [Google Scholar]
- 5.Cabot G, Ocampo-Sosa AA, Tubau F, Macia MD, Rodríguez C, Moya B, Zamorano L, Suárez C, Peña C, Martínez-Martínez L, Oliver A, Spanish Network for Research in Infectious Diseases (REIPI). 2011. Overexpression of AmpC and efflux pumps in Pseudomonas aeruginosa isolates from bloodstream infections: prevalence and impact on resistance in a Spanish multicenter study. Antimicrob Agents Chemother 55:1906–1911. doi: 10.1128/AAC.01645-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ocampo-Sosa AA, Cabot G, Rodríguez C, Roman E, Tubau F, Macia MD, Moya B, Zamorano L, Suárez C, Peña C, Domínguez MA, Moncalián G, Oliver A, Martínez-Martínez L, Spanish Network for Research in Infectious Diseases (REIPI). 2012. Alterations of OprD in carbapenem-intermediate and -susceptible strains of Pseudomonas aeruginosa isolated from patients with bacteremia in a Spanish multicenter study. Antimicrob Agents Chemother 56:1703–1713. doi: 10.1128/AAC.05451-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute (CLSI). 2016. Performance standards for antimicrobial susceptibility testing, 26th ed CLSI document M100-S26. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 8.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 9.Sader HS, Castanheira M, Mendes RE, Flamm RK, Farrell DJ, Jones RN. 2015. Ceftazidime-avibactam activity against multidrug-resistant Pseudomonas aeruginosa isolated in U.S. medical centers in 2012 and 2013. Antimicrob Agents Chemother 59:3656–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitart C, Marco F, Keating TA, Nichols WW, Vila J. 2015. Activity of ceftazidime-avibactam against fluoroquinolone-resistant Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 59:3059–3065. doi: 10.1128/AAC.05136-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winkler ML, Papp-Wallace KM, Hujer AM, Domitrovic TN, Hujer KM, Hurless KN, Tuohy M, Hall G, Bonomo RA. 2014. Unexpected challenges in treating multidrug-resistant Gram-negative bacteria: resistance to ceftazidime-avibactam in archived isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother 59:1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moya B, Zamorano L, Juan C, Pérez JL, Ge Y, Oliver A. 2010. Activity of a new cephalosporin, CXA-101 (FR264205), against beta-lactam-resistant Pseudomonas aeruginosa mutants selected in vitro and after antipseudomonal treatment of intensive care unit patients. Antimicrob Agents Chemother 54:1213–1217. doi: 10.1128/AAC.01104-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahiri SD, Walkup GK, Whiteaker JD, Palmer T, McCormack K, Tanudra MA, Nash TJ, Thresher J, Johnstone MR, Hajec L, Livchak S, McLaughlin RE, Alm RA. 2015. Selection and molecular characterization of ceftazidime/avibactam-resistant mutants in Pseudomonas aeruginosa strains containing derepressed AmpC. J Antimicrob Chemother 70:1650–1658. [DOI] [PubMed] [Google Scholar]
- 14.Mushtaq S, Warner M, Livermore DM. 2010. In vitro activity of ceftazidime+NXL104 against Pseudomonas aeruginosa and other non-fermenters. J Antimicrob Chemother 65:2376–2381. doi: 10.1093/jac/dkq306. [DOI] [PubMed] [Google Scholar]
- 15.Moya B, Dötsch A, Juan C, Blázquez J, Zamorano L, Haussler S, Oliver A. 2009. Beta-lactam resistance response triggered by inactivation of a nonessential penicillin-binding protein. PLoS Pathog 5:e1000353. doi: 10.1371/journal.ppat.1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ropy A, Cabot G, Sánchez-Diener I, Aguilera C, Moya B, Ayala JA, Oliver A. 2015. Role of Pseudomonas aeruginosa low-molecular-mass penicillin-binding proteins in AmpC expression, β-lactam resistance, and peptidoglycan structure. Antimicrob Agents Chemother 59:3925–3934. doi: 10.1128/AAC.05150-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juan C, Moyá B, Pérez JL, Oliver A. 2006. Stepwise upregulation of the Pseudomonas aeruginosa chromosomal cephalosporinase conferring high-level beta-lactam resistance involves three AmpD homologues. Antimicrob Agents Chemother 50:1780–1787. doi: 10.1128/AAC.50.5.1780-1787.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulet X, Moyá B, Juan C, Macià MD, Pérez JL, Blázquez J, Oliver A. 2011. Antagonistic interactions of Pseudomonas aeruginosa antibiotic resistance mechanisms in planktonic but not biofilm growth. Antimicrob Agents Chemother 55:4560–4568. doi: 10.1128/AAC.00519-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulet X, Maciá MD, Mena A, Juan C, Pérez JL, Oliver A. 2009. Azithromycin in Pseudomonas aeruginosa biofilms: bactericidal activity and selection of nfxB mutants. Antimicrob Agents Chemother 53:1552–1560. doi: 10.1128/AAC.01264-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martínez-Ramos I, Mulet X, Moyá B, Barbier M, Oliver A, Albertí S. 2014. Overexpression of MexCD-OprJ reduces Pseudomonas aeruginosa virulence by increasing its susceptibility to complement-mediated killing. Antimicrob Agents Chemother 58:2426–2429. doi: 10.1128/AAC.02012-13. [DOI] [PMC free article] [PubMed] [Google Scholar]