LETTER
Since the first description of the plasmid-mediated colistin resistance gene (mcr-1), over 30 follow-up reports have proved the worldwide geographical distribution of this gene (1, 2). The overall picture indicates a very low prevalence in animals, human beings, and retail food, with two exceptions, the first in China, where mcr-1 carriage was observed in 21% and 15% of the animals and raw meat samples, and the second in France, where 21% of the extended-spectrum-β-lactamase (ESBL)-producing Escherichia coli isolates from calves were mcr-1 positive (1, 3).
To date, only one study from China has provided dynamic information on the prevalence of the mcr-1 gene (4). The earliest mcr-1-positive E. coli isolate from chickens was identified in the 1980s, and a dramatic rise in mcr-1 prevalence was highlighted over the past 6 years (from 5.2% in 2009 to 30% in 2014) (4). Here, we provide annual figures of the proportion of mcr-1 among ESBL-producing E. coli isolates from French calves from 2006 to 2014. Using the recently published mcr-1-specific primers (1), we retrospectively screened our collection of 885 nonduplicate ESBL-producing E. coli isolates collected through the long-term French monitoring program Resapath (www.resapath.anses.fr). All isolates were obtained from different individuals and farms and mostly presented differing pulsed-field gel electrophoresis profiles.
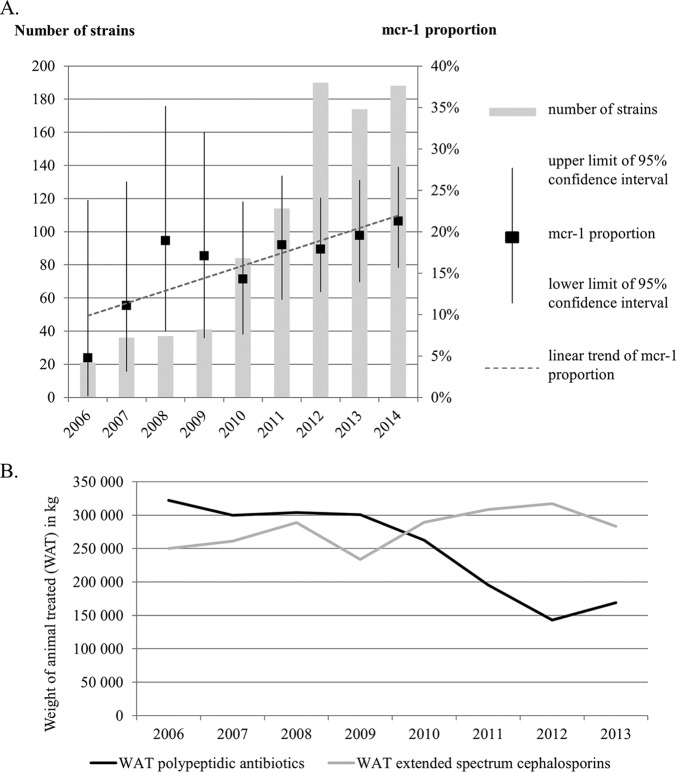
As shown in Fig. 1A, the proportion of mcr-1-positive E. coli strains among ESBL-producing E. coli isolates increased from 4.76% in 2006 to 21.28% in 2014. This corresponded to an increase from 1 mcr-1-positive strain recovered in 2006 to 37 in 2014. The proportion of mcr-1-positive strains increased until 2014, with an estimated rise of 1.28% per year (chi-square test for linear trend, P = 0.038). The number of strains tested from 2006 to 2009 was low due to the still limited number of ESBL-producing E. coli strains in bovines. Therefore, the confidence intervals of the proportions of mcr-1-positive E. coli strains among ESBL-producing E. coli isolates are wide, but the results of tests for linear trend, which take such variability into account, are statistically significant. Interestingly enough, the 2006–2007 period, which was seemingly a starting point of this rising trend, came shortly after the first reports of ESBL-producing E. coli in bovines in France (5). Taking the data together, the increasing mcr-1 prevalence among ESBL-producing E. coli strains clearly differs from the low mcr-1 prevalences in non-ESBL-producing E. coli strains, which stand at around 1.0% in healthy calves (M. Haenni, unpublished data), 0.5% in pigs, 1.8% in broilers, and 5.9% in turkeys (6). This suggests that the use of extended-spectrum cephalosporins may have simultaneously favored the spread of mcr-1. This hypothesis is also supported by previous data demonstrating the colocalization in E. coli of mcr-1 and blaESBL genes on a unique IncHI2/ST4 plasmid in French calves (3, 7).
FIG 1.
(A) Proportion of mcr-1-positive E. coli strains among ESBL-producing E. coli isolates from French calves and linear trend of the evolution between 2006 and 2014. Upper and lower limits of confidence intervals at 95% of the mcr-1 proportion are shown. (B) Trends in weights of animals treated (WAT) (bovines) with polypeptidic antibiotics (almost exclusively represented by colistin) or extended-spectrum cephalosporins.
In terms of usage, it was not possible to retrospectively trace the individual treatments, either with colistin or cephalosporins, of the mcr-1-positive calves, and this is a limitation of the study. However, as reported in the 2013 sales survey of veterinary medicinal products containing antimicrobials in France (https://www.anses.fr/en/content/monitoring-sales-veterinary-antimicrobials), the global exposure of bovines to polypeptidic antibiotics (almost exclusively represented by calves orally treated with colistin) decreased by 52.4% between 2005 and 2013 (Fig. 1B). In contrast, during the same period, the global exposure of bovines to extended-spectrum cephalosporins was constantly high (Fig. 1B) (https://www.anses.fr/en/content/monitoring-sales-veterinary-antimicrobials), with the main ESBL reservoir in bovines being found also in calves, as previously reported (8).
Shen et al. attributed the rise in mcr-1 prevalence in China to a parallel increased use of colistin in food animals (4). In line with recent data on mcr-1 in Brazilian poultry not exposed to polymyxins (9), our data strongly indicate driving forces for the spread of mcr-1 other than the use of colistin only, notably operating through the use of extended-spectrum cephalosporins. This reinforces the idea of the need for global intervention programs on the prudent use of all antibiotics in the Agri-Food sector worldwide, beyond the very recent advice of the European Medicine Agency focused on colistin use in food animals (10).
ACKNOWLEDGMENTS
We deeply acknowledge all peripheral laboratories of the Resapath network.
We declare that we have no conflicts of interest.
This study was supported by the Agency for Food, Environmental and Occupational Health and Safety (ANSES) and by a grant of the ANIWHA ERA-NET project (France).
REFERENCES
- 1.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. 18 November 2015. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 2.Skov RL, Monnet DL. 2016. Plasmid-mediated colistin resistance (mcr-1 gene): three months later, the story unfolds. Euro Surveill 21(9). doi: 10.2807/1560-7917.ES.2016.21.9.30155. [DOI] [PubMed] [Google Scholar]
- 3.Haenni M, Poirel L, Kieffer N, Châtre P, Saras E, Métayer V, Dumoulin R, Nordmann P, Madec J-Y. 7 January 2015. Co-occurrence of extended spectrum β-lactamase and MCR-1 encoding genes on plasmids. Lancet Infect Dis doi: 10.1016/S1473-3099(16)00007-4. [DOI] [PubMed] [Google Scholar]
- 4.Shen Z, Wang Y, Shen Y, Shen J, Wu C. 2016. Early emergence of mcr-1 in Escherichia coli from food-producing animals. Lancet Infect Dis 16:293. doi: 10.1016/S1473-3099(16)00061-X. [DOI] [PubMed] [Google Scholar]
- 5.Meunier D, Jouy E, Lazizzera C, Kobisch M, Madec JY. 2006. CTX-M-1- and CTX-M-15-type beta-lactamases in clinical Escherichia coli isolates recovered from food-producing animals in France. Int J Antimicrob Agents 28:402–407. doi: 10.1016/j.ijantimicag.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Perrin-Guyomard A, Bruneau M, Houée P, Deleurme K, Legrandois P, Poirier C, Soumet C, Sanders P. 2016. Prevalence of mcr-1 in commensal Escherichia coli from French livestock, 2007 to 2014. Euro Surveill 21(6). doi: 10.2807/1560-7917.ES.2016.21.6.30135. [DOI] [PubMed] [Google Scholar]
- 7.Grami R, Mansour W, Mehri W, Bouallègue O, Boujaâfar N, Madec JY, Haenni M. 2016. Impact of food animal trade on the spread of mcr-1-mediated colistin resistance, Tunisia, July 2015. Euro Surveill 21(8). doi: 10.2807/1560-7917.ES.2016.21.8.30144. [DOI] [PubMed] [Google Scholar]
- 8.Haenni M, Chatre P, Metayer V, Bour M, Signol E, Madec JY, Gay E. 2014. Comparative prevalence and characterization of ESBL-producing Enterobacteriaceae in dominant versus subdominant enteric flora in veal calves at slaughterhouse, France. Vet Microbiol 171:321–327. doi: 10.1016/j.vetmic.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Lentz SA, de Lima-Morales D, Cuppertino VM, de Nunes LS, da Motta AS, Zavascki AP, Barth AL, Martins AF. 2016. Escherichia coli harbouring mcr-1 gene isolated from poultry not exposed to polymyxins in Brazil. Euro Surveill 21(26). doi: 10.2807/1560-7917.ES.2016.21.26.30267. [DOI] [PubMed] [Google Scholar]
- 10.European Medicine Agency (EMA). 26 May 2016. Updated advice on the use of colistin products in animals within the European Union: development of resistance and possible impact on human and animal health. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2016/05/news_detail_002536.jsp&mid=WC0b01ac058004d5c1.