Abstract
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA), especially the USA300 pulsotype, is a frequent cause of skin and soft tissue infections and severe pneumonia. Despite appropriate antibiotic treatment, complications are common and pneumonia is associated with high mortality. S. aureus strains express multiple cytotoxins, including alpha-hemolysin (Hla) and up to five bicomponent leukocidins that specifically target phagocytic cells for lysis. CA-MRSA USA300 strains carry the genes for all six cytotoxins. Species specificity of the leukocidins greatly contributes to the ambiguity regarding their role in S. aureus pathogenesis. We performed a comparative analysis of the leukocidin susceptibility of human, rabbit, and mouse polymorphonuclear leukocytes (PMNs) to assess the translational value of mouse and rabbit S. aureus models. We found that mouse PMNs were largely resistant to LukSF-PV, HlgAB, and HlgCB and susceptible only to LukED, whereas rabbit and human PMNs were highly sensitive to all these cytotoxins. In the rabbit pneumonia model with a USA300 CA-MRSA strain, passive immunization with a previously identified human monoclonal antibody (MAb), Hla-F#5, which cross-neutralizes Hla, LukSF-PV, HlgAB, HlgCB, and LukED, provided full protection, whereas an Hla-specific MAb was only partially protective. In the mouse USA300 CA-MRSA pneumonia model, both types of antibodies demonstrated full protection, suggesting that Hla, but not leukocidin(s), is the principal virulence determinant in mice. As the rabbit recapitulates the high susceptibility to leukocidins characteristic of humans, this species represents a valuable model for assessing novel, cytotoxin-targeting anti-S. aureus therapeutic approaches.
INTRODUCTION
Staphylococcus aureus is one of the most common human pathogens. It is characterized by high versatility and adaptability, causing many types of infections with various levels of disease severity, with pneumonia being one of the most severe infections associated with high mortality (1). Its success as a pathogen relies on the great arsenal of virulence factors, many of which are employed to evade and counteract the immune system (2).
One of the most aggressive ways in which S. aureus interacts with the human host is through the production of tissue- and cell-damaging cytolytic toxins (3). The role of alpha-hemolysin (Hla, or alpha-toxin), a beta-barrel pore-forming toxin that damages epithelial and endothelial cells, in the pathogenesis of pneumonia is well characterized (4). Phagocytic cells, especially neutrophilic granulocytes, are the cornerstones of the immune defense against extracellular bacterial pathogens in general and S. aureus in particular (5). In recent years, the important role of the bicomponent leukocidins lysing white blood cells (WBCs), mainly phagocytic cells, became evident (3, 6). The fact that S. aureus is capable of producing up to five potent leukocidins underlines the importance of this type of immune evasion mechanism. These toxins—two gamma-hemolysins (HlgAB and HlgCB), the Panton-Valentine leukocidin (LukSF-PV), and LukED and LukGH (also known as LukAB)—belong to the bicomponent beta-barrel pore-forming toxin family and share structural homology with Hla (6). The most prevalent community-associated methicillin-resistant S. aureus (CA-MRSA) clone in the United States, the USA300 pulsotype, carries the genes for all five leukocidins.
The species specificity of the staphylococcal cytotoxins delayed the recognition of their important roles in S. aureus pathogenesis. The most studied of these toxins is LukSF-PV, which was the first identified leukocidin. It was shown to be inactive as a cytolysin with respect to mouse cells, while rabbit and human neutrophils are highly susceptible (7, 8). LukSF-PV was demonstrated to be a major contributor to acute lung injury in a rabbit model of necrotizing pneumonia induced by USA300 CA-MRSA (8), whereas deletion of the LukSF-PV gene did not attenuate S. aureus in a murine model of pneumonia (9). The role of gamma-hemolysins could not be convincingly demonstrated in mice in vivo, and these toxins are not lytic with respect to murine neutrophils in vitro (8). The lack of sensitivity of murine neutrophils to LukSF-PV and the gamma-hemolysins arises from the lack of evolutionary conservation of their cognate receptors, which have been identified as the complement receptors C5aR and C5L2 for LukSF-PV and HlgCB and as the chemokine receptors CXCR1 and CXCR2 (and CCR2) for HlgAB (10, 11). LukED, which also recognizes CXCR1 and CXCR2 (and, in addition, CCR5 on lymphocytes), was shown to contribute to pathogenesis in murine bacteremia models (12, 13), but its role in the pathogenesis of murine pneumonia has not been established. Mouse neutrophils are lysed by LukED, although at toxin concentrations approximately 10 times higher than those needed for human neutrophils (14). Interestingly, while HlgAB and LukED share receptors on human neutrophils, the mouse CXCR2 is not recognized by HlgAB, in contrast to LukED (10). The more distantly related bicomponent leukocidin LukGH/LukAB, which binds CD11b, the α-subunit of the complement receptor CR3 (CD11b/CD18 or Mac1), also displays species specificity, being very potent against human phagocytic cells but intermediately active and very weak against rabbit and mouse phagocytes, respectively (15, 16).
Previously, we reported the discovery of unique human monoclonal antibodies (MAbs) with simultaneous, high-affinity binding to Hla and to three F components (HlgB, LukF-PV, and LukD) of the leukocidin family, resulting in potent inhibition of lysis of human cells by Hla, HlgAB, HlgCB, LukSF-PV, and LukED in in vitro assays (17). Such an antibody was shown to be highly protective in mouse pneumonia models with several S. aureus strains (17). Considering the species specificity of the leukocidins and their low toxicity in mice, it appears that murine models have limitations with respect to evaluation of the efficacy of such antibodies. Moreover, mice were also reported to be significantly less sensitive or even resistant to other toxins, e.g., Hla, toxic shock syndrome toxin 1 (TSST-1), and enterotoxin superantigens, all of which are important S. aureus virulence factors (18).
Hla is known to be hemolytic and to cause damage to the lung in both mice and rabbits. However, the susceptibility of rabbits to gamma-hemolysins and LukED and the contribution of these toxins to disease have not been reported. Therefore, we aimed to determine the sensitivity of rabbit polymorphonuclear cells (PMNs) to these leukocidins in vitro and to compare the protective efficacies of Hla-specific and Hla-leukocidin cross-neutralizing MAbs in rabbit and mouse models of CA-MRSA pneumonia.
MATERIALS AND METHODS
Human monoclonal antibodies used in the study.
The Hla/HlgB/LukD/LukF-PV cross-reactive (Hla-F#5) and Hla-specific human MAbs were discovered using a human IgG1 library expressed by yeast as described previously (17). The two MAbs target the cell binding region (rim domain) of Hla and compete with each other for antigen binding. They display comparable affinities and neutralization potencies for Hla (17). The exact epitopes are different, resulting in quadruple- or monospecific antibodies. Motavizumab, specific for the respiratory syncytial virus (RSV), was used as an isotype-matched (human IgG1), negative-control antibody. The genes for the heavy and light chains of each MAb were cotransfected into HEK293 cells using the same plasmid vectors and transfection protocols. MAbs were purified by protein A affinity chromatography, dialyzed against phosphate-buffered saline (PBS), and stored at the same concentration at −80°C until use.
Mouse pneumonia model.
Female, 6-to-8-week-old BALB/cJRj mice (Janvier, France) were randomized for administration of one of the three monoclonal antibody preparations: Hla-F#5 MAb; Hla-specific MAb; or isotype-matched, control MAb. Each animal was dosed with 200 μg MAbs (approximately 10 mg/kg of body weight) via the intraperitoneal route. At 24 h postimmunization, animals were anesthetized by intraperitoneal injection of 200 μl of 10% ketamine (Ketamidor; Richter-Pharma) and 2% xylazine (Rompun; Bayer) and subsequently challenged intranasally with a lethal dose of 8 × 108 CFU (in 40 μl of saline solution) of the strain, a clinical isolate belonging to epidemic CA-MRSA clone USA300-0114. Survival of animals was monitored daily for 10 days postchallenge. Moribund animals were humanely euthanized by cervical dislocation prior to the experimental endpoint. The murine studies were performed according to Austrian law (BGBl Nr. 501/1989, approved by MA58, Vienna, Austria).
Rabbit model of necrotizing pneumonia.
Male New Zealand White rabbits (weighing between 2.0 and 2.4 kg) were randomly assigned for intravenous administration of one of the three blind monoclonal antibody preparations, corresponding to 10 mg/kg of Hla-F#5 MAb, Hla-specific MAb, or isotype-matched control MAb. At 24 h after antibody prophylaxis, animals were challenged endobronchially with 6.1 × 109 CFU (in 1.5 ml of saline solution) of SF8300, as previously described (8, 18). Rabbits were assessed for evidence of pulmonary dysfunction every 3 h for the first 48 h and three times daily thereafter until all survivors were euthanized at 90 h postinfection. Animals with pulmonary dysfunction—defined as a respiration rate of >75 breaths per minute and the presence of cyanosis and cough—were euthanized. Ratios of lung weight to body weight (LW/BW) and bacterial counts in lungs, spleens, and kidneys were determined at the time that rabbits were found dead or were euthanized (8, 19).
PMN lysis assays.
Mouse PMNs were purified from acid citrate dextrose (ACD)-treated whole blood of naive BALB/cJRj mice (Janvier, France) by negative selection using an EasySep mouse neutrophil enrichment kit (Stemcell Technologies) according to the manufacturer's instructions. Rabbit PMNs from ACD-treated blood of naive New Zealand White rabbits (Medical University of Vienna, Division for Laboratory Animal Science and Genetics, Vienna, Austria) were purified using Histopaque 1077 (Sigma-Aldrich) and HetaSep (Stemcell Technologies) as described elsewhere (20). Human PMNs were isolated from heparinized human whole blood according to a previously published protocol (17). Purification methods resulted in PMN purity of >90% for murine and >95% for human and rabbit PMNs as determined by flow cytometry (forward-scatter/side-scatter dot plot analysis) using an iCyt Eclipse flow cytometer (Sony Biotechnology) as described in reference 20. Viability was determined by trypan blue (Thermo Scientific) exclusion and was >98% for all three species. Purified PMNs were resuspended in RPMI 1640 (Gibco) supplemented with 10% fetal calf serum (FCS) (Sigma-Aldrich) and 2 mM l-glutamine (Invitrogen), referred to as “neutrophil medium” for all cell-based assays. For toxin activity assays, an equimolar mixture of the leukocidin F and S components was serially diluted in neutrophil medium and used for intoxication of 2.5 × 104 cells for 4 h at 37°C and 5% CO2 in 96-well, white half-area plates (Greiner). Cell viability was then measured using a CellTiter-Glo luminescent cell viability assay kit (Promega), and lactate dehydrogenase (LDH) release was determined using a CytoTox 96 nonradioactive cytotoxicity assay (Promega). Percent viability (ATP assay) was calculated relative to mock-treated control cell results, and LDH release was expressed as a percentage relative to a Triton X-100 (0.8%) lysis control. Values corresponding to 50% effective concentrations (EC50s) were calculated by nonlinear regression analysis using Prism 6 (GraphPad).
Hla neutralization assay—rabbit RBC hemolysis inhibition.
Red blood cells (RBCs) were purified from EDTA-treated whole blood obtained from naive New Zealand White rabbits (Preclinics; Germany) by Ficoll purification (Ficoll-Paque Plus; GE Healthcare). Pooled sera from rabbits treated with Hla-F#5 or Hla-specific or control MAbs, obtained 23 h after intravenous MAb administration (1 h before bacterial challenge), were serially diluted in PBS and mixed with recombinant Hla (10 nM). After preincubation at room temperature for 30 min, the mixture was applied to 5 × 107 RBCs per well for 1 h at 37°C. Cell lysis was detected by photometric measurement of released hemoglobin at 541 nm. Percent inhibition of toxin activity was calculated using the following formula: percent inhibition = [(normal activity − inhibited activity)/(normal activity)] × 100. Data were analyzed by nonlinear regression analysis using Prism 6 (GraphPad).
Leukocidin neutralization assays—PMN lysis inhibition.
For PMN cytotoxicity inhibition assays, pooled sera (described above) were serially diluted in neutrophil medium and mixed with recombinant leukocidins at concentrations selected to result in >90% cell death in the absence of neutralizing antibodies (as indicated in the figure legends). Sera and toxins were preincubated for 30 min prior to addition of rabbit PMNs (2.5 × 104 cells/well). Cell viability was measured after 4 h of incubation at 37°C in 5% CO2 using a CellTiter-Glo luminescent cell viability assay kit (Promega). Inhibition of toxin activity was calculated using the same formula as mentioned above. Data were analyzed by nonlinear regression analysis using Prism 6 (GraphPad).
Statistical analyses.
The survival rates of mice and rabbits in the different treatment groups, indicated in the respective Kaplan-Meier plots, were statistically compared by applying the two-sided log-rank (Mantel-Cox) test using the hypothesis that the survival curves of animals pretreated with Hla-specific MAb, Hla-F#5 MAb, or control MAb are not different from one another, with P values of <0.017 (the significance level of 0.05 divided by 3 different comparisons) being considered statistically significant to account for multiple comparisons performed using the Bonferroni method. LW/BW ratios and bacterial densities in lungs, spleens, and kidneys were compared to one another by nonparametric one-way analysis of variance (ANOVA) with the Kruskal-Wallis test followed by Dunn's multiple-comparison test using Prism 6 (GraphPad).
RESULTS
Rabbit PMNs, unlike mouse PMNs, are highly susceptible to leukocidins.
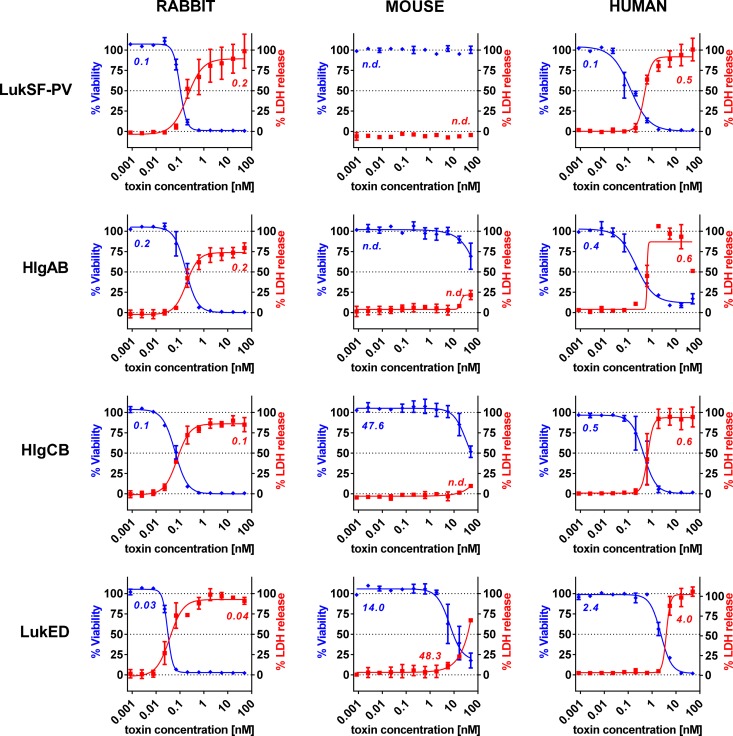
To determine the sensitivity of rabbit white blood cells to gamma hemolysin, LukED, and LukSF-PV and to directly compare it to that of mouse and human cells, polymorphonuclear cells (PMNs) were purified from freshly drawn rabbit, mouse, and human blood and incubated with recombinant leukocidins in a broad range of concentrations (50 to 0.001 nM). In these assays, two different readouts were used: cell viability measured by luminescent detection of cellular ATP levels and cell lysis determined by LDH release. The results of the two different methods were comparable, with slightly higher EC50 values calculated based on LDH release compared to the more sensitive ATP-based readout. Both methods revealed high sensitivity of rabbit PMNs to all four leukocidins, being in the same range as that observed with human PMNs, with the exception of LukED, which was even more potent for rabbit cells (Fig. 1). Mouse PMNs were resistant to LukSF-PV, and HlgAB and HlgCB did not reach half maximal killing in the LDH release assay even at the highest toxin concentration tested (50 nM; ∼3.5 μg/ml). The level of LukED-mediated lysis of mouse PMN was about 2 orders and 1 order of magnitude lower than those of rabbit and human PMNs, respectively.
FIG 1.
Greatly different sensitivities of rabbit, mouse, and human PMNs to S. aureus leukocidins. PMNs purified from rabbit, mouse, and human whole blood were coincubated with leukocidins in the indicated dose range for 4 hours, followed by parallel measurement of cellular ATP content (blue curves and EC50 values) and LDH release (red curves and EC50 values) as indicators for cell viability or pore formation, respectively. n.d., no EC50 value determined. Error bars represent SEM from at least 2 independent experiments for each toxin.
Comparable efficacies of Hla-specific and Hla-leukocidin cross-neutralizing MAbs in a murine pneumonia model.
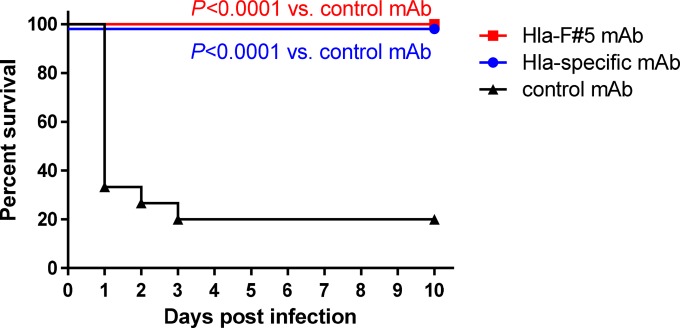
Hla-F#5 MAb, which cross-neutralizes Hla, HlgAB, HlgCB, LukSF-PV, and LukED, and an Hla-specific MAb were compared for their protective efficacies in a murine pneumonia model induced by intranasal challenge with the CA-MRSA USA300 type SF8300 strain. Mice were immunized intraperitoneally with Hla-F#5 or Hla-specific or isotype-matched negative-control MAbs at a 10 mg/kg dose 24 h prior to bacterial challenge. We detected 20% (3/15) survival in the control group, while both Hla-F#5 MAb and the Hla-specific MAb elicited full (100%; 15/15) protection (Fig. 2) (P < 0.0001 versus control MAb by log-rank [Mantel-Cox] test).
FIG 2.
Protection in murine CA-MRSA pneumonia model is dependent on alpha-hemolysin neutralization. Kaplan-Meier survival curves are shown for three independent experiments performed with 5 BALB/cJRj mice per group (n = 15 animals per experimental group). At 24 h prior to lethal intranasal challenge with 8.0 × 108 CFU S. aureus strain SF8300, animals were immunized intraperitoneally with 200 μg (∼10 mg/kg of body weight) of Hla-F#5, Hla-specific, or control MAb. Indicated P values were calculated by log-rank (Mantel-Cox) test.
Neutralization of leukocidins in addition to alpha-hemolysin increases survival in the rabbit necrotizing pneumonia model.
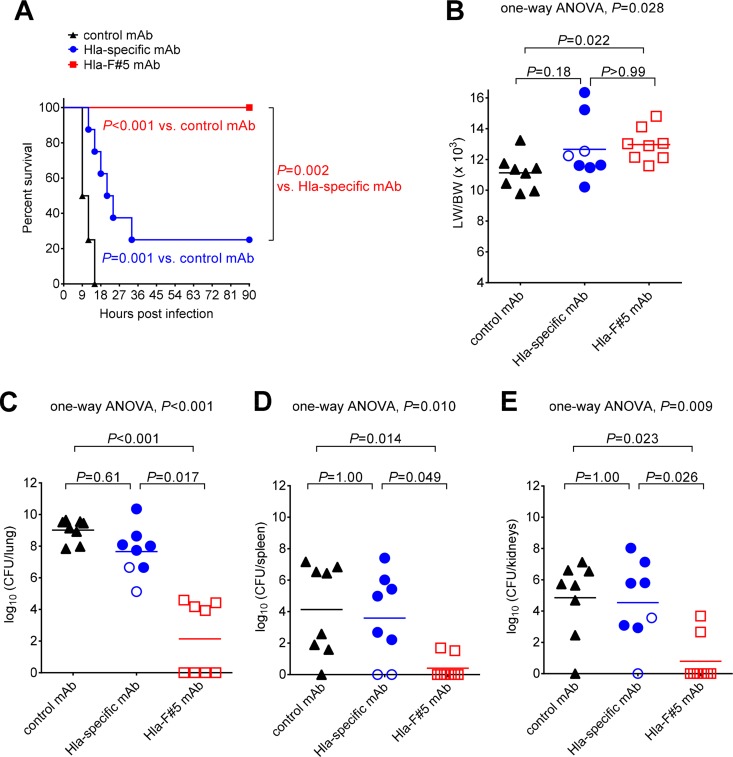
Since rabbit cells proved to be significantly more sensitive to S. aureus leukocidins than mouse cells, we aimed at evaluating survival outcomes for rabbits treated with Hla plus leukocidin neutralizing antibody in a CA-MRSA pneumonia model described previously (8, 18). Animals were randomized for prophylaxis with Hla-F#5 MAb, the Hla-specific MAb, or control MAb (10 mg/kg of the same purification batches used in the murine model) and were infected endobronchially 24 h later with a USA300 CA-MRSA strain (SF8300). The survival rates were 100% (8/8) for rabbits immunized with the Hla-F#5 MAb and only 25% (2/8) for the animals pretreated with the Hla-specific MAb and 0% (0/8) for those in the control group (Fig. 3A). Unlike in the mouse model, pretreatment with Hla-F#5 MAb resulted in a 75% reduction in the mortality rate compared to the Hla-specific MAb (Fig. 3A), suggesting that simultaneous neutralization of Hla and bicomponent leukocidins enhanced survival over targeting of Hla alone. Although rabbits passively immunized with Hla-specific MAb had statistically significantly greater protection than those immunized with control MAb (Fig. 3A), the overall difference in mortality rates was only 25%, further suggesting that targeting of Hla alone may not be sufficient to confer a significant survival benefit in an animal species susceptible to the bicomponent leukocidins.
FIG 3.
Superior in vivo efficacy achieved by simultaneous neutralization of Hla and leukocidins in the rabbit model of CA-MRSA necrotizing pneumonia. (A) Kaplan-Meier survival curves for animals (n = 8 per group) pretreated at 24 h before challenge with S. aureus strain SF8300 (6.1 × 109 CFU/ml) with 10 mg/kg of Hla-specific MAb, 10 mg/kg of Hla-F#5 MAb, or 10 mg/kg of control MAb. P values were calculated by log-rank (Mantel-Cox) test. (B) Comparisons of lung weight (in grams) to body weight (in kilograms) (LW/BW × 103). (C to E) Bacterial counts (log10 CFU) in lung (C), spleen (D), and kidneys (E). In panels B to E, filled symbols represent data from animals that died from respiratory failure or were euthanized for pulmonary dysfunction, and open symbols represent data from surviving rabbits that were euthanized at 90 h postinfection. Multiplicity-adjusted P values were calculated by nonparametric one-way ANOVA with the Kruskal-Wallis test followed by Dunn's multiple-comparison test.
The severity of pulmonary edema, as determined using the LW/BW ratios, was reduced in rabbits pretreated with control MAb compared to those pretreated with Hla-specific MAb or Hla-F#5 MAb (Fig. 3B). As PVL was shown previously to induce greater severity of pulmonary edema in the same model of rabbit pneumonia (6), it would be expected that animals pretreated with Hla-F#5 MAb, which neutralizes PVL, would have reduced pulmonary edema formation. A potential explanation is that rabbits pretreated with the control MAb succumbed rapidly to fatal infection, allowing less time for pulmonary edema formation, compared to those treated with the Hla-F#5 or Hla-specific MAbs that had prolonged survival time, which allowed more time for staphylococcal virulence factors other than those targeted by these MAbs to induce pulmonary edema formation. In support of this, the LW/BW ratio was 10.67 for the seven rabbits that died early (between 9 and 12 h postinfection) compared to 12.99 for the seven animals that died later (15 to 33 h postinfection; P = 0.016).
Bacterial counts in lungs, spleens, and kidneys were significantly lower in rabbits pretreated with Hla-F#5 MAb than in those pretreated with the Hla-specific MAb or control MAb (Fig. 3C to E). This reduction in CFU counts afforded by Hla-F#5 MAb was not due to any direct antimicrobial effect because this antibody targets secreted toxins but was rather an indirect effect on enhancing host survival, thereby allowing the host's cellular immune defense to clear the bacteria from lungs and distal organs. In support of this, rabbits that died of pneumonia had a bacterial burden in the lung that was ∼104-fold greater than that seen with those that were euthanized at 90 h postinfection (filled versus open symbols in Fig. 3C to E).
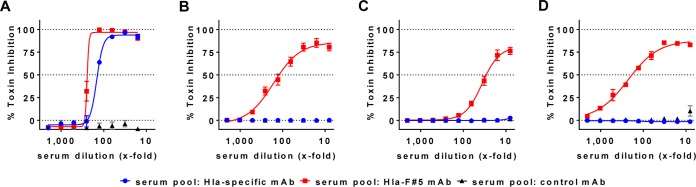
We wanted to confirm that sera of rabbits passively immunized with Hla-F#5 or the Hla-specific MAb were effective in neutralizing cytotoxins in vitro. Therefore, sera were collected 1 h before bacterial challenge, pooled for each treatment group (8 in each group), and characterized in toxin neutralization assays. The serum pools from animals treated with Hla-F#5 MAb or Hla-specific MAb exhibited comparable neutralization potencies in the rabbit RBC lysis assay performed with recombinant alpha-hemolysin (Fig. 4A), whereas only the Hla-F#5-containing serum pool exhibited neutralization activities against the bicomponent toxins, namely, LukSF-PV (Fig. 4B), LukED (Fig. 4C), and HlgCB (Fig. 4D). The serum pool from rabbits treated with control MAb did not exhibit any toxin neutralization activity.
FIG 4.
In vitro neutralization of cytotoxins with rabbit sera after passive immunization with human MAbs. Rabbit sera, collected at 1 h before bacterial challenge from animals treated with Hla-F#5, Hla-specific, or control MAbs, were pooled and tested for cytotoxin neutralization potency using rabbit cells. (A) Rabbit red blood cell (RBC) hemolysis inhibition assay using Hla at 10 nM. (B to D) Rabbit PMN lysis inhibition assay using LukSF-PV at 1 nM (B), LukED at 0.1 nM (C), and HlgCB at 0.25 nM (D). Error bars represent SEM of data from at least 2 independent experiments for each toxin.
These data confirmed that, compared to the Hla-specific MAb, the Hla-F#5 MAb provides improved protection against lethal pneumonia through its potent neutralizing activities against leukocidins.
DISCUSSION
In order to best predict the clinical value of human therapeutics, it is essential to use appropriate animal models. This is especially critical in light of repeated experiences showing that vaccines and antibody-based approaches targeting S. aureus failed to show clinical efficacy despite supportive preclinical in vivo data generated in rodent models (mainly in mice) (21). Recent immune approaches increasingly employ cytolysins as vaccine antigens and as targets for human monoclonal antibody therapeutics (22, 23). A previously described human MAb, Hla-F#5, binds to a region sufficiently conserved in alpha-hemolysin (Hla), LukF-PV, HlgB, and LukD for high-affinity interaction and therefore neutralizes Hla, LukSF-PV, HlgAB, HlgCB, and LukED (17). Due to the species specificity of the cytolysins targeted by Hla-F#5, the choice of animal species is pivotal for testing its efficacy and understanding the mechanism of protection. We observed that rabbit PMNs were not only highly susceptible to LukSF-PV, in line with earlier reports (7, 8), but were also sensitive to HlgAB, HlgCB, and LukED (Fig. 1). These data resembled those obtained with human PMNs, although LukED was even more potent toward rabbit cells. In contrast, mouse PMNs exhibited sensitivity to LukED that was 6-fold to 10-fold lower than that seen with human PMNs and were not lysed by LukSF-PV, and less than 20% of total LDH was released by HlgAB or HlgCB at the highest concentration tested. Therefore, we conclude that the rabbit is a more relevant host than the mouse for investigating the contribution of leukocidins to S. aureus pneumonia pathogenesis and thus for evaluating the potential beneficial effects of leukocidin neutralization in disease prevention.
We subsequently compared the protective efficacies of human monoclonal antibodies neutralizing Hla only (Hla-specific MAb) or cross-neutralizing four leukocidins in addition to Hla (Hla-F#5 MAb) in mouse and rabbit pneumonia models using the same CA-MRSA USA300 strain and the same batches and doses of MAbs. Although Hla-F#5 has previously been shown to protect against pneumonia and sepsis in mouse models (17), it was not evident whether its mechanism of protection included neutralization of any bicomponent leukocidins. It was also not clear whether neutralization of alpha-hemolysin alone was sufficient to confer protection in rabbits that are susceptible to LukSF-PV and other bicomponent toxins. Here, we have shown that Hla-F#5 MAb has protective efficacy comparable to that of an Hla-specific MAb in a mouse pneumonia model (Fig. 2), suggesting that targeting of Hla alone is sufficient to improve survival outcomes in the mouse. These data are in line with earlier observations indicating that S. aureus pathogenesis is mainly driven by Hla in mouse models (18). In contrast, Hla-F#5 MAb demonstrated efficacy that was superior to that of Hla-specific MAb in the rabbit pneumonia model (Fig. 3), indicating that neutralization of leukocidins contributes to prevention of pneumonia in a species that is highly susceptible to these cytolytic toxins. A limitation of the current study was that it focused on survival outcome and was not designed to evaluate the effects on lung edema and bacterial burden, which are evaluable only when animals are sacrificed at the same time point after bacterial challenge (e.g., see reference 8).
This rabbit pneumonia model reproduces some of the hallmark clinical features of fulminant necrotizing pneumonia in humans, including severe leukopenia, lung necrosis, respiratory failure, hemoptysis, and death (8). S. aureus necrotizing pneumonia is a rare clinical entity that is rapidly progressive and hemorrhagic and is associated with severe leukopenia and high mortality (24). It is often caused by S. aureus strains that produce LukSF-PV, most notably CA-MRSA strains, even in young, immunocompetent patients, typically after presenting with flu-like symptoms (25). S. aureus strains are very heterogeneous regarding their cytotoxin expression profiles. The SF8300 USA300 CA-MRSA strain used in this study carries the genes for Hla and all five leukocidins. We previously reported that LukSF-PV contributed to acute lung injury and death in a PMN-dependent manner (8). While lukSF-PV is present in only 5% to 10% of S. aureus clinical isolates, hlgABC and lukED can be found in 100% and ∼60% of S. aureus strains, respectively. Although CA-MRSA strain USA300 is a common cause of necrotizing pneumonia, it also causes nonnecrotizing nosocomial pneumonia. The majority of S. aureus pneumonia cases are not characterized as necrotizing. Moreover, hospital-associated MRSA strains, such as the USA100 clone, endemic in health care facilities across the United States, and methicillin-susceptible S. aureus (MSSA) strains are frequently isolated from pneumonia patients. The USA100 strains and the majority of MSSA strains do not express LukSF-PV; therefore, it will be important to evaluate whether the Hla-F#5 MAb would also confer greater survival benefits than Hla-specific MAbs in the rabbit pneumonia model with LukSF-PV-negative strains.
In patients who were receiving mechanical ventilation and were heavily colonized in their lower airways with S. aureus, Hla expression levels of MSSA strains were associated with progression to pneumonia (26). In the same study, none of the S. aureus isolates obtained from patients diagnosed with ventilator-associated pneumonia carried the gene for LukSF-PV. Based on the functionally equivalent roles of the other leukocidins in lysing human phagocytic cells with the same potency as LukSF-PV, their involvement in neutrophil-associated lung damage can be envisioned. However, it remains to be seen whether leukocidin neutralization can interfere with nonnecrotizing pneumonia progression. A derivative of the Hla-F#5 MAb (ASN-1) is an important component of MAb combination product candidate ASN100, which recently completed phase 1 safety and pharmacokinetic testing and is in development for use in a stand-alone preemptive approach in the prevention of pneumonia in patients receiving mechanical ventilation. Therefore, the prophylactic approach used in this study is relevant to support the intended clinical indication.
Cytotoxin neutralization may also reduce disease severity when used as an adjunctive therapy in addition to antibiotics administration in patients diagnosed with S. aureus pneumonia. The therapeutic efficacy of the Hla-F#5 MAb, as well as of two Hla-specific antibodies, was demonstrated in combination with different antibiotics in mice (17, 27–29). It will be necessary to perform similar therapeutic studies with Hla-F#5 in the rabbit necrotizing pneumonia model, particularly in light of the notion that certain commonly used antimicrobial agents such as oxacillin or vancomycin upregulate the cytotoxin production of S. aureus whereas others (e.g., bacterial protein synthesis inhibitors clindamycin and linezolid) are not expected to increase cytolysin production (19, 30, 31).
In sum, in vivo data, together with those from in vitro cytotoxin neutralization assays performed with sera from MAb-treated rabbits, confirm that the mechanism of protection afforded by Hla-F#5 MAb in necrotizing pneumonia is based on leukocidin neutralization in addition to alpha-hemolysin neutralization.
ACKNOWLEDGMENTS
Experiments were designed by E.N., B.A.D., H.R., and G.N. Data were generated by B.A.D., V.T.M.L., Z.C.V., H.R., and E.C.D., and L.S., E.N., and B.A.D. prepared the manuscript.
We thank Karin Gross and Susanne Weber for technical assistance in the in vitro assays and Jacqueline Steinhäuser and Manuel Zerbs for technical assistance in the mouse pneumonia model. IgGs were expressed and quality control was performed by the Molecular Core, High Throughput Expression and Analytical Group of Adimab.
A derivative of the cross-neutralizing Hla-F#5 MAb used in the present study is included in a MAb combination product candidate (ASN100), which recently completed a phase 1 clinical study by Arsanis. Z.C.V., H.R., L.S., G.N., and E.N. declare a potential conflict of interest, being employees of and shareholders in Arsanis.
This work was supported in part by FFG “Basisprogramm” grants (837128 and 841918) from the Austrian Research Promotion Agency, awarded to Arsanis Biosciences, and in part by United States Public Health Service grant NIH R01 AI087674 to B.A.D.
REFERENCES
- 1.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Thammavongsa V, Kim HK, Missiakas D, Schneewind O. 2015. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol 13:529–543. doi: 10.1038/nrmicro3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandenesch F, Lina G, Henry T. 2012. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Front Cell Infect Microbiol 2:12. doi: 10.3389/fcimb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berube BJ, Bubeck Wardenburg J. 2013. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel) 5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spaan AN, Surewaard BG, Nijland R, van Strijp JA. 2013. Neutrophils versus Staphylococcus aureus: a biological tug of war. Annu Rev Microbiol 67:629–650. doi: 10.1146/annurev-micro-092412-155746. [DOI] [PubMed] [Google Scholar]
- 6.Alonzo F III, Torres VJ. 2014. The bicomponent pore-forming leucocidins of Staphylococcus aureus. Microbiol Mol Biol Rev 78:199–230. doi: 10.1128/MMBR.00055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Löffler B, Hussain M, Grundmeier M, Brück M, Holzinger D, Varga G, Roth J, Kahl BC, Proctor RA, Peters G. 2010. Staphylococcus aureus Panton-Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog 6:e1000715. doi: 10.1371/journal.ppat.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diep BA, Chan L, Tattevin P, Kajikawa O, Martin TR, Basuino L, Mai TT, Marbach H, Braughton KR, Whitney AR, Gardner DJ, Fan X, Tseng CW, Liu GY, Badiou C, Etienne J, Lina G, Matthay MA, DeLeo FR, Chambers HF. 2010. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci U S A 107:5587–5592. doi: 10.1073/pnas.0912403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. 2007. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med 13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 10.Spaan AN, Vrieling M, Wallet P, Badiou C, Reyes-Robles T, Ohneck EA, Benito Y, de Haas CJ, Day CJ, Jennings MP, Lina G, Vandenesch F, van Kessel KP, Torres VJ, van Strijp JA, Henry T. 2014. The staphylococcal toxins γ-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat Commun 5:5438. doi: 10.1038/ncomms6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spaan AN, Henry T, van Rooijen WJ, Perret M, Badiou C, Aerts PC, Kemmink J, de Haas CJ, van Kessel KP, Vandenesch F, Lina G, van Strijp JA. 2013. The staphylococcal toxin Panton-Valentine Leukocidin targets human C5a receptors. Cell Host Microbe 13:584–594. doi: 10.1016/j.chom.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Reyes-Robles T, Alonzo F III, Kozhaya L, Lacy DB, Unutmaz D, Torres VJ. 2013. Staphylococcus aureus leukotoxin ED targets the chemokine receptors CXCR1 and CXCR2 to kill leukocytes and promote infection. Cell Host Microbe 14:453–459. doi: 10.1016/j.chom.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alonzo F III, Kozhaya L, Rawlings SA, Reyes-Robles T, DuMont AL, Myszka DG, Landau NR, Unutmaz D, Torres VJ. 2013. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature 493:51–55. doi: 10.1038/nature11724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alonzo F III, Benson MA, Chen J, Novick RP, Shopsin B, Torres VJ. 2012. Staphylococcus aureus leucocidin ED contributes to systemic infection by targeting neutrophils and promoting bacterial growth in vivo. Mol Microbiol 83:423–435. doi: 10.1111/j.1365-2958.2011.07942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malachowa N, Kobayashi SD, Braughton KR, Whitney AR, Parnell MJ, Gardner DJ, Deleo FR. 2012. Staphylococcus aureus leukotoxin GH promotes inflammation. J Infect Dis 206:1185–1193. doi: 10.1093/infdis/jis495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DuMont AL, Yoong P, Day CJ, Alonzo F, McDonald WH, Jennings MP, Torres VJ. 2013. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc Natl Acad Sci U S A 110:10794–10799. doi: 10.1073/pnas.1305121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rouha H, Badarau A, Visram ZC, Battles MB, Prinz B, Magyarics Z, Nagy G, Mirkina I, Stulik L, Zerbs M, Jägerhofer M, Maierhofer B, Teubenbacher A, Dolezilkova I, Gross K, Banerjee S, Zauner G, Malafa S, Zmajkovic J, Maier S, Mabry R, Krauland E, Wittrup KD, Gerngross TU, Nagy E. 2015. Five birds, one stone: neutralization of alpha-hemolysin and four bi-component leukocidins of Staphylococcus aureus with a single human monoclonal antibody. MAbs 7:243–254. doi: 10.4161/19420862.2014.985132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salgado-Pabón W, Schlievert PM. 2014. Models matter: the search for an effective Staphylococcus aureus vaccine. Nat Rev Microbiol 12:585–591. doi: 10.1038/nrmicro3308. [DOI] [PubMed] [Google Scholar]
- 19.Diep BA, Afasizheva A, Le HN, Kajikawa O, Matute-Bello G, Tkaczyk C, Sellman B, Badiou C, Lina G, Chambers HF. 2013. Effects of linezolid on suppressing in vivo production of staphylococcal toxins and improving survival outcomes in a rabbit model of methicillin-resistant Staphylococcus aureus necrotizing pneumonia. J Infect Dis 208:75–82. doi: 10.1093/infdis/jit129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siemsen DW, Malachowa N, Schepetkin IA, Whitney AR, Kirpotina LN, Lei B, Deleo FR, Quinn MT. 2014. Neutrophil isolation from nonhuman species. Methods Mol Biol 1124:19–37. doi: 10.1007/978-1-62703-845-4_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansen KU, Girgenti DQ, Scully IL, Anderson AS. 2013. Vaccine review: “Staphyloccocus aureus vaccines: problems and prospects.” Vaccine 31:2723–2730. doi: 10.1016/j.vaccine.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 22.DiGiandomenico A, Sellman BR. 2015. Antibacterial monoclonal antibodies: the next generation? Curr Opin Microbiol 27:78–85. doi: 10.1016/j.mib.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Sause WE, Buckley PT, Strohl WR, Lynch AS, Torres VJ. 2016. Antibody-based biologics and their promise to combat Staphylococcus aureus infections. Trends Pharmacol Sci 37:231–241. doi: 10.1016/j.tips.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai YF, Ku YH. 2012. Necrotizing pneumonia: a rare complication of pneumonia requiring special consideration. Curr Opin Pulm Med 18:246–252. doi: 10.1097/MCP.0b013e3283521022. [DOI] [PubMed] [Google Scholar]
- 25.Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, Vandenesch F, Piémont Y, Brousse N, Floret D, Etienne J. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359:753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 26.Stulik L, Malafa S, Hudcova J, Henics BZ, Craven D, Sonnevend AM, Nagy E. 2014. Hemolysin activity of methicillin-susceptible S. aureus predicts ventilator-associated pneumonia. Am J Respir Crit Care Med 190:1139–1148. doi: 10.1164/rccm.201406-1012OC. [DOI] [PubMed] [Google Scholar]
- 27.Foletti D, Strop P, Shaughnessy L, Hasa-Moreno A, Casas MG, Russell M, Bee C, Wu S, Pham A, Zeng Z, Pons J, Rajpal A, Shelton D. 2013. Mechanism of action and in vivo efficacy of a human-derived antibody against Staphylococcus aureus alpha-hemolysin. J Mol Biol 425:1641–1654. doi: 10.1016/j.jmb.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Hua L, Hilliard JJ, Shi Y, Tkaczyk C, Cheng LI, Yu X, Datta V, Ren S, Feng H, Zinsou R, Keller A, O'Day T, Du Q, Cheng L, Damschroder M, Robbie G, Suzich J, Stover CK, Sellman BR. 2014. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother 58:1108–1117. doi: 10.1128/AAC.02190-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hua L, Cohen TS, Shi Y, Datta V, Hilliard JJ, Tkaczyk C, Suzich J, Stover CK, Sellman BR. 2015. MEDI4893* promotes survival and extends the antibiotic treatment window in a Staphylococcus aureus immunocompromised pneumonia model. Antimicrob Agents Chemother 59:4526–4532. doi: 10.1128/AAC.00510-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dumitrescu O, Boisset S, Badiou C, Bes M, Benito Y, Reverdy ME, Vandenesch F, Etienne J, Lina G. 2007. Effect of antibiotics on Staphylococcus aureus producing Panton-Valentine leukocidin. Antimicrob Agents Chemother 51:1515–1519. doi: 10.1128/AAC.01201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otto MP, Martin E, Badiou C, Lebrun S, Bes M, Vandenesch F, Etienne J, Lina G, Dumitrescu O. 2013. Effects of subinhibitory concentrations of antibiotics on virulence factor expression by community-acquired methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 68:1524–1532. doi: 10.1093/jac/dkt073. [DOI] [PubMed] [Google Scholar]