Abstract
Tuberculosis is a major infectious disease that requires prolonged chemotherapy with a combination of four drugs. Here we present data suggesting that treatment of Mycobacterium tuberculosis, the causative agent of tuberculosis, and Mycobacterium smegmatis, a model organism widely used for the screening of antituberculosis agents, with first-line drugs resulted in the generation of substantial populations that could be recovered only by the addition of a culture supernatant from growing mycobacteria. These bacilli failed to grow in standard media, resulting in significant underestimation of the numbers of viable mycobacteria in treated samples. We generated M. smegmatis strains overexpressing M. tuberculosis resuscitation-promoting factors (Rpfs) and demonstrated their application for the detection of Rpf-dependent mycobacteria generated after drug exposure. Our data offer novel opportunities for validation of the sterilizing activity of antituberculosis agents.
INTRODUCTION
Approximately one-third of the world's population is latently infected with Mycobacterium tuberculosis, the causative agent of human tuberculosis (TB) (1). M. tuberculosis bacilli can adapt to stressful conditions occurring at sites of host defense by reducing their metabolic activity and adopting a dormant state, often referred to as nonreplicating persistence (NRP) (2). Dormancy, or NRP, enables mycobacteria to survive environmental stresses and reduces the initiation of the immune response (3). Hence, dormancy can be defined as “a reversible state of low metabolic activity in which cells can exist for extended periods without division” (4).
Dormant bacilli, which are associated with latent infection, are believed to be less susceptible to antimicrobial treatment than actively growing bacilli due to physiological changes and therefore are of clinical significance in the eradication of TB (5). Nonreplicating M. tuberculosis bacilli generated in various in vitro models were more tolerant to rifampin and isoniazid than actively growing bacilli. These systems include the Wayne hypoxia model (2), starvation in phosphate buffer (6), a multiple-stress model (7), the persistence of a streptomycin-dependent strain (8), and a “nonculturability” model induced by potassium limitation (9). Thus, investigation of drug action on NRP bacteria and the development of in vitro models for the generation of such bacteria have been considered important for successful drug discovery programs. However, recent comparative studies on drug activities on NRP mycobacteria in in vitro systems showed significant differences (10). The presence of multiple mycobacterial populations with various physiological characteristics in NRP models may contribute to variability in the killing activity of drugs. Furthermore, the in vivo environment and exposure to stressful conditions may spawn heterogeneous mycobacterial populations with distinct cultivation requirements (11) and biochemical characteristics (12). Of particular interest are so-called resuscitation-promoting factor (Rpf)-dependent mycobacteria. Rpfs are a family of secreted peptidoglycan-remodeling enzymes, which are required for the regrowth of dormant bacteria (13). Rpf-dependent mycobacteria can be generated in vitro (14, 15), but most importantly, they are highly abundant in tuberculosis sputum (16) and can be recovered from infected lungs (11). Rpf-dependent M. tuberculosis bacilli from sputum have been shown to be more tolerant to rifampin (16), streptomycin, and isoniazid (17) than actively growing bacilli. Moreover, Hu and colleagues have recently demonstrated that Rpf-dependent M. tuberculosis bacilli were present in apparently sterile murine lungs in the Cornell model of latent tuberculosis infection and that the abundance of these bacilli correlated with tuberculosis reactivation (18). These observations suggest the necessity of monitoring the dynamics of Rpf-dependent M. tuberculosis during infection and treatment in order to predict chemotherapy outcomes for TB patients. Thus, there is strong demand for the development of simple assays to detect Rpf-dependent M. tuberculosis in clinical and experimental samples. The major challenge in Rpf dependency assays is posed by the high instability and unreliable activity of recombinant Rpf (19). Culture supernatants (SN) obtained from growing M. tuberculosis have been used as an alternative source for the detection of Rpf-dependent mycobacteria (16, 20, 21); however, this experimental setup is highly laborious and requires additional controls, preventing large-scale clinical studies and high-throughput drug testing. Moreover, other factors released into the culture supernatants of growing bacteria may contribute to the resuscitation of nonplatable (NP) bacteria and complicate the interpretation of results.
In this study, we demonstrate that treatment of mycobacterial cultures with first-line drugs results in the generation of bacteria that could be grown only in media supplemented with the culture supernatant. We have generated Mycobacterium smegmatis strains overexpressing M. tuberculosis Rpfs and have shown that these strains can be used for the detection of Rpf-dependent cells that arose after exposure to drugs. Our simple in vitro system can be used for assessing the efficacy of drugs and their propensity to generate Rpf-dependent mycobacteria.
MATERIALS AND METHODS
Bacteria and media.
M. smegmatis mc2155 or M. tuberculosis H37Rv was grown at 37°C. Bacteria were cultivated either in 7H9 Middlebrook medium (Becton, Dickinson and Company Ltd., Oxford, United Kingdom) supplemented with 0.2% (vol/vol) glycerol, 10% (vol/vol) oleic acid-albumin-dextrose-catalase (OADC), and 0.05% (wt/vol) Tween 80 or in Sauton's medium, unless otherwise stated. In some experiments, the OADC supplement was replaced with ADC; no significant difference in mycobacterial growth was found. Liquid cultures were shaken either at 100 rpm (M. tuberculosis) or at 200 rpm (M. smegmatis). For CFU assessment, mycobacteria were plated onto 7H10 Middlebrook (Becton, Dickinson and Company Ltd., Oxford, United Kingdom) agar. For maintenance of the pMind plasmid, kanamycin was added to a final concentration of 50 μg/ml; tetracycline (20 ng/ml) was added for induction of the expression of rpf genes.
Assessment of viable counts.
CFU counts, using a standard liquid droplet method with serially diluted bacterial cultures, were performed in triplicate. Sealed agar plates were incubated at 37°C for as long as 1 week (M. smegmatis) or as long as 6 weeks (M. tuberculosis). Most probable number (MPN) counts were performed as described previously (16). Briefly, mycobacteria were serially diluted in 96-well (M. smegmatis) or 48-well (M. tuberculosis) microtiter plates containing growth broth with or without a culture supernatant prepared from actively growing M. tuberculosis cultures (16). For M. tuberculosis, 50 μl of mycobacteria was inoculated in 450 μl of 7H9 medium supplemented with 10% (vol/vol) OADC and 0.05% (vol/vol) Tween or in 450 μl of medium diluted with a culture supernatant (50% [vol/vol]). For M. smegmatis experiments, 20 μl of a bacterial suspension was inoculated in 180 μl of Sauton's medium with or without a culture supernatant. Four wells were used for each dilution and each medium. Plates were incubated for 2 weeks (M. smegmatis) or 12 weeks (M. tuberculosis) statically at 37°C before the number of positive wells was recorded and MPN calculated as described previously (16). Each sample was analyzed in triplicate; duplicate biological replicas were used per assay, and each independent assay was performed at least twice. The following terminology was introduced to describe mycobacteria in different physiological states: platable (CFU-producing) bacteria formed on solid media; “nonplatable” (NP) forms could grow only in liquid media; SN-dependent mycobacteria could be grown only in the presence of an Rpf-containing culture supernatant; Rpf-dependent mycobacteria required Rpf for growth (17). NP, SN-dependent, or Rpf-dependent mycobacteria were quantified in an MPN assay, and the resuscitation potential of mycobacterial populations was expressed as the resuscitation index (RI), calculated as log10(MPN) − log10(CFU).
Growth studies.
Growth studies were carried out in supplemented 7H9 medium using an automated Bioscreen C MBR plate reader (Thermo Fisher Scientific) as described previously (22, 23). Cultures from the late-logarithmic phase were inoculated into supplemented 7H9 medium containing 50 μg/ml kanamycin and 20 ng/ml tetracycline to a final optical density (OD) of 0.1. Numbers of inoculated bacteria were confirmed by CFU counts. Plates were then incubated at 37°C with continuous shaking, and OD measurements were taken automatically every 2 h at 600 nm. Ten replicas were used per sample, and each experiment was performed a minimum of three times.
The growth rate (per hour) was calculated by plotting a log scale graph of linear growth and generating an exponential line showing the equation of the line, the gradient (slope) of which is equal to the growth rate. All trend lines had an R2 value of at least 0.99. The apparent lag phase was measured as the time taken for the first OD doubling (determined as an OD of 0.2). The final OD at 600 nm (OD600) corresponded to the highest optical density reached by cultures during growth.
Exposure to antimicrobials.
M. tuberculosis from the logarithmic-growth stage was inoculated into 10 ml of supplemented 7H9 medium containing an antimicrobial agent and was incubated at 37°C with shaking for 3 days. The initial inocula in all experiments were (4 ± 1.5) × 107 CFU/ml. After centrifugation at 4,000 × g, pellets were washed once with 7H9 medium, resuspended in 1 ml of supplemented 7H9 medium, and used for the estimation of CFU, MPN, and MNP counts in the presence of culture supernatant (MPN_SN). Antimicrobial concentrations (in micrograms per milliliter) were as follows: streptomycin, 20; isoniazid, 10; ethambutol, 20; rifampin, 5. The exposure of M. tuberculosis to these drug concentrations resulted in marked decreases in CFU counts (>2 log10).
M. smegmatis was inoculated from a single colony into 5 ml of LB supplemented with 0.05% (wt/vol) Tween 80 and was grown at 37°C with shaking at 200 rpm for 24 h. The culture (1 ml) was then centrifuged for 10 min at 14,000 × g and was resuspended in 1 ml of Sauton's medium supplemented with one of the following drugs (with concentrations given in micrograms per milliliter): amikacin (100), meropenem (50), rifampin (5, 25, or 100), ethambutol (20), isoniazid (1, 10, or 100), or streptomycin (10 or 20). Samples were incubated at 37°C with shaking for 24 h. After washing with Sauton's medium, the treated cells were resuspended in 1 ml of Sauton's medium and were used for CFU, MPN, and MPN_SN assays. When strains containing pMind plasmids were assessed, kanamycin was added at a final concentration of 50 μg/ml at all stages of the experiment. Plates were incubated for 2 weeks statically at 37°C before the number of positive wells was recorded. Each sample was analyzed in triplicate; duplicate biological replicas were used per assay; and each independent assay was performed at least twice
Generation of Rpf-overexpressing strains.
The rpfABCDE genes were amplified from the M. tuberculosis genome by PCR using the gene-specific primers listed in Table S1 in the supplemental material. The PCR products were cloned into the BamHI and MluI (24) sites of the pMind plasmid or pMind-6×His-Myc (23), and the constructs were sequenced by GATC Biotech (Germany) before electroporation into M. smegmatis. The transformants were selected by incorporating kanamycin at a final concentration of 50 μg/ml into the growth medium. The identities of the resultant strains were confirmed using colony PCR and the pMind-specific primers MindF and MindR. A control M. smegmatis strain contained an empty pMind plasmid. rpf expression was induced by the addition of 20 ng/ml tetracycline. The plasmids and strains generated in this study are shown in Table 1. The MICs of rifampin and isoniazid for pMind and the RpfD-overexpressing strain were determined by the microdilution method as described previously (23).
TABLE 1.
Strains generated in this study
| Plasmid name | Strain | Insert size (bp)a | Primers | Description |
|---|---|---|---|---|
| pMind::rpfA | RpfA overexpression | 1,224 | RpfAF and RpfAR | M. tuberculosis rpfA gene cloned into pMind |
| pMind::rpfB | RpfB overexpression | 1,089 | RpfBF and RpfBR | M. tuberculosis rpfB gene cloned into pMind |
| pMind::rpfC | RpfC overexpression | 531 | RpfCF and RpfCR | M. tuberculosis rpfC gene cloned into pMind |
| pMind::rpfD | RpfD overexpression | 465 | RpfDF and RpfDR | M. tuberculosis rpfD gene cloned into pMind |
| pMind::rpfE | RpfE overexpression | 519 | RpfEF and RpfER | M. tuberculosis rpfE gene cloned into pMind |
| pMind::rpf | Rpf overexpression | 696 | RpfF and RpfR | M. luteus rpf gene cloned into pMind |
| pMind | MIND | N/A | N/A | Empty plasmid control |
N/A, not applicable.
RNA extraction and qRT-PCR.
RNA was extracted from 10 ml of mid-log-phase mycobacterial cultures using a previously described TRIzol method (25). To remove DNA contamination, RNA samples were cleaned using the Turbo DNA-free kit (Invitrogen). cDNA synthesis, using the commercially available SuperScript II reverse transcriptase kit (Invitrogen), was performed according to the manufacturer's instructions. Quantitative real-time PCR (qRT-PCR) experiments were carried out in a Corbett Rotor Gene 6000 real-time thermocycler. Absolute qPCR SYBR green mix (ABgene) and gene-specific primers were used as described previously (26). The level of expression of each individual M. tuberculosis rpf gene was normalized to that of 16S rRNA (26). Amplification efficiencies were automatically calculated using Rotor Gene 6000 series software. Melting curves were used to confirm the specificity of the qPCR product.
Detection of Rpf in culture supernatants.
Enzyme-linked immunosorbent assays (ELISAs) were carried out using a method adapted from previously published work (15). Briefly, supernatants (10 ml) were obtained from logarithmic-phase cultures grown in Sauton's medium. After centrifugation and filtration, supernatants were concentrated by freeze-drying. Dried samples were resuspended in 1 ml phosphate-buffered saline (PBS). ELISA was performed in 96-well plates with 100 μl of a concentrated culture supernatant. Primary monoclonal anti-polyhistidine (Sigma-Aldrich) or polyclonal anti-Rpf (13) antibodies and alkaline phosphatase-conjugated secondary anti-mouse or anti-sheep antibodies (respectively) were used at dilutions of 1:3,000 and 1:10,000, respectively. A p-nitrophenyl phosphate substrate (Sigma-Aldrich) was used for the detection of alkaline phosphatase activity. The reaction was measured spectrophotometrically at 405 nm, using a Model 680 microplate reader (Bio-Rad).
RESULTS
Drug treatment induces culture supernatant dependency in mycobacteria.
Previous studies have shown that antimicrobial treatment of mycobacteria can result in the generation of metabolically distinct forms (12). Thus, the presence of NP mycobacteria in the drug-treated population was investigated. M. tuberculosis cultures were treated with isoniazid, rifampin, ethambutol, or streptomycin for 3 days, and viable counts of surviving cells were determined using CFU and MPN counts with or without the addition of Rpf-containing culture supernatants (Rpf-SN) (see Fig. S1 in the supplemental material). As shown in Fig. 1A and Fig. S1, no significant resuscitation was observed in 7H9 medium after treatment with any of these drugs. However, the addition of Rpf-SN caused significant increases in viable counts after treatment with ethambutol, isoniazid, or rifampin. Interestingly, streptomycin was very efficient at eliminating mycobacteria and did not result in the generation of resuscitatable mycobacteria. Treatment of mycobacteria with 10 or 20 μg/ml of streptomycin resulted in similar outcomes (data not shown). Further incubation of mycobacteria with drugs led to reductions in resuscitation indices for isoniazid and ethambutol but not for rifampin (Fig. 1B; also Fig. S1), showing that cell wall-targeting antimicrobials induce temporary SN dependency.
FIG 1.
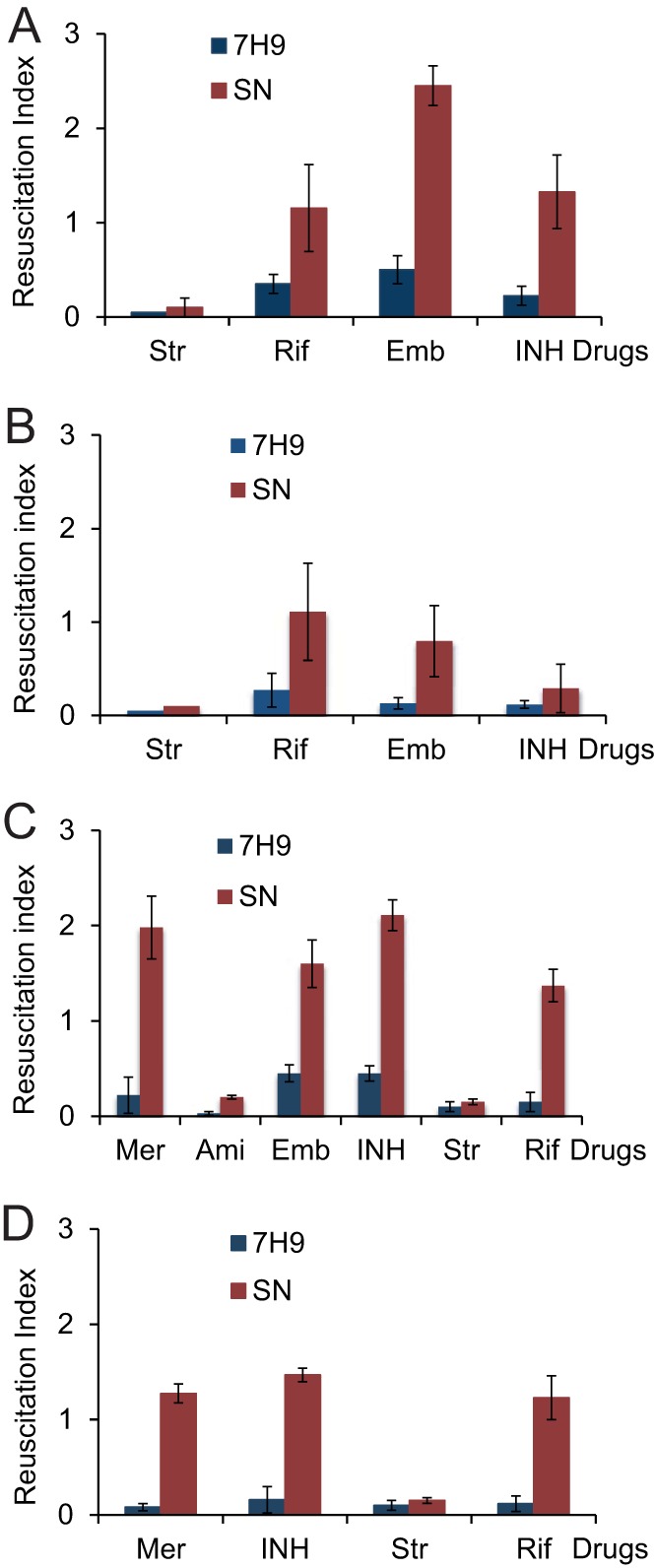
Antimicrobial treatment induces SN dependency in mycobacteria. M. tuberculosis (A and B) or M. smegmatis (C and D) was treated with antimicrobial agents as described in Materials and Methods. M. tuberculosis was exposed to drugs for 3 (A) or 7 (B) days. M. smegmatis was treated for 24 h (C) or 48 h (D). The resuscitation index was calculated as log10(MPN) − log10(CFU). Str, streptomycin; Rif, rifampin; Emb, ethambutol; INH, isoniazid; Mer, meropenem; Ami, amikacin. Average values for two independent experiments with three biological replicates are shown; error bars indicate standard deviations.
Next, the propensity of drugs to induce SN dependency was investigated using M. smegmatis cultures (Fig. 1C and D; see also Fig. S2 in the supplemental material). While streptomycin and amikacin did not induce SN dependency, meropenem, ethambutol, isoniazid, and rifampin generated high numbers of mycobacteria that could be resuscitated only in the presence of a culture supernatant. However, as with M. tuberculosis culture, further incubation with drugs reduced the numbers of resuscitating mycobacteria in samples treated with meropenem or isoniazid (Fig. 1D). These data indicate that certain drugs can result in the generation of NP mycobacteria, which can be detected by the application of a liquid culture supplemented with Rpf-containing culture supernatants. To simplify this assay and prove the resuscitating effect of Rpfs, we generated M. smegmatis strains that artificially overproduce M. tuberculosis Rpfs and validated their usefulness for the detection of Rpf-dependent mycobacteria induced by drug treatment.
Generation and validation of strains overexpressing rpf genes.
Each of the five M. tuberculosis rpf genes (rpfABCDE) was cloned into the pMind or pMind-6×His-Myc plasmid, and the level of rpf expression was assessed using qRT-PCR. In separate experiments, the amounts of Rpfs released into culture supernatants were determined by ELISAs using anti-polyhistidine or anti-Rpf (13) antibodies. As can be observed in Fig. 2A, the M. tuberculosis rpf genes were expressed from the pMind plasmid at different levels: rpfD was expressed at the highest level, followed by rpfE, rpfC, rpfA, and rpfB. Measurement of Rpfs in culture supernatants using polyclonal anti-polyhistidine antibodies confirmed the increases in Rpf levels in the overexpressing strains: those overexpressing RpfA and RpfE showed the greatest absorbance, followed by those with RpfB and RpfD (Fig. 2B). Similar results were obtained with anti-Rpf antibodies (data not shown). The apparent discrepancy between the qRT-PCR data and the ELISA results could be explained by the attachment of Rpfs to the cell wall, as demonstrated previously (27).
FIG 2.
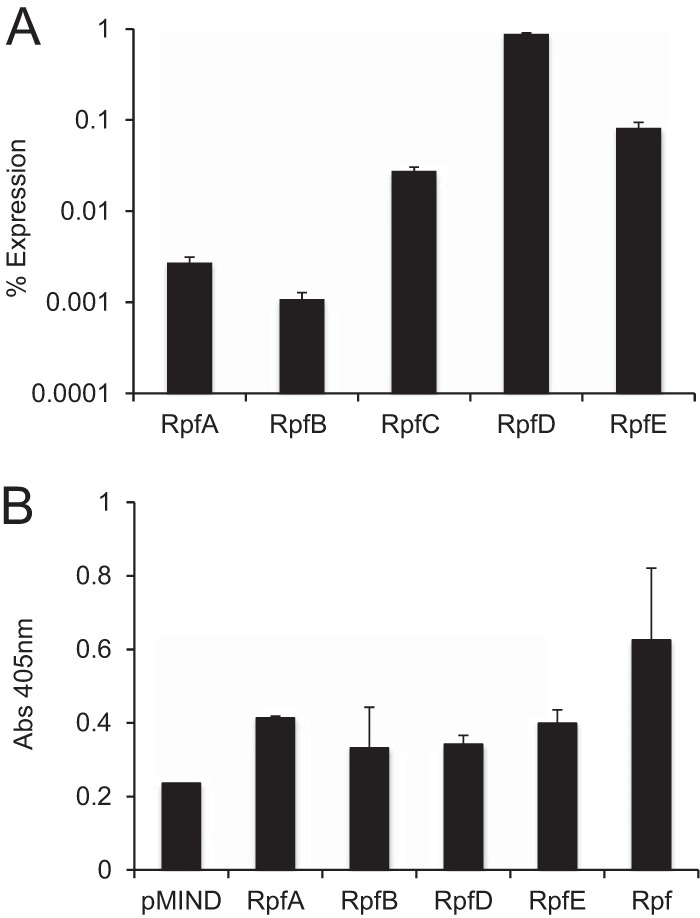
Confirmation of M. tuberculosis rpf gene overexpression and Rpf production in M. smegmatis strains. (A) Expression of rpfA through rpfE as a percentage of 16S rRNA expression. (B) Detection of RpfA, RpfB, RpfD, and RpfE in M. smegmatis culture supernatants by ELISA with monoclonal anti-polyhistidine antibodies. A recombinant His-tagged form of RpfB (designated Rpf) was used as a positive control. RpfC production was not assessed.
Next, the effects of M. tuberculosis rpf overexpression on the growth of M. smegmatis were examined. Growth experiments were carried out in supplemented 7H9 medium, and growth parameters were calculated (Table 2). The overexpression of any M. tuberculosis rpf gene resulted in a slight but statistically significant increase in the final OD over that for the control strain (P, <0.05 by the t test). Differences in the growth rate were also observed; RpfA, RpfB, and RpfC led to increases in the growth rate, while RpfE led to a slightly reduced growth rate. RpfD and Micrococcus luteus Rpf led to growth rates comparable to that of the control strain. Examination of the apparent lag phase revealed a slight reduction in strains overexpressing rpfC or rpfD and a smaller reduction in strains overexpressing rpfE or M. luteus rpf (Table 2). Overexpression of M. tuberculosis Rpfs also altered the growth of M. smegmatis in Sauton's broth (see Fig. S3 in the supplemental material).
TABLE 2.
Comparative analysis of growth parameters of strains overexpressing rpf genes
| Strain or description | Apparent lag phase (h)a | Final ODb | Growth rate (h−1)b,c |
|---|---|---|---|
| MIND | 20 ± 0.66 | 0.71 | 0.19 |
| RpfA overexpression | 20 ± 0.88 | 0.82* | 0.22* |
| RpfB overexpression | 20 ± 0.71 | 0.82* | 0.21* |
| RpfC overexpression | 18 ± 0.88 | 0.81* | 0.20* |
| RpfD overexpression | 18 ± 0.66 | 0.81* | 0.19 |
| RpfE overexpression | 19 ± 0.5 | 0.81* | 0.18* |
| M. luteus Rpf overexpression | 19 ± 0.53 | 0.84* | 0.19 |
Values are means ± standard deviations.
Asterisks indicate values significantly different from that for the control strain, MIND (P, <0.05 by the t test).
Growth parameters were calculated as described in Materials and Methods.
Rpfs have previously been implicated in the resuscitation of NP M. tuberculosis cells and have shown cross-species reactivity, including reactivity in M. smegmatis and Mycobacterium bovis BCG (13, 14). The resuscitation abilities of the individual M. tuberculosis Rpfs in the M. smegmatis Rpf-overexpressing strains were established in a control system prior to assessment of the front-line antituberculosis drugs. To determine the resuscitation capabilities of rpf-overproducing strains, we used a well-established M. smegmatis NP model (15). This assay uses potassium-limiting conditions to generate NP cells that are unable to grow in standard media and can be resuscitated only by the addition of SN or recombinant Rpf. All five strains overexpressing M. tuberculosis Rpfs produced no colonies on agar but resuscitated in liquid medium. In agreement with the data published previously, M. smegmatis overexpressing M. luteus Rpf (15) was also able to grow in liquid culture, while the empty-plasmid control failed to resuscitate (Fig. 3). RpfA and RpfD had the greatest resuscitation capacity, as reflected by the highest resuscitation index values (4.7 and 5.3, respectively), followed by RpfB, RpfC, and RpfE. Interestingly, rpfD was the most overexpressed gene by qRT-PCR, while RpfA was the most abundant Rpf protein detected in culture supernatants by ELISA. These results indicated that Rpf-overexpressing strains could be used for the detection of Rpf-dependent mycobacteria.
FIG 3.
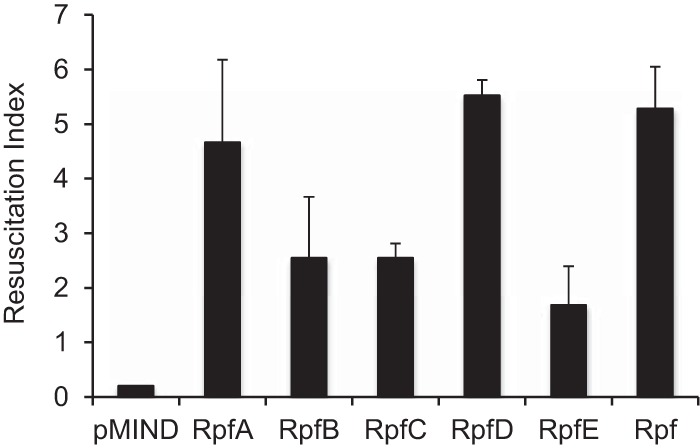
Overexpressed M. tuberculosis rpf genes resuscitate nonplatable M. smegmatis. NP mycobacteria were generated in potassium-limited medium. Resuscitation was carried out in Sauton's medium supplemented with 50 μg/ml of kanamycin and 20 ng/ml of tetracycline. The resuscitation index was calculated as log10(MPN) − log10(CFU). Average values for three independent experiments are shown; error bars indicate standard deviations. The limit of detection was 0.2. The limit-of-detection value is shown for the MIND strain.
Rpf overexpression rescues nonculturable mycobacteria generated by treatment with front-line drugs.
To investigate the abilities of individual Rpfs to resuscitate drug-treated mycobacteria, M. smegmatis Rpf-overexpressing strains and the control strain MIND were treated with rifampin, ethambutol, or meropenem, and the numbers of surviving bacteria were determined by CFU and MPN counts. CFU counts in all treated populations decreased by ∼2 orders of magnitude from those with the initial inocula (see Fig. S4 in the supplemental material). Figure 4A shows resuscitation indices calculated for rifampin-, meropenem-, and ethambutol-treated samples. The highest values were obtained for meropenem-treated samples, confirming the data for M. smegmatis presented in Fig. 1C. Rifampin and ethambutol treatment also led to the generation of Rpf-dependent mycobacteria. On the other hand, CFU counts for streptomycin-treated samples were below the limit of detection, corresponding to 25 cells/ml, and no resuscitation was observed for any of the Rpf overexpression strains, suggesting that this drug treatment does not induce Rpf dependency (data not shown). Reducing the streptomycin concentration to 10 μg/ml did not increase viable counts. This apparently increased sensitivity of Rpf overexpression strains to streptomycin may be caused by the presence of the plasmids or the administration of kanamycin for their maintenance, or both.
FIG 4.
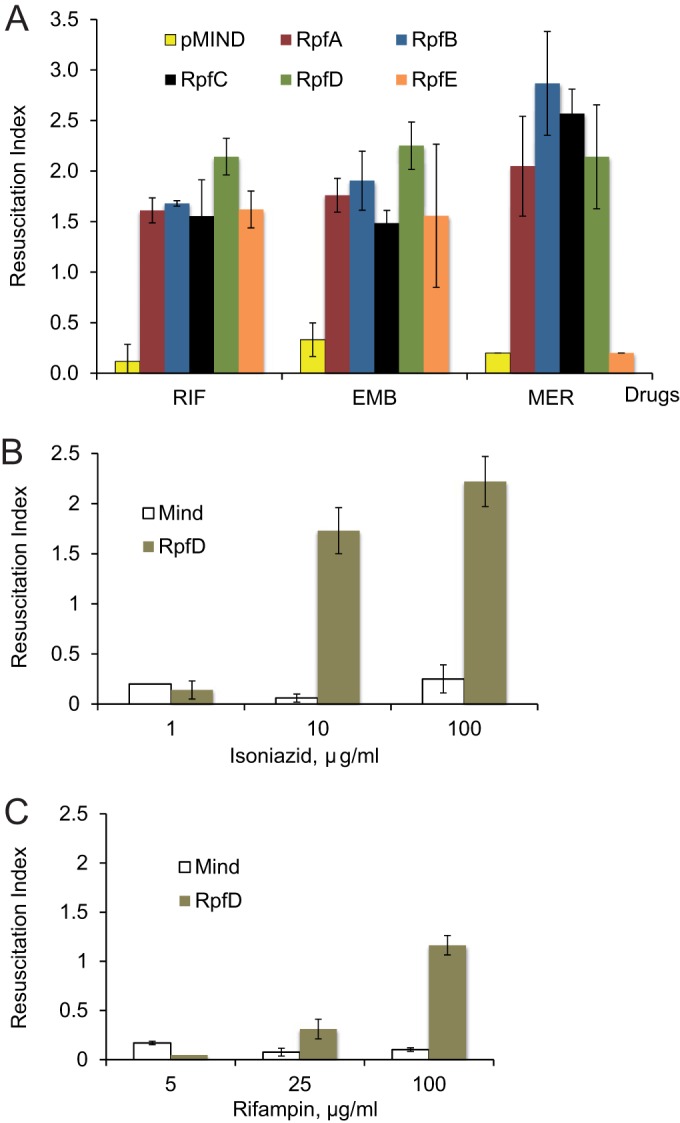
Use of strains overexpressing rpf genes for the detection of Rpf-dependent mycobacteria induced by treatment with antituberculosis drugs. Strains were treated with ethambutol (EMB), meropenem (MER), or rifampin (RIF) (A), isoniazid (B), or rifampin (C). The resuscitation index was calculated as log10(MPN) − log10(CFU). Average values for three independent experiments are shown; error bars indicate standard deviations. The limit of detection was 0.2.
Among the strains overexpressing different Rpfs, the RpfD-overexpressing strain showed consistently higher resuscitation than the others, while the RpfE-overexpressing strain showed the lowest resuscitation potential. In accordance with previously published data, the empty-plasmid strain did not resuscitate in liquid medium, while the positive-control strain overexpressing M. luteus Rpf showed high resuscitation potential (15).
We next investigated how different concentrations of drug influence the generation of Rpf-dependent mycobacteria. We treated the pMind control strain and the RpfD-overexpressing strain with three different concentrations of isoniazid (1, 10, and 100 μg/ml). According to our data, the isoniazid MICs for the pMind and RpfD-overexpressing strains were identical (5 μg/ml). As Fig. S5 in the supplemental material shows, at a concentration of 1 μg/ml, isoniazid did not influence the viability of M. smegmatis and did not result in the generation of RpfD-dependent mycobacteria, while high numbers of RpfD-dependent mycobacteria arose after exposure to 10 or 100 μg/ml of isoniazid (Fig. 4B). A similar pattern was obtained with rifampin-treated mycobacteria. The rifampin MIC was 16 μg/ml for both strains. At 5 μg/ml rifampin, mycobacteria were able to grow without a loss of viability and did not produce RpfD-dependent forms (Fig. 4C; also Fig. S5). Treatment with 25 μg/ml rifampin stopped the growth of mycobacteria but did not stimulate RpfD dependency; exposure to 100 μg/ml of rifampin resulted in the accumulation of RpfD-dependent M. smegmatis cells. An increase in the drug exposure time to 48 h did not significantly change the viable counts (data not shown); we noted outgrowth of mycobacteria if the time of incubation was extended beyond 72 h. The observed outgrowth of mycobacteria could be caused by the multiplication of drug-resistant mutants or the degradation of drugs.
DISCUSSION
Tuberculosis is a worldwide health threat, and recent global efforts have been directed to the development of novel agents to eradicate M. tuberculosis and especially its nonreplicating forms, which are believed to be responsible for prolonged treatment and disease relapse. Populations of nonreplicating, Rpf-dependent, and SN-dependent cells were recently identified in patient sputum samples and were apparently enriched in treated samples (16, 28), leading to our hypothesis that antituberculosis drugs may induce Rpf dependency. Accordingly, we investigated whether mycobacteria from growing cultures could become NP or SN dependent after exposure to antimicrobial agents. In our experiments, we did not detect substantial numbers of NP mycobacteria that could be regrown in liquid medium (Fig. 1). However, mycobacterial populations treated with either rifampin, ethambutol, isoniazid, or meropenem did indeed develop SN dependency. This phenomenon was observed for M. tuberculosis and M. smegmatis, suggesting that it is not associated with slow-growing mycobacteria. The numbers of SN-dependent mycobacteria obtained after treatment with cell wall-targeting drugs decreased after extended incubation (Fig. 1B and D), though rifampin-induced SN-dependent populations remained stable even during prolonged treatment (Fig. 1B and D). We have shown previously that the resuscitation effect of culture supernatant was caused mainly by Rpf proteins (11, 14, 16). Therefore, we hypothesized that artificial overexpression of Rpf in treated mycobacteria might induce their resuscitation, and consequently, we developed a model system using M. smegmatis strains overexpressing M. tuberculosis Rpfs.
The expression of each M. tuberculosis rpf gene was confirmed at the RNA level by qRT-PCR (Fig. 2A); the release of Rpf proteins was confirmed by ELISA (Fig. 2B). The Rpf-overexpressing strains showed a growth advantage in 7H9 medium (Table 2) and in Sauton's broth (Fig. S3 in the supplemental material); they were also able to resuscitate in an NP model (Fig. 3), suggesting their potential utility for the investigation of drug-induced Rpf-dependent mycobacteria. Our results indicate that overexpressed Rpfs resuscitate Rpf-dependent M. smegmatis (Fig. 4). Moreover, the resuscitation effects of overexpressed Rpfs were very similar to those of culture supernatant (Fig. 1B).
While the biological significance of these Rpf-dependent populations awaits further evaluation, our findings indicate that the bactericidal actions of meropenem, isoniazid, ethambutol, and rifampin may have been overestimated. Interestingly, neither streptomycin nor amikacin induced Rpf-dependent mycobacteria in any of our assays, suggesting that aminoglycosides are potent drugs for the prevention of Rpf dependency in mycobacteria. The Rpf proteins are the enzymes involved in the remodeling, biosynthesis, and (potentially) repair of bacterial cell walls. Any environmental insult, including drug treatment, leading to alteration or damage of the cell wall may put bacteria in a situation where the role of cell wall-modifying enzymes, such as Rpfs, becomes vital. Thus, the data presented indicate that drug-induced Rpf dependency may be associated with inhibition or damage of cell wall biosynthesis but not with a shutdown of protein biosynthesis. We also cannot exclude the possibility that drug treatment helps to reveal some “preexisting” Rpf-dependent populations by eliminating most of the platable bacteria from actively growing cultures. The phenomenon of “persisters” has long been recognized, and persisters are believed to arise stochastically in growing cultures (29).
We have demonstrated recently that SN-dependent mycobacteria from sputum are more tolerant to isoniazid and streptomycin than actively growing mycobacteria (17). This potentially means that Rpf-dependent M. tuberculosis bacilli generated in vivo are more resistant to protein biosynthesis inhibition than bacteria from actively growing cultures and that the enrichment of SN- or Rpf-dependent M. tuberculosis bacilli in samples from treated patients is due to their higher tolerance to drugs. Interestingly, treatment of growing cultures with ethambutol induced SN dependency both in M. tuberculosis and in M. smegmatis; however, SN-dependent mycobacteria from sputum did not show higher tolerance to this drug than actively growing mycobacteria (17), suggesting the complex nature of the SN dependency phenomenon. Nevertheless, it is possible that some Rpf-dependent M. tuberculosis bacilli isolated from posttreatment samples resulted from drug exposure. Thus, our M. smegmatis model system can be used for the detection of drugs with a high propensity to induce Rpf dependency. Our assay using Rpf-overexpressing strains in the nonpathogenic bacterium M. smegmatis has been shown to accurately model the effects observed in M. tuberculosis. Drug resistance in TB is on the rise, and current efforts are focused on developing new antibiotics that will be effective against multidrug-resistant TB (MDR-TB) and extensively drug resistant TB (XDR-TB). It is therefore worthwhile to investigate a simple in vitro method for assessing the Rpf-dependent populations produced by antimicrobial treatments in order to help prevent resistance in the future.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by MRC-DTG (to J.L.) and BBSRC grants BB/H008586/1 and BB/K000330/1 (to G.V.M.). The research leading to these results has received funding from the Innovative Medicines Initiative Joint Undertaking under grant agreement 115337, resources for which are composed of financial contributions from the European Union's Seventh Framework Programme (FP7/2007-2013) and EFPIA companies' in-kind contributions (www.imi.europa.eu). We acknowledge the Centre for Core Biotechnology Services at the University of Leicester for the provision of a level 3 containment laboratory.
Funding Statement
This work, including the efforts of Jessica Loraine, was funded by Medical Research Council (MRC) (MRC-DTG). This work, including the efforts of Obolbek Turapov, was funded by the Innovative Medicines Initiative Joint Undertaking under grant agreement N115337, resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and EFPIA companies′ in-kind contribution. This work, including the efforts of Galina V. Mukamolova, was funded by Biotechnology and Biological Sciences Research Council (BBSRC) (BB/H008586/1 and BB/K000330/1).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00518-16.
REFERENCES
- 1.Zumla A, George A, Sharma V, Herbert RH, Baroness Masham of Ilton, Oxley IA, Oliver M. 2015. The WHO 2014 global tuberculosis report—further to go. Lancet Glob Health 3:e10–e12. doi: 10.1016/S2214-109X(14)70361-4. [DOI] [PubMed] [Google Scholar]
- 2.Wayne LG, Sohaskey CD. 2001. Non-replicating persistence of Mycobacterium tuberculosis. Annu Rev Microbiol 55:139–163. doi: 10.1146/annurev.micro.55.1.139. [DOI] [PubMed] [Google Scholar]
- 3.Barry CE III, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ, Young D. 2009. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 7:845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaprelyants AS, Gottschal JC, Kell DB. 1993. Dormancy in non-sporulating bacteria. FEMS Microbiol Rev 10:271–285. [DOI] [PubMed] [Google Scholar]
- 5.Chao MC, Rubin EJ. 2010. Letting sleeping dos lie: does dormancy play a role in tuberculosis? Annu Rev Microbiol 64:293–311. doi: 10.1146/annurev.micro.112408.134043. [DOI] [PubMed] [Google Scholar]
- 6.Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol 43:717–731. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- 7.Deb C, Lee CM, Dubey VS, Daniel J, Abomoelak B, Sirakova TD, Pawar S, Rogers L, Kolattukudy PE. 2009. A novel in vitro multiple-stress dormancy model for Mycobacterium tuberculosis generates a lipid-loaded, drug-tolerant, dormant pathogen. PLoS One 4:e6077. doi: 10.1371/journal.pone.0006077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sala C, Dhar N, Hartkoorn RC, Zhang M, Ha YH, Schneider P, Cole ST. 2010. Simple model for testing drugs against nonreplicating Mycobacterium tuberculosis. Antimicrob Agents Chemother 54:4150–4158. doi: 10.1128/AAC.00821-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salina E, Ryabova O, Kaprelyants A, Makarov V. 2014. New 2-thiopyridines as potential candidates for killing both actively growing and dormant Mycobacterium tuberculosis cells. Antimicrob Agents Chemother 58:55–60. doi: 10.1128/AAC.01308-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franzblau SG, DeGroote MA, Cho SH, Andries K, Nuermberger E, Orme IM, Mdluli K, Angulo-Barturen I, Dick T, Dartois V, Lenaerts AJ. 2012. Comprehensive analysis of methods used for the evaluation of compounds against Mycobacterium tuberculosis. Tuberculosis (Edinb) 92:453–488. doi: 10.1016/j.tube.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Turapov O, Glenn S, Kana B, Makarov V, Andrew PW, Mukamolova GV. 2014. The in vivo environment accelerates generation of resuscitation-promoting factor-dependent mycobacteria. Am J Respir Crit Care Med 190:1455–1457. doi: 10.1164/rccm.201407-1289LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manina G, Dhar N, McKinney JD. 2015. Stress and host immunity amplify Mycobacterium tuberculosis phenotypic heterogeneity and induce nongrowing metabolically active forms. Cell Host Microbe 17:32–46. doi: 10.1016/j.chom.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Mukamolova GV, Turapov OA, Young DI, Kaprelyants AS, Kell DB, Young M. 2002. A family of autocrine growth factors in Mycobacterium tuberculosis. Mol Microbiol 46:623–635. doi: 10.1046/j.1365-2958.2002.03184.x. [DOI] [PubMed] [Google Scholar]
- 14.Shleeva MO, Bagramyan K, Telkov MV, Mukamolova GV, Young M, Kell DB, Kaprelyants AS. 2002. Formation and resuscitation of “non-culturable” cells of Rhodococcus rhodochrous and Mycobacterium tuberculosis in prolonged stationary phase. Microbiology 148:1581–1591. doi: 10.1099/00221287-148-5-1581. [DOI] [PubMed] [Google Scholar]
- 15.Shleeva M, Mukamolova GV, Young M, Williams HD, Kaprelyants AS. 2004. Formation of ‘non-culturable’ cells of Mycobacterium smegmatis in stationary phase in response to growth under suboptimal conditions and their Rpf-mediated resuscitation. Microbiology 150:1687–1697. doi: 10.1099/mic.0.26893-0. [DOI] [PubMed] [Google Scholar]
- 16.Mukamolova GV, Turapov O, Malkin J, Woltmann G, Barer MR. 2010. Resuscitation-promoting factors reveal an occult population of tubercle bacilli in sputum. Am J Respir Crit Care Med 181:174–180. doi: 10.1164/rccm.200905-0661OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turapov O, O'Connor BD, Sarybaeva AA, Williams C, Patel H, Kadyrov AS, Sarybaev AS, Woltmann G, Barer MR, Mukamolova GV. 16 February 2016. Phenotypically adapted Mycobacterium tuberculosis populations from sputum are tolerant to first-line drugs. Antimicrob Agents Chemother doi: 10.1128/AAC.01380-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Y, Liu A, Ortega-Muro F, Alameda-Martin L, Mitchison D, Coates A. 2015. High-dose rifampicin kills persisters, shortens treatment duration, and reduces relapse rate in vitro and in vivo. Front Microbiol 6:641. doi: 10.3389/fmicb.2015.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukamolova GV, Murzin AG, Salina EG, Demina GR, Kell DB, Kaprelyants AS, Young M. 2006. Muralytic activity of Micrococcus luteus Rpf and its relationship to physiological activity in promoting bacterial growth and resuscitation. Mol Microbiol 59:84–98. doi: 10.1111/j.1365-2958.2005.04930.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaprelyants AS, Mukamolova GV, Ruggiero A, Makarov VA, Demina GR, Shleeva MO, Potapov VD, Shramko PA. 2012. Resuscitation-promoting factors (Rpf): in search of inhibitors. Protein Pept Lett 19:1026–1034. doi: 10.2174/092986612802762723. [DOI] [PubMed] [Google Scholar]
- 21.O'Connor BD, Woltmann G, Patel H, Turapov O, Haldar P, Mukamolova GV. 2015. Can resuscitation-promoting factors be used to improve culture rates of extra-pulmonary tuberculosis? Int J Tuberc Lung Dis 19:1556–1557. doi: 10.5588/ijtld.15.0682. [DOI] [PubMed] [Google Scholar]
- 22.Mukamolova GV, Turapov OA, Kazarian K, Telkov M, Kaprelyants AS, Kell DB, Young M. 2002. The rpf gene of Micrococcus luteus encodes an essential secreted growth factor. Mol Microbiol 46:611–621. doi: 10.1046/j.1365-2958.2002.03183.x. [DOI] [PubMed] [Google Scholar]
- 23.Turapov O, Loraine J, Jenkins CH, Barthe P, McFeely D, Forti F, Ghisotti D, Hesek D, Lee M, Bottrill AR, Vollmer W, Mobashery S, Cohen-Gonsaud M, Mukamolova GV. 2015. The external PASTA domain of the essential serine/threonine protein kinase PknB regulates mycobacterial growth. Open Biol 5:150025. doi: 10.1098/rsob.150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blokpoel MC, Murphy HN, O'Toole R, Wiles S, Runn ES, Stewart GR, Young DB, Robertson BD. 2005. Tetracycline-inducible gene regulation in mycobacteria. Nucleic Acids Res 33:e22. doi: 10.1093/nar/gni023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waddell SJ, Butcher PD. 2010. Use of DNA arrays to study transcriptional responses to antimycobacterial compounds. Methods Mol Biol 642:75–91. doi: 10.1007/978-1-60327-279-7_6. [DOI] [PubMed] [Google Scholar]
- 26.Turapov O, Waddell SJ, Burke B, Glenn S, Sarybaeva AA, Tudo G, Labesse G, Young DI, Young M, Andrew PW, Butcher PD, Cohen-Gonsaud M, Mukamolova GV. 2014. Antimicrobial treatment improves mycobacterial survival in nonpermissive growth conditions. Antimicrob Agents Chemother 58:2798–2806. doi: 10.1128/AAC.02774-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hett EC, Chao MC, Deng LL, Rubin EJ. 2008. A mycobacterial enzyme essential for cell division synergizes with resuscitation-promoting factor. PLoS Pathog 4:e1000001. doi: 10.1371/journal.ppat.1000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolwijck E, Friedrich SO, Karinja MN, van Ingen J, Warren RM, Diacon AH. 2014. Early stationary phase culture supernatant accelerates growth of sputum cultures collected after initiation of anti-tuberculosis treatment. Clin Microbiol Infect 20:O418–O420. doi: 10.1111/1469-0691.12441. [DOI] [PubMed] [Google Scholar]
- 29.Lewis K. 2010. Persister cells. Annu Rev Microbiol 64:357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


