Abstract
Plasmodium falciparum, the deadliest species of malaria parasites, is dependent on glycolysis for the generation of ATP during the pathogenic red blood cell stage. Hexokinase (HK) catalyzes the first step in glycolysis, transferring the γ-phosphoryl group of ATP to glucose to yield glucose-6-phosphate. Here, we describe the validation of a high-throughput assay for screening small-molecule collections to identify inhibitors of the P. falciparum HK (PfHK). The assay, which employed an ADP-Glo reporter system in a 1,536-well-plate format, was robust with a signal-to-background ratio of 3.4 ± 1.2, a coefficient of variation of 6.8% ± 2.9%, and a Z′-factor of 0.75 ± 0.08. Using this assay, we screened 57,654 molecules from multiple small-molecule collections. Confirmed hits were resolved into four clusters on the basis of structural relatedness. Multiple singleton hits were also identified. The most potent inhibitors had 50% inhibitory concentrations as low as ∼1 μM, and several were found to have low-micromolar 50% effective concentrations against asexual intraerythrocytic-stage P. falciparum parasites. These molecules additionally demonstrated limited toxicity against a panel of mammalian cells. The identification of PfHK inhibitors with antiparasitic activity using this validated screening assay is encouraging, as it justifies additional HTS campaigns with more structurally amenable libraries for the identification of potential leads for future therapeutic development.
INTRODUCTION
Malaria is one of the world's deadliest infectious diseases, causing the deaths of hundreds of thousands of people annually. This burden is borne primarily by children (1). The malaria parasite has a complex life cycle, with the asexual intraerythrocytic stage largely being responsible for the development of pathologies. During this stage, glycolysis plays a central metabolic role (2, 3). In support of this, glucose consumption is increased up to 100-fold in infected erythrocytes (4) and lactate levels are ∼20 to 100 times higher in infected erythrocytes than in uninfected erythrocytes (5, 6). Additionally, knockout of the hexose transporter responsible for importing glucose is lethal to the parasite, and inhibition of glycolysis with glucose analogs rapidly depletes parasite ATP (7, 8).
The first enzymatic step in glycolysis, catalyzed by Plasmodium falciparum hexokinase (PfHK), is the transfer of the γ-phosphoryl group from ATP to glucose, yielding glucose-6-phosphate (G-6-P). This intermediate has several potential fates, including consumption by glycolysis. Additionally, it can enter the pentose phosphate pathway for the generation of NADPH, a key component in the P. falciparum antioxidant defense and de novo nucleotide triphosphate biosynthesis pathways (9). While the 55.3-kDa PfHK shares several biochemical characteristics with mammalian hexokinases (HKs), including being inhibited by its products, it has limited amino acid identity to the human HKs, suggesting that PfHK-specific therapeutics can be developed (10). While there are, to date, no PfHK-specific inhibitors available, such inhibitors would serve as valuable research tools to dissect the importance of the enzyme for parasite growth and viability and help validate PfHK as a therapeutic target.
We have previously described the cloning, expression, and partial characterization of PfHK (10). As part of that work, we screened a limited collection of small-molecule PfHK inhibitors and made two key observations. First, PfHK inhibitors were confirmed to be toxic to asexual intraerythrocytic-stage parasites (10), although concern about the potential promiscuity of these HK inhibitors limited further work on these molecules. Second, the potential for the development of highly selective inhibitors for a parasitic HK was suggested by the work, as previously identified potent inhibitors of the African trypanosome HK1 (Trypanosoma brucei HK1 [TbHK1]) (11) lacked detectable activity against the Plasmodium enzyme (10).
Here, we describe the pursuit of novel scaffolds for further optimization via a validated PfHK biochemical high-throughput screening (HTS) campaign with a total of 57,654 compounds. This effort, which has served to justify continued screening of the target, has yielded inhibitors of PfHK biochemical activity that also have antiparasitic activity. Secondary assays, including determination of the activities of the compounds against a panel of human cell lines, have been used to assess the specificity of the identified inhibitors and explore the potential off-target effects of the observed parasite toxicity.
MATERIALS AND METHODS
Chemicals, reagents, and libraries.
Glucose-6-phosphate dehydrogenase, β-NAD (NAD+), ATP, and glucose were purchased from Sigma (St. Louis, MO). Dimethyl sulfoxide (DMSO) was purchased from Fisher Scientific (Pittsburgh, PA), and phosphoenolpyruvate (PEP) was obtained from VWR International (West Chester, PA). Isobenzothiazolinones and benzamides were obtained from the University of Kansas Specialized Chemistry Center. The libraries screened included the Library of 1,280 Pharmacologically Active Compounds (LOPAC1,280; Sigma-Aldrich, St. Louis, MO) and the Tocris (Tocris Bioscience, Bristol, United Kingdom), Prestwick (Prestwick Chemical, San Diego, CA), BIOMOL (Enzo Life Sciences, Farmingdale, NY), MicroSource (MicroSource Discovery Systems, Gaylordsville, CT), KINACore (ChemBridge, San Diego, CA), Roche (Roche Library, Basel, Switzerland), NPC (the National Center for Advancing Translational Sciences [NCATS] Pharmaceutical Collection [https://tripod.nih.gov/npc/]), MIPE 3.0 (Mechanism Interrogation PlatE [https://ncats.nih.gov/pubs/features/screening-platform]), Sytravon (a chemically diverse in-house collection), and NPACT (NCATS Pharmacologically Active Chemical Toolbox) libraries. This group of libraries includes those that focus on drug and drug-like compounds, as well as kinase-targeted libraries. The libraries selected include bioactive and chemically diverse compounds. Compound concentrations varied from library to library. For most libraries, compounds were tested at concentrations ranging from 0.02 to 76 μM, while compounds in the MIPE 3.0 collection were tested at concentrations ranging from 1 nM to 76 μM. The compound plates were prepared as described previously (12).
Recombinant enzyme purification and assay conditions.
The P. falciparum HK (UniProt accession no. Q02155) was expressed and purified from a codon-optimized open reading frame cloned into the expression vector pQE30 (Qiagen, Valencia, CA) as described previously (10), with a minor modification. Briefly, recombinant PfHK was purified following a protocol developed for heterologous expression and purification of an HK from the African trypanosome, with the protocol modified by expression of protein in an Escherichia coli BL21 background instead of E. coli M15(pREP), which has led to improved yields (from ∼0.1 mg/ml to concentrations routinely of ∼1 mg/ml; see Fig. S1 in the supplemental material) compared to those in our previous work (10).
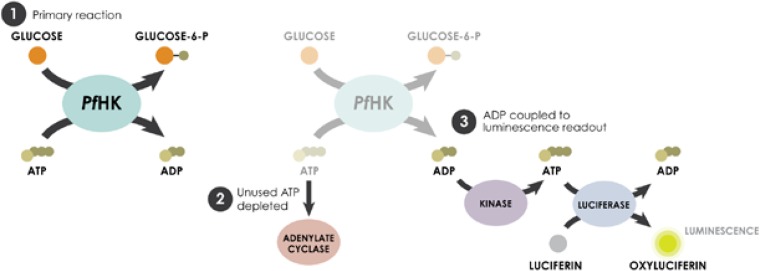
HK assays were performed in a 1,536-well format with white medium-binding solid-bottom plates (Greiner Bio One, Monroe, NC) and ADP-Glo (Promega, Madison, WI) to measure ADP formation as a product of PfHK activity (see Table S1 in the supplemental material). First, 2 μl of enzyme and glucose in assay buffer (0.1 M triethanolamine, pH 7.9, 10 mM MgCl2, 0.01% Tween 20, 1.5 mM glucose with enzyme [22.5 μg/ml PfHK]) was added to columns 1 and 2 and 5 to 48 of 1,536-well plates. A no-PfHK control (0.1 M triethanolamine, pH 7.9, 10 mM MgCl2, 0.01% Tween 20, 1.5 mM glucose) was added to columns 3 and 4. Compounds (23 nl, library and control compounds) were then added with a pin tool (Kalypsys, San Diego, CA), and the plates were incubated (30 min, room temperature [RT]) prior to the addition of 1 μl ATP in assay buffer (0.1 M triethanolamine, pH 7.9, 10 mM MgCl2, 0.01% Tween 20, 1 mM ATP) to initiate the reaction. The plates were incubated at RT for 60 min, followed by the two-step ADP-Glo detection process, which quantifies the ADP generated by the reaction, as described previously (13) (Fig. 1). In step 1, 2 μl of the ADP detection reagent was added and the plates were incubated for 40 min at RT. In step 2, 4 μl of the kinase detection reagent was added and the plates were incubated for 30 min prior to reading of the luminescence on a ViewLux multimodal detector (PerkinElmer, Waltham, MA) using previously described methodologies (13–16).
FIG 1.
Reaction scheme for qHTS. PfHK activity was measured using a two-step ADP-Glo detection method where the ADP produced by PfHK activity is converted back to ATP for quantification via luciferase activity.
To identify compounds that interfere with the ADP-Glo reagents, counterassay mixtures were assembled without PfHK but with 100% ATP (approximating full enzyme inhibition; columns 3 and 4) or a 20% ADP–80% ATP mix (which approximates 20% substrate conversion or the uninhibited enzyme reaction; columns 1 and 2 and 5 to 48) prior to addition of the ADP-Glo reagents. A compound interfering with detection would induce a change in the observed luminescence relative to that for a DMSO control. One of the ADP-Glo components, luciferase, has indeed previously been shown to be inhibited (leading to a decrease in luminescence) or stabilized (leading to an increase in luminescence) by a small fraction of compounds contained within screening libraries (12). An alternative approach to the measurement of PfHK activity, in which Promega Kinase-Glo is used to detect unused ATP substrate in the assay, was also considered. However, this approach was unsuitable as a consequence of the PfHK high apparent Km for ATP, which is beyond the product specifications.
Using an interplate dose-response approach, called quantitative high-throughput screening (qHTS), concentration-response curves (CRCs) have been resolved (12). With automated CRC fitting and classification, compounds were grouped as full inhibitors, partial modulators, or inactive in order to select compounds for reconfirmation. Identification of active compounds in this manner limited the number of reacquired compounds to a few hundred. Compounds were scored for activity against the assay components alone, with compounds specific to PfHK reacquired either by resupply from the library source or through resynthesis at the NCATS, U.S. National Institutes of Health (NIH). Overall assay performance metrics, including the Z′-factor trend, were confirmed and 50% inhibitory concentrations (IC50s) were obtained from 11-point dose-response assays.
Percent activity was computed after normalization using the median values for the DMSO-treated uninhibited enzyme control (64 wells per plate) and the DMSO-treated, no-enzyme, or 100% inhibited control (32 wells per plate). Additionally, ebselen (10) was plated in one column (32 wells per plate) as a control in the dose-response assay. An NCATS in-house database was used to track sample concentrations across plates, while ActivityBase software (IDBS, Alameda, CA) was used for compound and plate registrations (17). A four-parameter Hill equation was fit to the concentration-response data by minimizing the residual error between the modeled and observed responses. After curve fitting, active compounds were organized on the basis of their potency and concentration-response curve as described previously (12), with frequent hitters being removed prior to cherry-picking.
Enzyme counterscreens to evaluate specificity.
Specificity assays were performed as described previously using human HK4 (human glucokinase [hGlk]; GenBank accession no. BC001890), the human sugar kinase with the highest amino acid sequence identity with the amino acid sequence of PfHK (albeit limited at 29%). We successfully expressed and purified hGlk for use as a secondary screening reagent during a previous HTS campaign (11). For additional specificity evaluation, another sugar kinase was examined using a previously described HTS protocol for TbHK1 (11). Like hGlk, TbHK1 shares limited sequence identity (28%) with the Plasmodium enzyme.
Whole-cell parasite viability assays.
P. falciparum 3D7 parasites were grown in type O-positive red blood cells at 1% hematocrit cultured in RPMI (plus l-glutamine without NaHCO3) under a 5% CO2, 5% O2, and 90% N2 atmosphere at 37°C. The medium was supplemented with 0.5% AlbuMAX, 0.37 mM hypoxanthine, 27 mM NaHCO3, 11 mM glucose, and 10 μg/ml gentamicin. Cultures were assayed in triplicate in a total volume of 100 μl, and the DMSO vehicle was used as a negative control. The assays were initiated at approximately 0.5% parasitemia (percent infected erythrocytes). Following 72 h of incubation, parasitemias were enumerated by flow cytometry of 30,000 erythrocytes stained with acridine orange (1.5 μg/ml in phosphate-buffered saline, 5 min, RT) with a FACSCanto II flow cytometer (BD Biosystems, Franklin Lakes, NJ).
Dose-response curves for compounds that elicited >50% growth inhibition at 10 μM were pursued in a 96-well plate format, with 50% effective concentrations (EC50s) being determined from the results of incubation with a 2-fold dilution series of inhibitor starting at 40 μM. Percent inhibition was calculated by comparison to the growth of parasites grown with the DMSO controls from each plate. Chloroquine was included in each assay as a positive control starting at 1 μM. Averages of the duplicates were calculated and fit to the dose-response curves for the determination of IC50s using GraphPad Prism (version 6.0) software (GraphPad Software, Inc., La Jolla, CA).
Additional whole-cell follow-on assays of inhibitors included tests against cultured bloodstream-form T. brucei and Leishmania amazonensis parasites, using previously described methods (11, 18). The findings from these assays lend insight into compound specificity, as both T. brucei and Leishmania have HKs with limited similarity to PfHK (<35%).
Human cell line toxicity testing.
The toxicities of the compounds against a panel of human cells (e.g., IMR90, HeLa, and HEK293 cells) were scored as described previously (11). Briefly, cell line viability was determined in assay mixtures with a 25-μl final volume using a 384-well plate-based assay. Cells were cultured in complete growth medium (according to ATCC specifications; ATCC, Manassas, VA), with 500 cells (HEK292 cells) or 1,000 cells (IMR90 and HeLa cells)/22 μl being seeded into each well. Test and control compounds (3 μl) were then added, with vehicle and positive controls being 1% and 10% DMSO, respectively. The plates were incubated for 44 to 46 h (37°C, 5% CO2), and 5 μl CellTiter Blue (Promega) reagent was added. Following an additional 2 to 4 h of incubation, data were captured on a SpectraMax M5 multimode plate reader (Molecular Devices, LLC, Sunnyvale, CA) with excitation at A560 and emission at A590. EC50s were calculated using GraphPad Prism (version 6.0) software.
RESULTS
Assay design and miniaturization.
To identify new scaffolds for further development, the PfHK assay was modified for HTS. We have expressed, purified, and characterized recombinant active PfHK using an E. coli codon-optimized version of the P. falciparum cDNA (PF3D7_0624000), as described previously (10). The protein was suitably stable, with activity being unaltered after at least 3 months at 4°C and at least 6 months at −80°C. At room temperature, 85% ± 3% of the initial protein activity was maintained after 6 h.
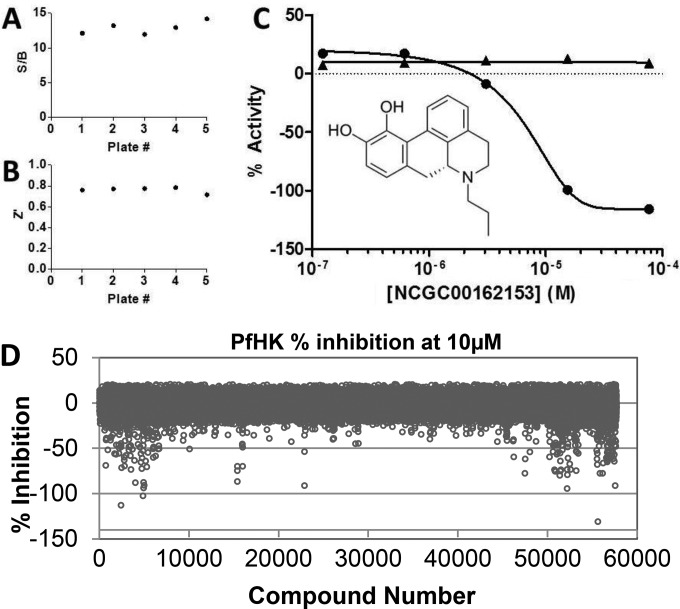
In optimizing the assay, the enzyme concentration was selected such that the amount of protein used produced a robust signal-to-background ratio (12.9 ± 0.9) in a 60-min reaction while remaining at <20% substrate conversion (Fig. 2A). See Table S1 in the supplemental material for the assay protocol. Because we sought to identify small molecules representing all types of inhibition modes, assays were performed with substrate concentrations near the Km values for the PfHK substrates (glucose Km = 0.62 ± 0.06 mM, ATP Km = 0.66 ± 0.08 mM [10]). There was no appreciable change in assay performance by the presence of up to 3.1% DMSO, representing addition of quadruple aliquots of DMSO to the assay plate (via the fixed 23-nl volume dispensed by the pin tool). Additionally, the stability of reagents under dilute assay conditions was examined, and a less than 2% change in activity was observed when a freshly prepared PfHK mix was compared with a mix incubated for 22 h at 4°C (data not shown).
FIG 2.
Validation of the PfHK primary assay and counterassay in a qHTS screen. The individual statistics from five 1,536-well plates of the primary screen show a signal-to-background ratio (S/B) of 12.9 ± 0.9 (A) and a Z′-factor of 0.76 ± 0.02 (B), which demonstrate the suitability of the assay for HTS. (C) A selected hit from the screen of the compounds in LOPAC1,280 shows the ability of the primary assay (circles) and the counterassay (triangles; ADP-Glo reagents alone) to distinguish PfHK inhibitors. (D) Results from the expanded screen of 57,654 molecules, with the percent inhibition of activity by all compounds at 10 μM being plotted.
Validation of the primary assay through pilot screens.
Validation of the PfHK assay was performed in a 1,536-well-format qHTS screen against the Library of 1,280 Pharmacologically Active Compounds (LOPAC1,280) as a five-point titration series in 3-μl volumes. Additionally, a DMSO-only control plate was included. This approach was robust, yielding an average Z′-factor of 0.76 ± 0.02 for 5 plates and a hit rate from the LOPAC1,280 library of 3%. Data from one LOPAC1,280 hit are included in Fig. 2C to illustrate how the primary assay (filled circles) and the counterscreen against the ADP-Glo reagents alone (filled triangles) distinguish PfHK inhibition by (R)-(−)-propylnorapomorphine hydrochloride.
HTS screening and counterscreening.
The promising results of the pilot screen led us to expand the effort, with 57,654 molecules being tested in total. The assay was robust, with a signal-to-background ratio of 3.4 ± 1.2, a coefficient of variation of 6.8% ± 2.9%, and a Z′-factor of 0.75 ± 0.08. From the library, 201 compounds at 10 μM inhibited PfHK by more than 50% without interfering with the ADP-Glo reporter system (verified by a counterscreen), with the resulting initial hit rate being 0.35% (Fig. 2D). See Table S2 in the supplemental material for a description of the 201 hits. The hit rate in terms of the individual libraries tested was also explored (see Table S3 in the supplemental material), with rates of 0 to 4.37% being obtained, reflecting the diversity of the small-molecule libraries explored.
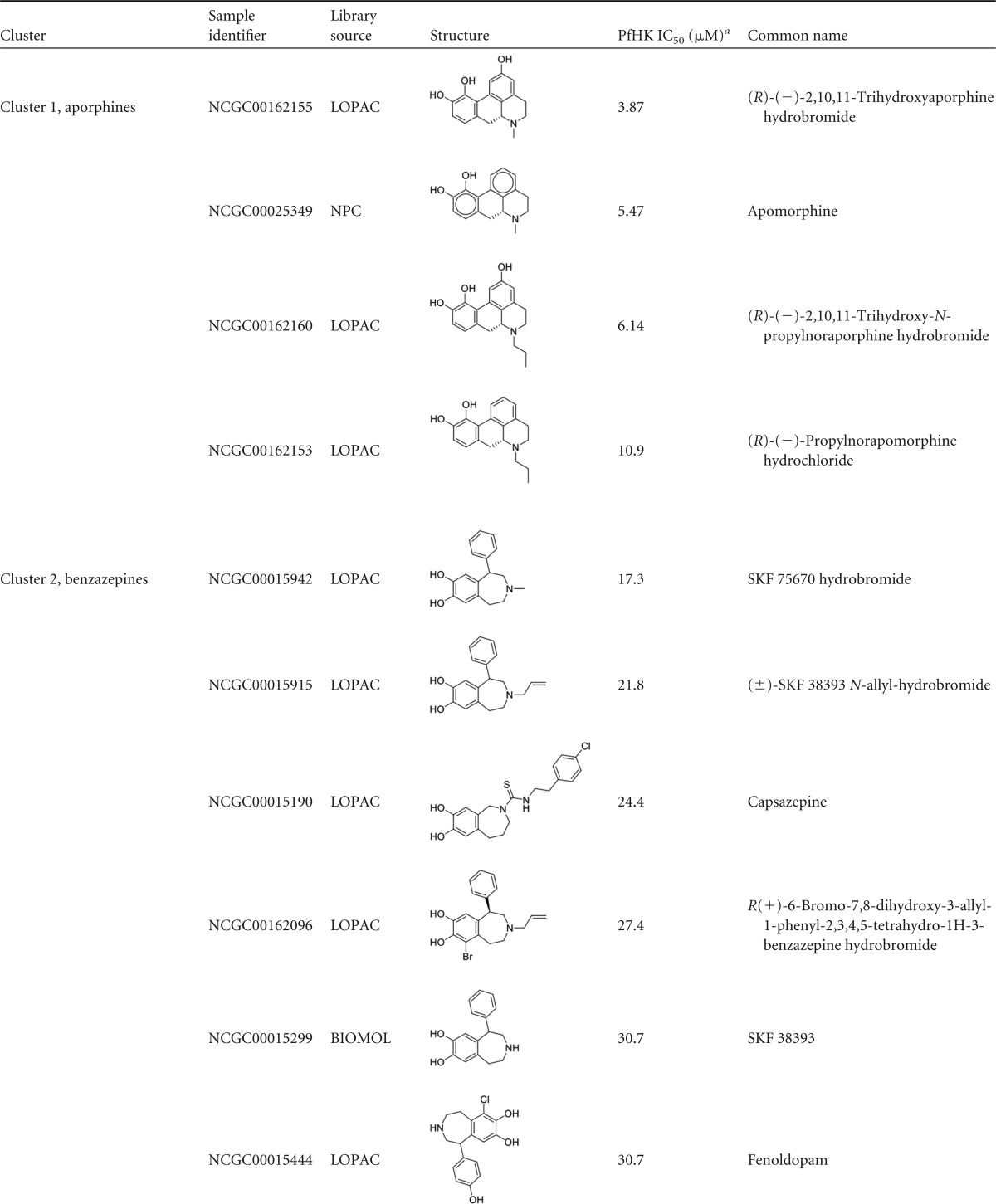
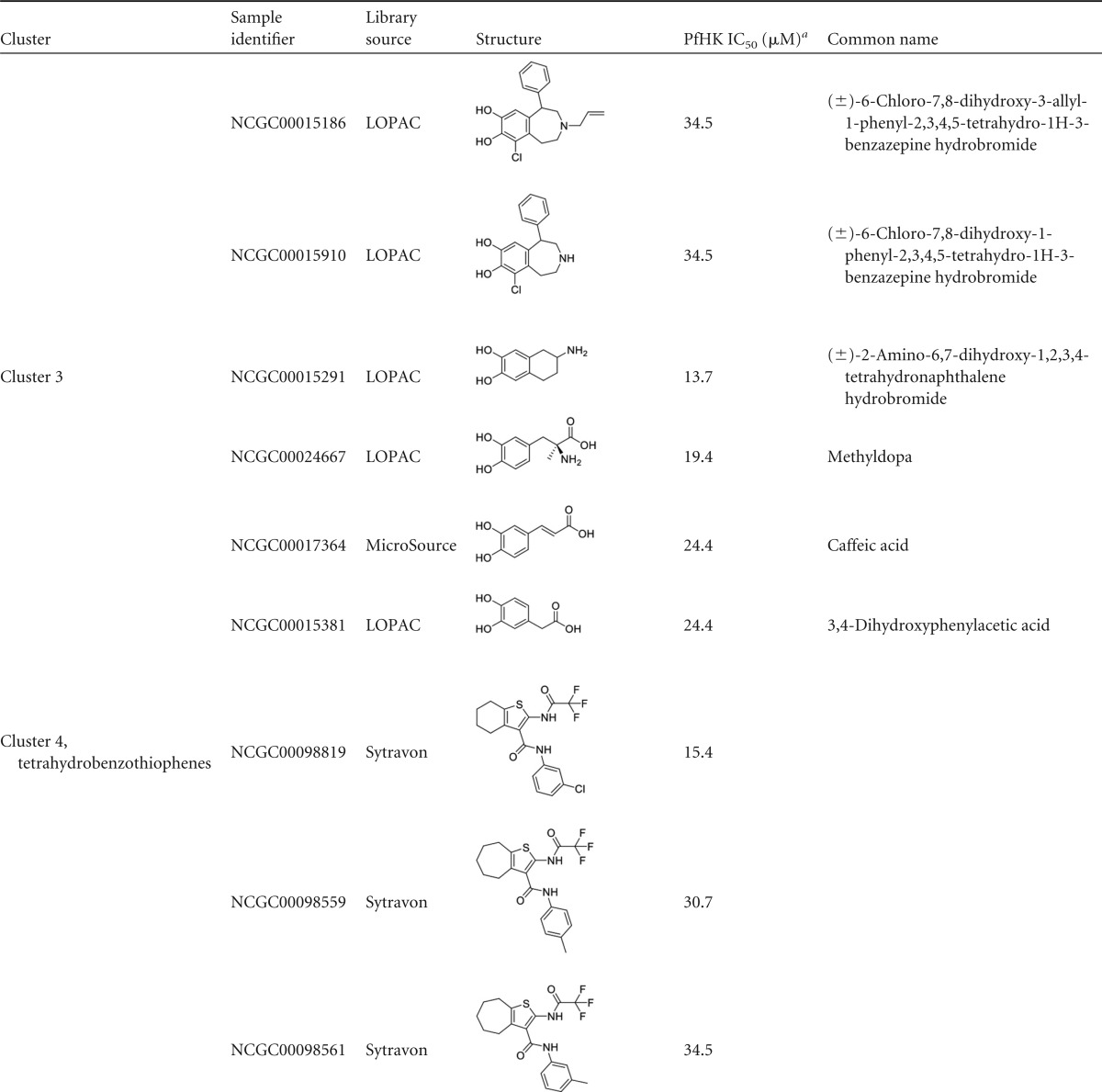
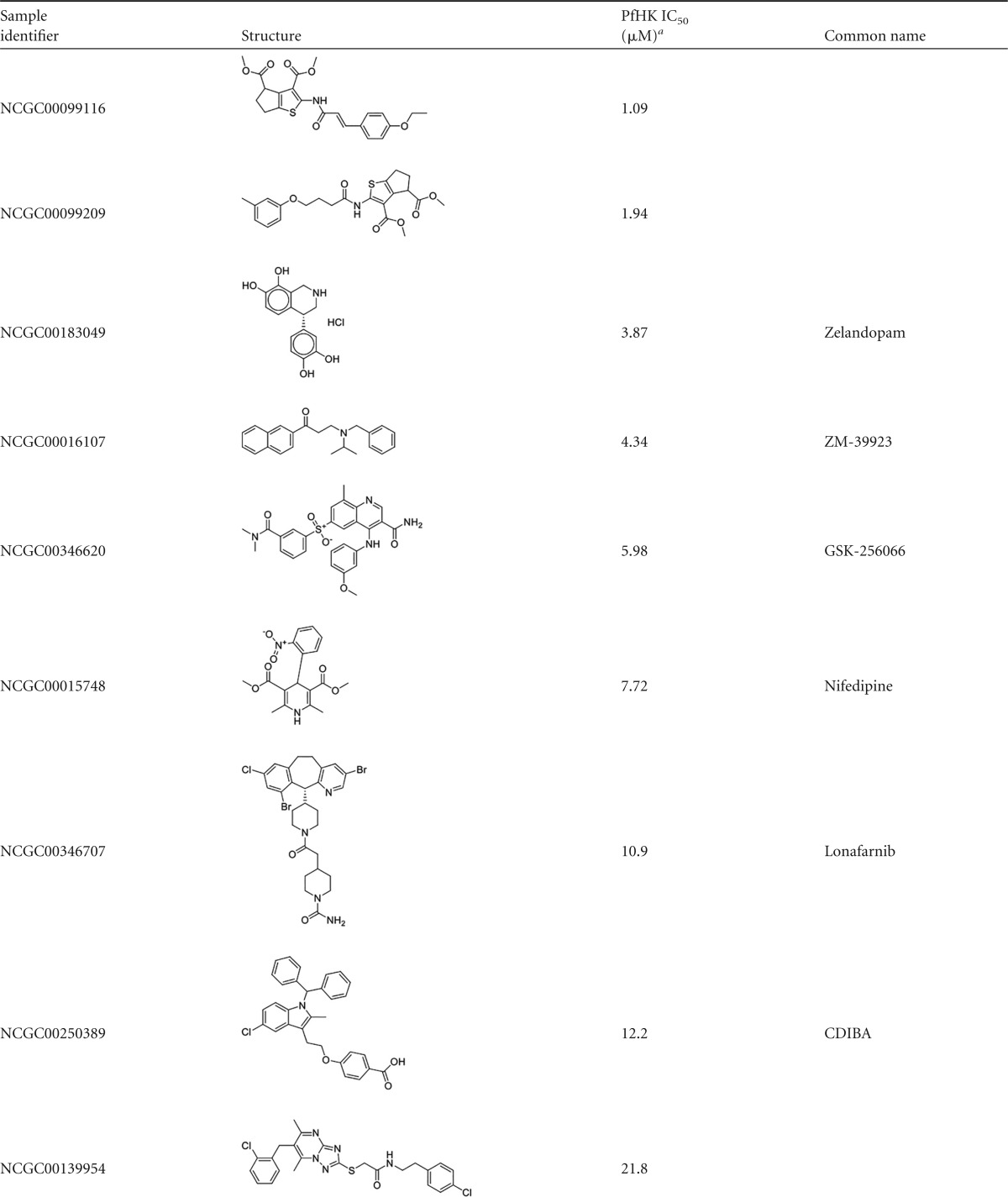
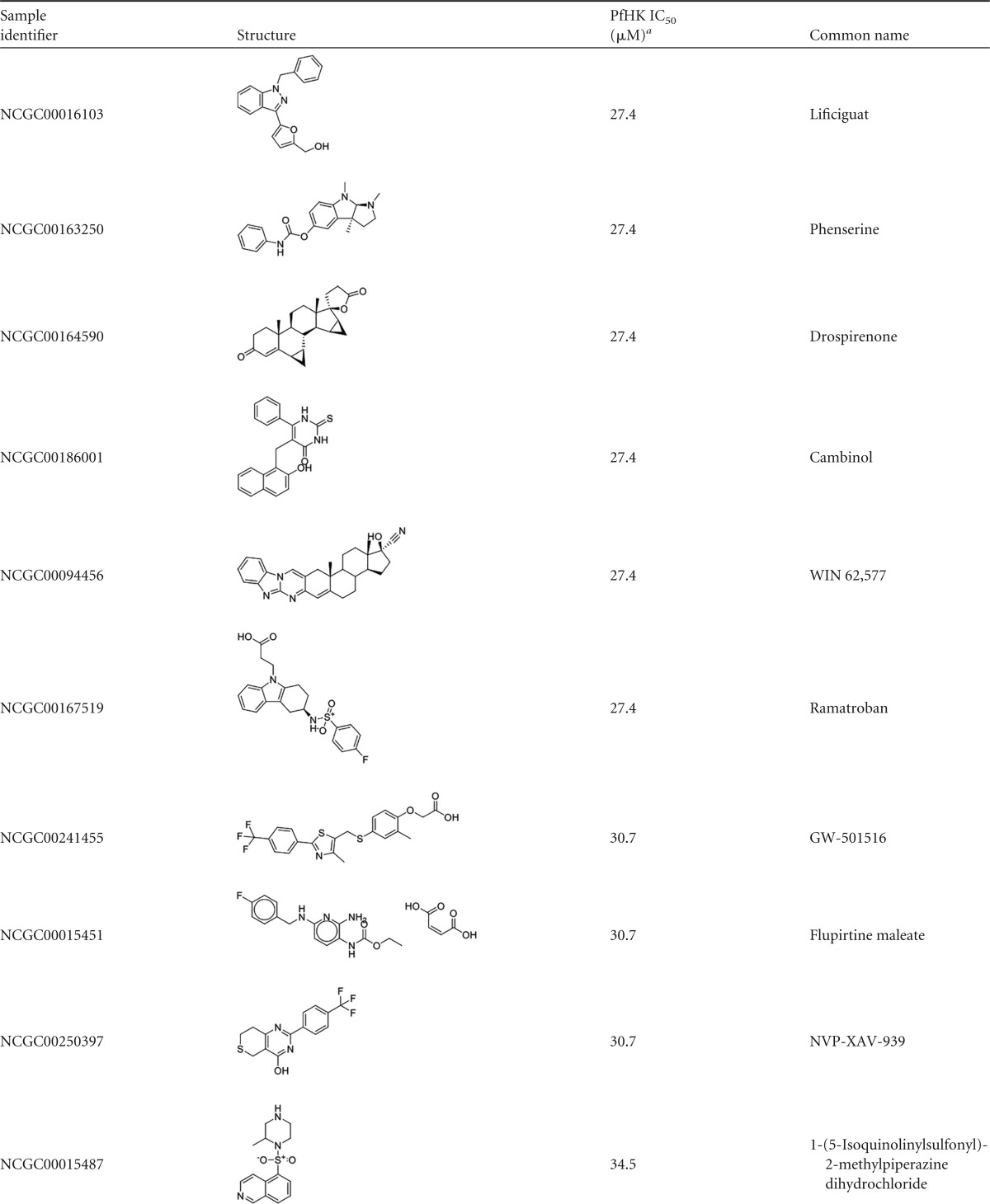
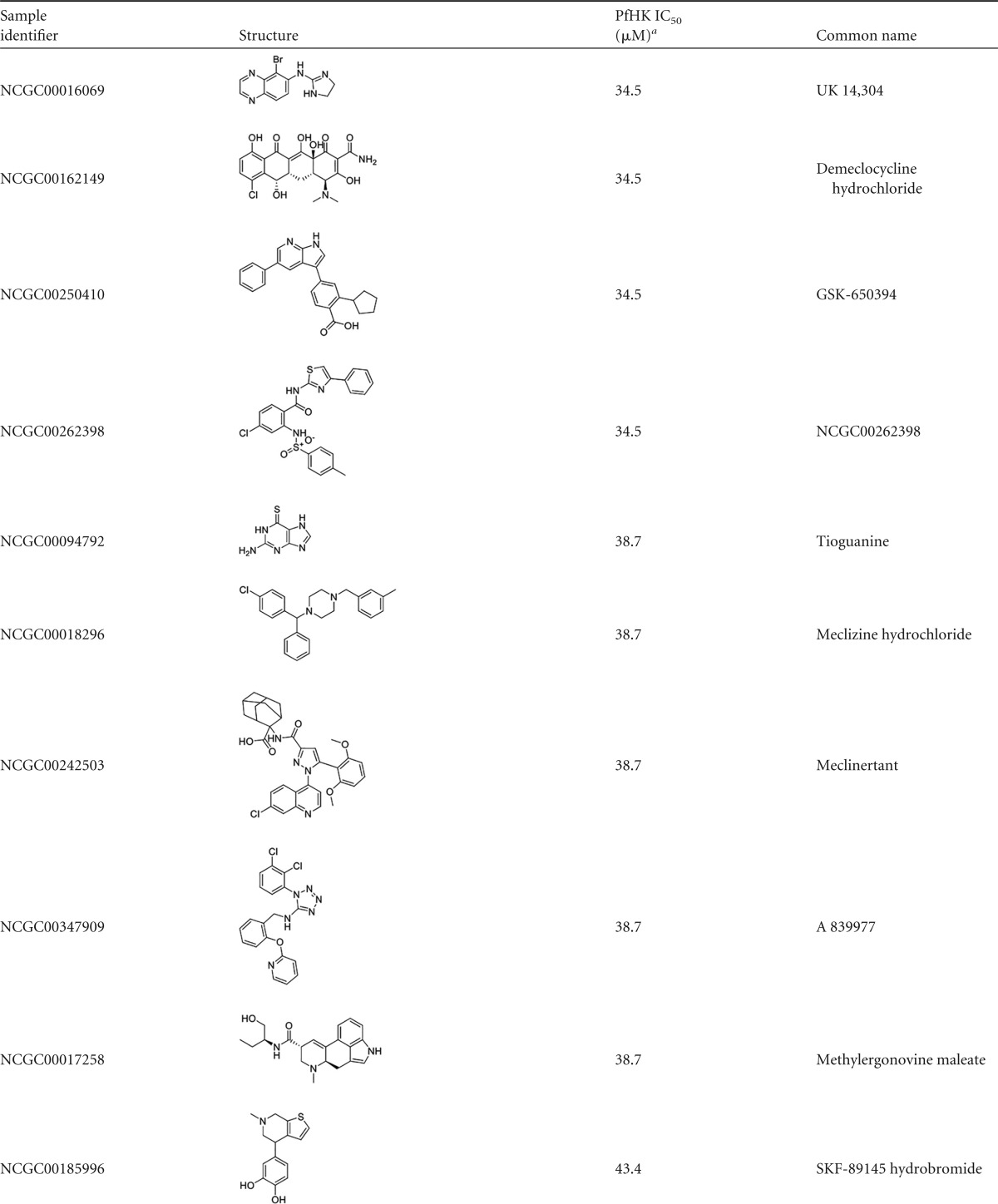
Reconfirmed hits were resolved into four structurally related clusters of molecules (Table 1) and singleton compounds (Table 2). Cluster 1 was composed of four aporphine compounds, which were active against PfHK with IC50s ranging from 3.9 to 11 μM. These molecules are notable as members of the most potent enzyme inhibitor hit cluster, with the weakest inhibitor, NCGC00162153, being more potent than any members of the remaining three clusters. Cluster 2 was the largest group of clustered PfHK inhibitors, with eight benzazepine members. None of these were particularly potent PfHK inhibitors, with all compounds having IC50s greater than 17 μM (Table 1). Cluster 3 comprised four of the smallest molecules identified in the screen, on the basis of molecular weight. These compounds varied in potency, with IC50s being between 13.7 and 24.4 μM. The final cluster, cluster 4, contained three tetrahydrobenzothiophenes. Only one, NCGC00098819, had an IC50 of less than 30 μM, at 15.4 μM. Finally, several singleton hits were PfHK inhibitors at low-micromolar concentrations, with NCGC00099116 and NCGC00099209 yielding IC50s of 1.09 μM and 1.94 μM, respectively (Table 2).
TABLE 1.
Clustered hits from validation screens for PfHK
Enzyme inhibition validation assay results reflect averages from triplicate experiments with cherry-picked inhibitors. IC50s greater than 10 μM are included for comparison of activities within a cluster. Note that all compounds had IC50s of >10 μM for hGlk.
TABLE 2.
Singleton hits from validation screens for PfHK
Enzyme inhibition validation assay results reflect averages from triplicate experiments with cherry-picked inhibitors. IC50s greater than 10 μM are included for comparison of activities within a cluster. Note that all compounds had IC50s of >10 μM for hGlk.
Hits from the LOPAC1,280 validation screen and pooled libraries were tested in a series of counterscreens to explore the specificity of inhibition. In the first counterscreen, compounds were assessed for their propensity to interfere with the ADP-Glo reagents (with ADP added as the substrate), and none of the hits had an impact on the reaction. Additionally, none of the compounds were active at 10 μM in counterscreens against human glucokinase (hGlk), supporting the specificity of compounds with IC50s against PfHK lower than this arbitrary threshold. Ideal candidate compounds for further development as PfHK inhibitors would also have limited activity against other hexokinases. Like hGlk, TbHK1, which shares limited (28%) amino acid sequence identity with PfHK, was not impacted by 10 μM inhibitor.
Parasite assays.
We anticipated that small molecules that interfere with parasite glucose metabolism would have antiparasitic activity against the disease-causing, asexual intraerythrocytic stages. To explore this, hits were tested in whole-cell parasite viability assays. While cluster 1 members were modestly active against asexual intraerythrocytic-stage parasites, with the EC50s of the PfHK inhibitors NCGC00162155 and NCGC00025349 being 4.1 μM and 1.3 μM, respectively (Table 3), the majority of PfHK inhibitors had poor antiparasitic activity. For example, cluster 2 compounds displayed weak activity, with only one compound (NCGC00162096) inhibiting parasite growth by more than 45% at 10 μM (Table 3). Further, cluster 3 compounds lacked measurable activity, and cluster 4 inhibitors had limited activity, showing <30% inhibition of parasite growth at 10 μM. Cluster 1 members also stood out as having values of the calculated partition coefficient between n-octanol and water (clogP) that suggested that they are sufficiently drug-like to be bioavailable (Table 3).
TABLE 3.
Activity of hits from validation screens against asexual-stage P. falciparum parasitesa
| Cluster | Sample identifier | cLogPb | % growth inhibition at 5 μM | EC50c (μM) |
||||
|---|---|---|---|---|---|---|---|---|
| P. falciparum 3D7 | HeLa cells | HEK293 cells | IMR90 cells | L. amazonensis promastigotes | ||||
| Cluster 1, aporphines | NCGC00162155 | 1.82 | 48.38 ± 1.44 | 4.1 ± 0.18 | 19.0 ± 6.1 | >25 | >25 | 19.4 ± 0.8 |
| NCGC00025349 | 2.49 | 47.1 ± 6.28 | 1.3 ± 0.1 | >25 | >25 | >25 | 7.32 ± 0.3 | |
| NCGC00162160 | 2.88 | 17.88 ± 2.67 | 11.6 ± 1.7 | >25 | >25 | 10.7 ± 0.1 | ||
| Cluster 2, benzazepines | NCGC00162096 | 4.32 | 51.41 ± 1.44 | 31 ± 6.4 | >25 | >25 | >25 | 13.1 ± 0.6 |
| NCGC00015186 | 4.12 | 38.03 ± 1.57 | >25 | 24.8 ± 0.1 | >25 | 21.3 ± 3.1 | ||
| Cluster 4, tetrahydrobenzothiophenes | NCGC00098819 | 5.69 | 12.64 ± 1.75 | 5.3 ± 2 | 15.4 ± 0.9 | 3.1 ± 1.4 | >25 | |
| NCGC00098559 | 5.87 | 13.2 ± 1.11 | 4.7 ± 1.4 | 2.8 ± 0.3 | 7.2 ± 1.5 | >25 | ||
| NCGC00098561 | 5.87 | 27.18 ± 2.19 | 2.7 ± 1.1 | 2.7 ± 0.5 | 5.4 ± 1.2 | >25 | ||
| Singletons | NCGC00099116 | 4.38 | 10.53 ± 1.84 | >25 | 10.3 ± 1.1 | >25 | 22.2 ± 0.8 | |
| NCGC00346707 | 3.49 | 80.97 ± 0.63 | 5.1 ± 0.17 | >25 | 5.2 ± 0.6 | >25 | 6.3 ± 0.04 | |
| NCGC00163250 | 4.13 | 80.14 ± 1.75 | 1.7 ± 0.22 | >25 | 10.0 ± 3.4 | >25 | >25 | |
| NCGC00094456 | 5.38 | 87.62 ± 0.87 | 1.9 ± 0.14 | 12 ± 0.5 | 7.3 ± 1.0 | 17.6 ± 3.6 | 2.3 ± 0.1 | |
| NCGC00162149 | −0.58 | 29.69 ± 2.31 | >25 | >25 | >25 | 16.4 ± 3.5 | ||
| NCGC00250410 | 5.67 | 91.12 ± 0.33 | 0.78 ± 0.04 | 12.8 ± 4.2 | >25 | >25 | 20.1 ± 5 | |
| NCGC00262398 | 5.02 | 23.42 ± 1.13 | 10.1 ± 0.2 | 8.5 ± 1.7 | 23.6 ± 1.5 | 21.5 ± 2.7 | ||
| NCGC00018296 | 6.73 | 52.19 ± 2.48 | 5.0 ± 0.41 | >25 | >25 | >25 | 3.5 ± 0.7 | |
| NCGC00242503 | 5.52 | 15.01 ± 5.17 | >25 | 24.3 ± 1.0 | >25 | 12.1 ± 0.3 | ||
| NCGC00016069 | 1.49 | 11.47 ± 3.49 | >25 | >25 | >25 | >25 | ||
Only compounds that inhibited P. falciparum growth by more than 10% at 5 μM are included.
cLogP values were calculated using ChemBridge software.
P. falciparum dose-response curves for compounds that caused >45% growth inhibition at 10 μM were performed in duplicate, and averages were used to resolve EC50s. All human cell lines were evaluated in three independent experiments.
The singleton hits NCGC00099116 and NCGC00099209, the most potent PfHK inhibitors identified in this screen, lacked activity against Plasmodium parasites. Interestingly, the singleton NCGC00250410, a rather modest PfHK inhibitor (IC50 = 34.5 μM), was the most potent compound identified, with an EC50 of 0.78 μM, indicating off-target activity or possible accumulation within the parasite allowing enzyme-inhibitory concentrations to be reached. Such is the case for chloroquine, which, due to its dibasic nature, concentrates within the parasite's acidic digestive vacuole.
Some of the PfHK inhibitors were active against other parasites. For example, NCGC00094456 and NCGC00018296 displayed similar activity against both L. amazonensis promastigotes and P. falciparum (Table 3). Both were poor inhibitors of PfHK (IC50s, >10 μM) and did not inhibit the T. brucei enzyme (which is very similar to the Leishmania enzyme), suggesting that toxicity was the result of off-target effects. NCGC00162155 was also active against Plasmodium and Leishmania parasites (EC50 = 19.4 ± 0.8 μM). However, the similar sensitivities of Leishmania and human cells (see below) toward this compound suggest that the potential therapeutic value might be extremely limited, a theme that holds true for most of the compounds with activity against Leishmania, as they tend to be toxic toward human cells.
Human cell line toxicity.
The hits demonstrated a spectrum of activity against the human cell lines tested. PfHK inhibitors in cluster 1 were not particularly toxic to human cells, with EC50s against both immortal and primary human cells lines being greater than 19 μM (Table 3). The active benzazepines in cluster 2 were similarly nontoxic to human cells. In contrast, the antiparasitic cluster 4 compounds tended to be more toxic to the human cell panel members than to the parasite. This is reflected by single-digit micromolar EC50s, suggesting too many off-target activities for further pursuit. Singletons with activity against Plasmodium parasites were active against one or more human cell lines, with only NCGC00018296 lacking measurable activity against human cells.
DISCUSSION
In the ongoing battle to develop new and effective treatments for malaria, two general screening approaches have been employed for early lead compound identification. In the first, antiparasitic compounds are identified by phenotypic screening of Plasmodium parasites, followed by iterative rounds of structure-activity-based analog optimization. One major advantage of this approach is that it selects for compounds that have the ability to access parasitic targets and, consequently, kill parasites. However, the identification of the parasite cellular target(s) can be challenging, limiting target structure-based drug design, structure-activity relationship (SAR)-based optimization, and directed evolution of the target to assess resistance risks. Nevertheless, this method has yielded a suite of promising potential therapeutic leads, with several first-in-class small molecules being identified from this approach (19).
The second general screening approach begins with a validated target, typically, a purified protein with in vitro activity, and then uses iterative SAR analyses to improve the activities of hit compounds. While this approach is appealing because it allows improvement of inhibitors on the basis of interactions with the known target (often with information about the atomic structure of the target), there are several drawbacks. Hurdles with this approach include balancing of the structural requirements for target inhibition with those required for delivery of the inhibitor to the target in vivo and the need to understand the function and role of the target in order to develop an appropriate assay. Further, confirming that observed cellular toxicity is due to target inhibition and not the result of unpredicted off-target activity is challenging. Successful targets have been described, however, with the dihydroorotate dehydrogenase (DHODH) inhibitors in development being a notable example (20).
Here, we have pursued a target-based screen for inhibitors of the P. falciparum HK. PfHK catalyzes the first step in glycolysis, a pathway critical for parasite survival. Previous screening of compounds from a small collection of HK inhibitors with activity against enzymes from other parasites and humans revealed that PfHK inhibitors are toxic to cultured parasites, suggesting that the enzyme can be accessed by small-molecule inhibitors, supporting the drive to identify new inhibitor chemotypes for further development.
Four structurally related groups of inhibitors were identified from the screen. Cluster 1 aporphine compounds belong to a family of compounds with an interesting and contentious history as antimalarial compounds. More than 120 years ago, morphine was appreciated to improve the effectiveness of quinine in the treatment of malarial neuralgia, although it is unclear if this was a consequence of antiparasitic activity or analgesic behavior (21). More recently, the complex alkaloid cocktail that makes up opium (of which morphine is a key component) has yielded other antiparasitic compounds. For example, structural analogs of the benzylisoquinoline alkaloid precursor of morphine, reticuline, have antimalaria properties (22, 23).
We tested ∼20 aporphine analogs in our library, and this class of compounds showed some promising results in the PfHK biochemical assay. In terms of their specificity and selectivity, we assessed their activity profiles in >300 different assays, including both biochemical and phenotypic screenings, and found that they were active in ∼15 to 20% of the assays, indicating a level of promiscuity. This observation, along with the very modest antiplasmodial activity of the cluster (Table 3), suggests that further investment in SAR analysis may be of limited value.
The screen also identified several previously described antiplasmodial compounds or structural analogs of antiparasitics as PfHK inhibitors. Lonafarnib, belonging to a group of protein farnesyltransferase (PFT) inhibitors (PFTIs) with reported potencies against several parasite species, including P. falciparum (EC50 = 3 μM) (24), was a modest inhibitor of PfHK (IC50 = 10.9 μM). Unlike other reported potent PFTIs displaying low-nanomolar EC50s against the Plasmodium parasites, the limited potency of lonafarnib against the Plasmodium PFT (IC50 > 250 nM) makes it unclear if the modest activity against the parasite (EC50 = 3 μM) is a consequence of PFT inhibition or perhaps the result of polypharmacology, including PfHK inhibition (25).
The limited antiparasitic activity of the PfHK inhibitors (8 inhibitors at 10 μM demonstrated >45% inhibition of parasite growth) suggests that access to the target may be a limiting factor. However, structural comparison of the hits with the hits from Tres Cantos Antimalarial Set (TCAMS) public database of antiplasmodial compounds (https://www.ebi.ac.uk/chemblntd/download/#tcams_dataset) revealed that several of the compounds in the TCAMS database share scaffolds with the hits identified here. These findings, along with the active and inactive analogs, will be taken together to support future lead optimization that may overcome liabilities of target access.
Conclusions.
The primary assay described herein is robust and has allowed the identification of scaffolds that could be the starting point for optimization of the SARs of inhibitors of PfHK, a target in the parasite's glycolytic pathway. Indeed, some of the compounds identified were active against the parasite in secondary assays and had little to no toxicity toward human cells. The availability of this qHTS assay and ADP-Glo counterassay will enable screening of additional libraries, such as the Molecular Libraries Small Molecule Repository (MLSMR), which contains nearly 400,000 compounds.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sam Michael and Paul Shinn at NCATS and their teams for assistance with compound management and automation. We also thank Kyle R. Brimacombe for generating Fig. 1.
Funding Statement
This work was supported in part by U.S. National Institutes of Health grant 1R03HD081723 to J.C.M. and in part by the Intramural Research Program of the NIH, NCATS, and the Fiske Drug Discovery Lab at the University of Virginia.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00914-16.
REFERENCES
- 1.World Health Organization. 2015. World malaria report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Olszewski KL, Mather MW, Morrisey JM, Garcia BA, Vaidya AB, Rabinowitz JD, Llinas M. 2010. Branched tricarboxylic acid metabolism in Plasmodium falciparum. Nature 466:774–778. doi: 10.1038/nature09301. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Vaidya AB, Mather MW. 2009. Mitochondrial evolution and functions in malaria parasites. Annu Rev Microbiol 63:249–267. doi: 10.1146/annurev.micro.091208.073424. [DOI] [PubMed] [Google Scholar]
- 4.Roth EF Jr, Raventos-Suarez C, Perkins M, Nagel RL. 1982. Glutathione stability and oxidative stress in P. falciparum infection in vitro: responses of normal and G6PD deficient cells. Biochem Biophys Res Commun 109:355–362. doi: 10.1016/0006-291X(82)91728-4. [DOI] [PubMed] [Google Scholar]
- 5.Pfaller MA, Krogstad DJ, Parquette AR, Nguyen-Dinh P. 1982. Plasmodium falciparum: stage-specific lactate production in synchronized cultures. Exp Parasitol 54:391–396. doi: 10.1016/0014-4894(82)90048-0. [DOI] [PubMed] [Google Scholar]
- 6.Vander Jagt DL, Hunsaker LA, Campos NM, Baack BR. 1990. d-Lactate production in erythrocytes infected with Plasmodium falciparum. Mol Biochem Parasitol 42:277–284. doi: 10.1016/0166-6851(90)90171-H. [DOI] [PubMed] [Google Scholar]
- 7.Slavic K, Straschil U, Reininger L, Doerig C, Morin C, Tewari R, Krishna S. 2010. Life cycle studies of the hexose transporter of Plasmodium species and genetic validation of their essentiality. Mol Microbiol 75:1402–1413. doi: 10.1111/j.1365-2958.2010.07060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Schalkwyk DA, Priebe W, Saliba KJ. 2008. The inhibitory effect of 2-halo derivatives of d-glucose on glycolysis and on the proliferation of the human malaria parasite Plasmodium falciparum. J Pharmacol Exp Ther 327:511–517. doi: 10.1124/jpet.108.141929. [DOI] [PubMed] [Google Scholar]
- 9.Muller S. 2004. Redox and antioxidant systems of the malaria parasite Plasmodium falciparum. Mol Microbiol 53:1291–1305. doi: 10.1111/j.1365-2958.2004.04257.x. [DOI] [PubMed] [Google Scholar]
- 10.Harris MT, Walker DM, Drew ME, Mitchell WG, Dao K, Schroeder CE, Flaherty DP, Weiner WS, Golden JE, Morris JC. 2013. Interrogating a hexokinase-selected small-molecule library for inhibitors of Plasmodium falciparum hexokinase. Antimicrob Agents Chemother 57:3731–3737. doi: 10.1128/AAC.00662-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharlow ER, Lyda TA, Dodson HC, Mustata G, Morris MT, Leimgruber SS, Lee KH, Kashiwada Y, Close D, Lazo JS, Morris JC. 2010. A target-based high throughput screen yields Trypanosoma brucei hexokinase small molecule inhibitors with antiparasitic activity. PLoS Negl Trop Dis 4:e659. doi: 10.1371/journal.pntd.0000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, Zheng W, Austin CP. 2006. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci U S A 103:11473–11478. doi: 10.1073/pnas.0604348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanega C, Shen M, Mott BT, Thomas CJ, MacArthur R, Inglese J, Auld DS. 2009. Comparison of bioluminescent kinase assays using substrate depletion and product formation. Assay Drug Dev Technol 7:606–614. doi: 10.1089/adt.2009.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis MI, Auld DS, Inglese J. 2016. Bioluminescence methods for assaying kinases in quantitative high-throughput screening (qHTS) format applied to Yes1 tyrosine kinase, glucokinase, and PI5P4Kalpha lipid kinase. Methods Mol Biol 1360:47–58. doi: 10.1007/978-1-4939-3073-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis MI, Sasaki AT, Shen M, Emerling BM, Thorne N, Michael S, Pragani R, Boxer M, Sumita K, Takeuchi K, Auld DS, Li Z, Cantley LC, Simeonov A. 2013. A homogeneous, high-throughput assay for phosphatidylinositol 5-phosphate 4-kinase with a novel, rapid substrate preparation. PLoS One 8:e54127. doi: 10.1371/journal.pone.0054127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel PR, Sun H, Li SQ, Shen M, Khan J, Thomas CJ, Davis MI. 2013. Identification of potent Yes1 kinase inhibitors using a library screening approach. Bioorg Med Chem Lett 23:4398–4403. doi: 10.1016/j.bmcl.2013.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasgar A, Shinn P, Jadhav A, Auld D, Michael S, Zheng W, Austin CP, Inglese J, Simeonov A. 2008. Compound management for quantitative high-throughput screening. JALA Charlottesville VA 13:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chambers JW, Fowler ML, Morris MT, Morris JC. 2008. The anti-trypanosomal agent lonidamine inhibits Trypanosoma brucei hexokinase 1. Mol Biochem Parasitol 158:202–207. doi: 10.1016/j.molbiopara.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Guiguemde WA, Shelat AA, Garcia-Bustos JF, Diagana TT, Gamo FJ, Guy RK. 2012. Global phenotypic screening for antimalarials. Chem Biol 19:116–129. doi: 10.1016/j.chembiol.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coteron JM, Marco M, Esquivias J, Deng X, White KL, White J, Koltun M, El Mazouni F, Kokkonda S, Katneni K, Bhamidipati R, Shackleford DM, Angulo-Barturen I, Ferrer SB, Jimenez-Diaz MB, Gamo FJ, Goldsmith EJ, Charman WN, Bathurst I, Floyd D, Matthews D, Burrows JN, Rathod PK, Charman SA, Phillips MA. 2011. Structure-guided lead optimization of triazolopyrimidine-ring substituents identifies potent Plasmodium falciparum dihydroorotate dehydrogenase inhibitors with clinical candidate potential. J Med Chem 54:5540–5561. doi: 10.1021/jm200592f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartholow R. 1878. A practical treatise on materia medica and therapeutics, New and enlarged edition. D. Appleton, New York, NY. [Google Scholar]
- 22.Allen RS, Millgate AG, Chitty JA, Thisleton J, Miller JA, Fist AJ, Gerlach WL, Larkin PJ. 2004. RNAi-mediated replacement of morphine with the nonnarcotic alkaloid reticuline in opium poppy. Nat Biotechnol 22:1559–1566. doi: 10.1038/nbt1033. [DOI] [PubMed] [Google Scholar]
- 23.Angerhofer CK, Guinaudeau H, Wongpanich V, Pezzuto JM, Cordell GA. 1999. Antiplasmodial and cytotoxic activity of natural bisbenzylisoquinoline alkaloids. J Nat Prod 62:59–66. doi: 10.1021/np980144f. [DOI] [PubMed] [Google Scholar]
- 24.Nallan L, Bauer KD, Bendale P, Rivas K, Yokoyama K, Horney CP, Pendyala PR, Floyd D, Lombardo LJ, Williams DK, Hamilton A, Sebti S, Windsor WT, Weber PC, Buckner FS, Chakrabarti D, Gelb MH, Van Voorhis WC. 2005. Protein farnesyltransferase inhibitors exhibit potent antimalarial activity. J Med Chem 48:3704–3713. doi: 10.1021/jm0491039. [DOI] [PubMed] [Google Scholar]
- 25.Wiesner J, Kettler K, Sakowski J, Ortmann R, Katzin AM, Kimura EA, Silber K, Klebe G, Jomaa H, Schlitzer M. 2004. Farnesyltransferase inhibitors inhibit the growth of malaria parasites in vitro and in vivo. Angew Chem Int Ed Engl 43:251–254. doi: 10.1002/anie.200351169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.