Abstract
Teicoplanin is a key drug for the treatment of multiresistant staphylococcal bone and joint infections (BJI), yet can only be administered via a parenteral route. The objective of this study was to evaluate the safety and tolerability of subcutaneous (s.c.) teicoplanin for that indication over 42 days. Thirty patients with Gram-positive cocci BJI were included. Once the target of 25 to 40 mg/liter trough serum concentration was achieved, treatment was switched from an intravenous to an s.c. route. No discontinuation of teicoplanin related to injection site reaction and no severe local adverse event were observed. On multivariate analysis, better tolerability was observed at the beginning of treatment, in patients over 70 years old, and for dosages less than 600 mg. In conclusion, we recommend s.c. administration of teicoplanin when needed.
TEXT
The use of a glycopeptide (teicoplanin or vancomycin) for 6 to 12 weeks is recommended to treat infection due to Gram-positive bacteria, most commonly involved in bone and joint infections (BJI), when the isolated strain is resistant to several class of antibiotics (1). Teicoplanin, generally preferred over vancomycin because of lower nephrotoxicity and longer elimination half-life (2, 3), has a marketing authorization exclusively for intramuscular (i.m.) or intravenous (i.v.) administration; the painful i.m. route is not routinely used, and long-term daily i.v. administration usually requires placement of a central venous catheter or an implantable chamber (1), which constitutes an invasive procedure and exposes the patient to the risk of mechanical, infectious, and thrombotic complications (4, 5).
The efficacy and nephrotoxicity of teicoplanin depend on plasma levels, and a trough serum concentration (Cmin) between 25 and 40 mg/liter appears to achieve the best benefit/risk balance (2, 6, 7).
When teicoplanin is needed, subcutaneous (s.c.) administration should offer several advantages: preservation of peripheral veins, absence of implantable device-related complications, easier outpatient management, reduction of health care costs, and possibly improved patient comfort.
Few clinical studies demonstrated comparable efficacy of the s.c. route to the i.v. route (8, 9). However, the tolerability of treatment administered for more than 2 weeks has not been evaluated.
We therefore conducted a prospective open-label study to evaluate the tolerability of s.c. teicoplanin for the treatment of BJI and the usefulness of drug concentration monitoring in dosage adjustments.
(Portions of the results of this study have been presented previously at Réunion Interdisciplinaire de Chimiothérapie Anti-Infectieuse 2014, Paris, France [64-O], at Journées Nationales d'Infectiologie 2015, Nancy, France [IOS 04], and at Congrès des Centres de Référence pour le Traitement des Infections Ostéo-Articulaires Complexes 2015, Lille, France [oral communication]).
This study was conducted at the Amiens University Hospital (France) from March 2013 to June 2015, the institutional ethics review board of which provided ethics approval.
The primary objective was to evaluate the tolerability at the injection site of subcutaneous teicoplanin for the treatment of BJI. Each evaluation was scored according to the Common Terminology Criteria for Adverse Events (CTCAE) (10). The secondary objective was to analyze the usefulness of teicoplanin dosage in maintaining its Cmin within a therapeutic range.
We prospectively included adult patients hospitalized for BJI (arthritis, spondylodiscitis, and osteitis, with or without foreign bodies) with Gram-positive bacteria susceptible to teicoplanin (MIC, ≤4 mg/liter) and resistant to beta-lactams, lincosamides, and quinolones, or patients who were allergic to penicillin or rifampin. Remission was defined as the absence of relapse 10 months after the end of treatment. Failure was defined by the association of clinical and radiological signs of culture-proven infection with the same strain. Remission was defined as the absence of infectious symptoms in patients on long-term oral antimicrobial suppression after surgery and teicoplanin therapy.
Treatment was initiated with 5 doses of 12 mg/kg of body weight twice daily (loading phase) and then administered once daily (maintenance dose), adjusted to renal function (1).
The first Cmin was determined 24 h after the end of the loading phase and then every 48 h. The dosage was then adapted to obtain a Cmin between 25 and 40 mg/liter; once it reached this target, the treatment was switched to the s.c. route at the same dosage. The Cmin was determined after 48 to 96 h and then every 10 to 15 days for 42 days.
After reconstituting, a maximum of 800 mg of teicoplanin solution was diluted into 50-ml bags of 0.9% sodium chloride (NaCl) and then administered over 30 min (1.7 ml/min). For teicoplanin doses of >800 mg, the total dose, divided in two, was administered at 2 different subcutaneous injection sites.
The injection site was changed daily, with rotation between various zones as well as within the same zone and varied from one patient to another depending on local tolerance. Teicoplanin was administered by gravity i.v. infusion (from i.v. pole) in inpatients and by nonelectronic elastomeric infusers for outpatients.
Clinical assessment of tolerability was performed by the same clinician for all patients; in-site reactions (ISR) were scored according to the Common Terminology Criteria for Adverse Events (CTCAE) (10). Pain, evaluated by numerical rating scale, was considered to be significant for a score strictly greater than 3. Safety was evaluated on inclusion, during the first 48 h following initiation of treatment via the s.c. route (D2), and then at each visit (D14, D28, and D42) on the presence or absence of clinical sign of hypersensitivity, and on the dosage of plasma creatinine levels and estimation of clearance by the simplified Modification of Diet in Renal Disease (MDRD) formula (11).
Serum teicoplanin assays were performed by an automated quantitative microsphere system (QMS on Indiko apparatus; Thermo Fisher), which is based on competition between teicoplanin present in samples and teicoplanin bound to microspheres for anti-teicoplanin antibodies present in the reagent. The lower limit of quantification is 4 μg/ml, the upper limit of linearity is 80 μg/ml, and the uncertainty is 15%.
For a description of patient characteristics, quantitative variables were described by the mean and range. The association between tolerability during treatment and the various clinical parameters was analyzed with a proportional hazards mixed model. All statistical analyses were performed with SAS 9.2 software (SAS Institute, Inc., Cary, NC, USA).
Thirty patients were included, and 25 completed the study (Fig. 1). The detailed characteristics of the population are provided in Table 1.
FIG 1.
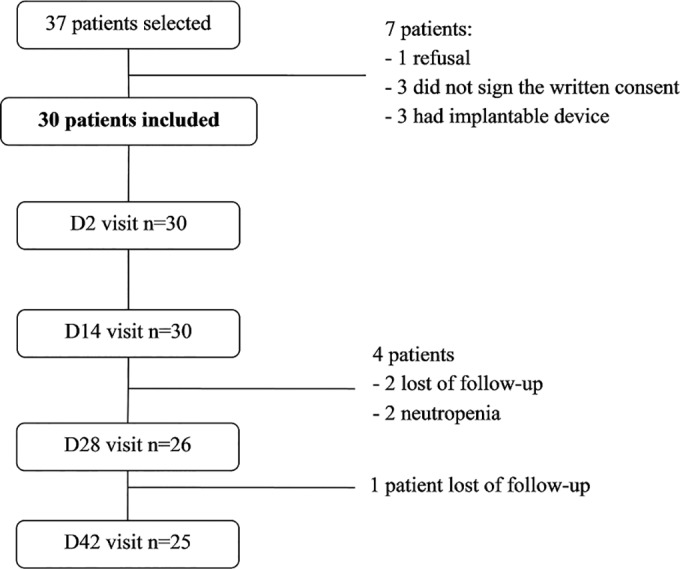
Flow chart of patient groups and visits.
TABLE 1.
Population characteristics
| Characteristica | Value (n = 30) |
|---|---|
| Age (mean [range]) (yr) | 62.4 (24–89) |
| Sex ratio (male/female) | 2.33 |
| BMI (mean [range]) (kg/m2) | 27.3 (19.6–41.8) |
| Wt (mean [range]) (kg) | 78.7 (50–130) |
| Characteristics of infection (no. [%]) | |
| Prosthesis | 20 (67) |
| Hip | 12 (40) |
| Knee | 8 (27) |
| Nonprosthetic material | 5 (17) |
| Absence of material | 5 (17) |
| Course of infection (no. [%]) | |
| Acute | 6 (20) |
| Chronic | 24 (80) |
| Bacteria isolated (n = 34) (no. [%]) | |
| Methicillin-resistant CoNS | 13 (38) |
| Methicillin-resistant S. aureus | 12 (35) |
| Enterococcus spp. | 4 (12) |
| Corynebacterium spp. | 4 (12) |
| Propionibacterium acnes | 1 (3) |
| Associated surgical treatment (no. [%]) | 26 (87) |
| Antibiotic in association (no. [%]) | |
| Rifampin | 18 (60) |
| Linezolid | 5 (17) |
| Clindamycin | 3 (10) |
| Fusidic acid | 2 (7) |
| Amoxicillin | 1 (3) |
| None | 1 (3) |
| Mean estimated creatinine clearance by MDRD formula rate (ml/min) | 108.6 |
BMI, body mass index.
A single bacterial strain was isolated in 26 cases of BJI, and multiple strains were isolated in 4 cases. The teicoplanin MIC was between 0.75 and 2 mg/liter and between 0.19 and 4 mg/liter for Staphylococcus aureus and coagulase-negative staphylococci (CoNS), respectively. All staphylococci isolated in our study were methicillin resistant.
Teicoplanin was also prescribed when other antibiotics were inappropriate for 5 patients: 3 patients were allergic or intolerant to penicillin or rifampin, and 2 failed to respond to initial treatment.
The clinical, biological, and radiological assessment showed cure in 18/25 patients (72%) after a mean follow-up of 19 months (range, 10 to 29 months), remission in 3/25 patients (12%), and failure in 4/25 patients (16%).
A total of 1,460 s.c. injections were performed in this study. The proportion of patients for whom the daily dose was divided into 2 s.c. injections per day was 12/30 patients at D2, 10/30 patients at D14, 6/26 patients at D28, and 4/25 patients at D42.
No severe in-site reaction and no discontinuation of teicoplanin due to ISRs were observed (Table 2). On multivariate analysis, better tolerability was observed at the beginning of treatment (P = 0.0017), in patients over 70 years old (P = 0.0273), and when using a daily dosage of <600 mg (P = 0.0195). The ISRs (apart from local warmth) were less frequent at the start of treatment (D2) than at other evaluation time points (Table 2).
TABLE 2.
Injection site reactions observed during the study
| Reaction | No. (%) by visita |
|||
|---|---|---|---|---|
| D2 | D14 | D28 | D42 | |
| n | 30 | 30 | 26 | 25 |
| Grade | ||||
| No local reaction | 7 (23) | 3 (10) | 3 (12) | 3 (12) |
| 1 | 9 (30) | 5 (17) | 5 (19) | 6 (24) |
| 2 | 14 (47) | 22 (73) | 18 (69) | 16 (64) |
| ≥3 | 0 | 0 | 0 | 0 |
| Pain | 11 (37) | 15 (50) | 13 (50) | 9 (36) |
| Swelling | 11 (37) | 18 (60) | 19 (73) | 17 (68) |
| >5 cm | 7 (23) | 9 (30) | 9 (35) | 11 (44) |
| Erythema | 15 (50) | 21 (70) | 14 (54) | 13 (52) |
| >5 cm | 9 (30) | 10 (33) | 8 (31) | 6 (24) |
| Warmth | 12 (40) | 11 (36.7) | 10 (39) | 6 (24) |
| Itching | 5 (17) | 9 (30) | 7 (27) | 7 (28) |
| Hematoma | (0) | 3 (10) | 3 (12) | 2 (8) |
| Telangiectasia | 1 (3) | (0) | (0) | (0) |
D2, D14, D28, and D42 are 2, 14, 28, and 42 days after s.c. teicoplanin initiation, respectively.
Local injection site reactions usually lasted only a few minutes and were mainly mild (Table 3). Adverse reactions were recurrent in one-third of cases throughout the study. Thirty percent (9/30) of patients never experienced any pain during the study, while 26.7% (8/30) experienced pain throughout the study. Discontinuation of treatment was necessary in two cases because of neutropenia, and three patients with chronic renal failure and vascular disease presented a slight deterioration of renal function with return to their baseline state after the study.
TABLE 3.
Proportion of patients according to the duration of local reactions during and after s.c. administration of teicoplanin
| Visit (n) | No. (%) by reaction type |
||||
|---|---|---|---|---|---|
| Not concerned, no local reaction | Only during injection | During and for several min after injection | During and for several h after injection | >1 day after injection | |
| D2 (30) | 20 (67) | 3 (10) | 5 (17) | 1 (3) | 1 (3) |
| D14 (30) | 14 (47) | 3 (10) | 5 (17) | 3 (10) | 5 (17) |
| D28 (26) | 14 (54) | 2 (8) | 7 (27) | 2 (8) | 1 (4) |
| D42 (25) | 15 (60) | 4 (16) | 2 (8) | 4 (16) | 0 |
The Cmin was higher after 48 h of s.c. administration than via the i.v. route: a mean difference of +6.51 mg/liter was observed (confidence interval, 3.05 to 9.97; P < 0.001) after adjustment for dosage (P = 0.5823) and glomerular filtration rate (GFR) (P = 0.0180).
The mean ± standard deviation (SD) dosage during treatment decreased from 11.69 ± 2.77 mg/kg (range, 6.06 to 19.04 mg/kg) to 8.03 ± 4.06 mg/kg (range, 3.84 to 16.66 mg/kg) between D2 and D42 (P < 0.0001), with no significant difference in creatinine clearance (P = 0.16; n = 27) and serum protein levels (P = 0.27; n = 27) (Fig. 2).
FIG 2.
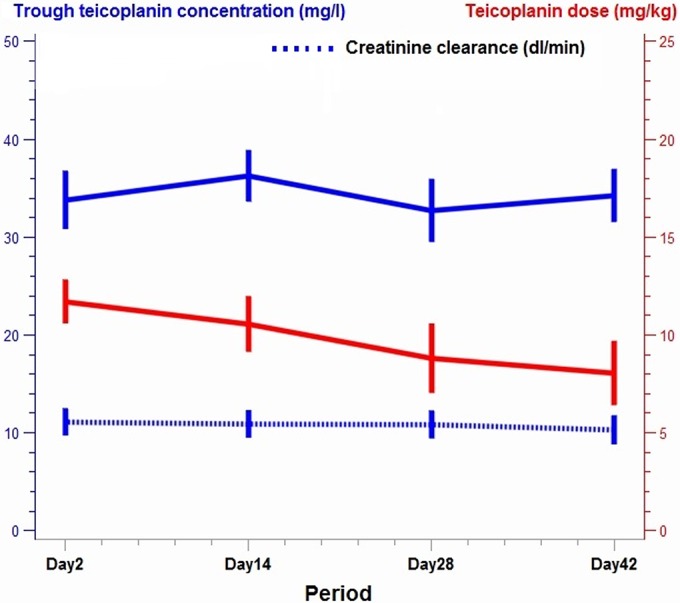
Course of Cmin, creatinine clearance, and dosage during s.c. teicoplanin therapy.
This prospective study found a good tolerability of s.c. administration of teicoplanin for the management of multiresistant Gram-positive cocci BJI, with very mild pain and transitory side effects, like swelling and erythema. Although these side effects seemed to increase during the study, no treatment interruption because of intolerance was observed.
Not surprisingly, we observed a better local tolerance at the beginning of s.c. injections of teicoplanin, probably due to lesser local trauma at the injection sites at the beginning of antibiotic therapy than at the end. However, while ISRs increased during the study, better long-term tolerance was observed among the oldest patients, possibly related to looser s.c. tissue in older patients. Doses lower than 600 mg improved tolerability, and at the beginning of treatment, more patients had the divided doses in two sites. On the contrary, tolerability was not improved by the end of the study, probably due to more-frequent injections in a single site as the daily dose decreased below 800 mg but remained above 600 mg.
Intravenous administration of teicoplanin results in higher peak concentrations than with the s.c. route, but higher trough concentrations are obtained with the s.c. route (7). This is confirmed in the present study, as the teicoplanin Cmin at the time of administration route switch was significantly higher with the s.c. route than with the i.v. route (mean difference, 6.51 mg/liter), which could be explained by an accumulation of teicoplanin. This study confirms the need for therapeutic drug monitoring of teicoplanin in order to offer an optimal dosage and avoid toxicity. No significant variation of GFR or serum proteins was observed throughout treatment, allowing accurate analysis of the various teicoplanin Cmin results.
In conclusion, this first prospective study performed in patients treated with subcutaneous injections of teicoplanin for 6 weeks showed acceptable tolerability and safety. Our results stress the importance of drug monitoring to avoid toxicity as teicoplanin accumulates over time. We therefore recommend the use of s.c. teicoplanin for long-term treatment of BJI when needed.
ACKNOWLEDGMENTS
We declare no conflicts of interest.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Société de Pathologie Infectieuse de Langue Française (SPILF), Collège des Universitaires de Maladies Infectieuses et Tropicales (CMIT), Groupe de Pathologie Infectieuse Pédiatrique (GPIP), Société Française d'Anesthésie et de Réanimation (SFAR), Société Française de Chirurgie Orthopédique et Traumatologique (SOFCOT), Société Française D' Nospace Hygiènehospitalière (SFHH), Société Française de Médecine Nucléaire (SFMN), Société Française de Médecine Physique et de Réadaptation (SOFMER), Société Française de Microbiologie (SFM), Société Française de Radiologie (SFR-Rad), Société Française de Rhumatologie (SFR-Rhu) 2009. Clinical practice recommendations. Osteoarticular infections on materials (prosthesis, implant, osteosynthesis). Med Mal Infect 39:815–863. (In French.) [DOI] [PubMed] [Google Scholar]
- 2.Svetitsky S, Leibovici L, Paul M. 2009. Comparative efficacy and safety of vancomycin versus teicoplanin: systematic review and meta-analysis. Antimicrob Agents Chemother 53:4069–4079. doi: 10.1128/AAC.00341-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood MJ. 2000. Comparative safety of teicoplanin and vancomycin. J Chemother 12(Suppl 5):S21–S25. [DOI] [PubMed] [Google Scholar]
- 4.Mimoz O, Lucet JC, Kerforne T, Pascal J, Souweine B, Goudet V, Mercat A, Bouadma L, Lasocki S, Alfandari S, Friggeri A, Wallet F, Allou N, Ruckly S, Balayn D, Lepape A, Timsit JF, CLEAN Trial Investigators. 2015. Skin antisepsis with chlorhexidine-alcohol versus povidone iodine-alcohol, with and without skin scrubbing, for prevention of intravascular-catheter-related infection (CLEAN): an open-label, multicentre, randomised, controlled, two-by-two factorial trial. Lancet 386: 2069–77. doi: 10.1016/S0140-6736(15)00244-5. [DOI] [PubMed] [Google Scholar]
- 5.Parienti JJ, Mongardon N, Mégarbane B, Mira JP, Kalfon P, Gros A, Marqué S, Thuong M, Pottier V, Ramakers M, Savary B, Seguin A, Valette X, Terzi N, Sauneuf B, Cattoir V, Mermel L, du Cheyron D, 3SITES Study Group. 2016. Intravascular complications of central venous catheterization by insertion site. N Engl J Med 373:1220–1229. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg R. 1990. Treatment of bone, joint and vascular-access-associated Gram-positive bacterial infections with teicoplanin. Antimicrob Agents Chemother 34:2392–2397. doi: 10.1128/AAC.34.12.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson AP. 2000. Clinical pharmacokinetics of teicoplanin. Clin Pharmacokinet 39:167–183. doi: 10.2165/00003088-200039030-00001. [DOI] [PubMed] [Google Scholar]
- 8.Barbot A, Venisse N, Rayeh F, Bouquet S, Debaene B, Mimoz O. 2003. Pharmacokinetics and pharmacodynamics of sequential intravenous and subcutaneous teicoplanin in critically ill patients without vasopressors. Intensive Care Med 29:1528–1534. doi: 10.1007/s00134-003-1859-z. [DOI] [PubMed] [Google Scholar]
- 9.Carpentier E, Roméo B, El Samad Y, Geslin-Lichtenberger L, Maingourd Y, Tourneux P. 2013. Subcutaneous teicoplanin for children with infectious endocarditis. Arch Pediatr 20:775–778. (In French.) doi: 10.1016/j.arcped.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Department of Health and Human Services. 2016. Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. U.S. Department of Health and Human Services, Washington, DC: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470. [DOI] [PubMed] [Google Scholar]


