Abstract
Interleukin-23 (IL-23) is a survival factor for a newly described population of T lymphocytes, namely Th-17 cells, that secrete IL-17, tumor necrosis factor- alpha (TNFα) and IL-6. It has been shown that Th-17 cells are a pathogenic T cell subset involved in autoimmune and chronic inflammatory diseases. Based on the increasing evidence of immune dysfunction in autism, including possible autoimmune and inflammatory processes, we hypothesized that Th-17 cells, a T cell lineage that has not been previously examined in this disorder, may be altered in autism. To assess the potential role, if any, of Th-17 cells in autism, we analyzed plasma samples obtained from children ranging in age from 2-5 years with a diagnosis of autism and age-matched typically developing controls for the presence of IL-17 and IL-23 cytokines. Plasma samples from 40 children with autism including 20 children with a regressive form of autism, 20 with early onset and no regression and 20 typically developing age-matched control children were analyzed for IL-17 and IL-23, under the hypothesis that altered number and function of Th-17 cells would directly correlate with altered levels of IL-17 and IL-23 in the plasma. In this study, we were able to demonstrate that IL-23 cytokine levels were significantly different in children with autism compared with age-matched controls, a finding primarily driven by children with early onset autism. In contrast, there were no statistical differences in IL-17 levels autism compared with age-matched typically developing controls. This is the first study to report altered IL-23 production in autism. The decreased plasma IL-23 production observed in children with autism warrants further research as to its affect on the generation and survival of Th-17 cells, a subset important in neuroinflammatory conditions that may include autism.
Keywords: Autism, Th-17 cells, inflammation, IL-23, neurodevelopment
Introduction
Autism (AU) is a neurodevelopmental disorder presenting in early childhood with specific social and behavioral abnormalities. Autism is generally evident by 3 years of age. A regressive form of autism has been described for children who meet early developmental milestones up to 12-18 months of age but then lose acquired speech and/or social skills. The occurrence and timing of regression has led to an interest in early life infectious and environmental exposures that occur within this same period and have been hypothesized to play a role in the genesis of autism disorder. To date, no concrete evidence for a direct causative early life exposure has been defined. However, many researchers have described various immunological abnormalities in subjects with autism, specifically in levels of inflammatory mediators and autoimmune responses[1-3]. This suggests that the immune system in some children with autism may be dysregulated. The neuroimmune hypothesis of autism suggests that these dysregulated immune responses either directly or indirectly adversely affect the course of neurodevelopment in the brain, leading to the development of autism[3]. Immune abnormalities include increased inflammatory cytokines in the plasma and CNS, specifically neuroinflammatory cytokine interleukin-6 (IL-6)[4, 5], proinflammatory cytokine tumor necrosis factor alpha (TNFα)[4-6] and chemoattractant cytokine macrophage chemotactic protein-1 (MCP-1)[7]. Despite this evidence, a clear role for the inflammatory process in the pathogenesis of autism has yet to be defined.
In recent studies, a distinct subset of T helper cells (Th-17) has been described that are unique from the traditional paradigm of Th1 and Th2 cells. For more than 30 years, CD4+ T-helper cells have been divided into two main subsets, Th1 and Th2 cells, based on their cytokine production patterns and biological effects. Th1 cells are associated with an anti-intracellular pathogen-driven cell-mediated immune response, produce interferon-gamma (IFNγ) and IL-2 and are long thought to initiate and mediate tissue damage. Conversely, Th2 cells are efficient at directing primarily humoral responses against extracellular pathogens and produce IL-4, IL-5 and IL-13. In contrast, Th-17 cells are characterized by their production of IL-17 in response to the cytokine IL-23[8, 9] and produce a distinct pattern of cytokines, including IL-17, IL-6, TNFα but not IFNγ and IL-4 [9-13]. IL-17 production by Th-17 cells has been associated with inflammation and development of autoimmune disease, such as chronic intestinal inflammation[14-16], rheumatoid arthritis[17-19] and multiple sclerosis[20-22]. Neutralization of IL-17 can decrease disease severity[22-24].
Further research into the Th-17 lineage has revealed that IL-23 acts as a survival factor for memory and effector Th-17 cells[8-9]. IL-23 is a heterodimer that has structural similarities with IL-12, both sharing a IL-12 p40 subunit. However, IL-23 has a distinctive second subunit, p19, that is not shared with IL-12[25]. Since IFNγ, which is increased in many chronic inflammatory and autoimmune disease models, induces production of IL-12, Th1 cells were interpreted as playing a decisive role in disease pathogenesis; however, a more pivotal role may actually be attributable to IL-23 production[17, 26, 27]. In co-culture cellular studies, IL-23 stimulated CD4+ T cells produced an increased expression of the proinflammatory cytokines IL-17, IL-6 and TNFα[8-9]. Current research examining IL-12p40 compared with IL-23p19 in autoimmune disease pathogenesis revealed that disease progression was driven by IL-23 and not by IL-12[17, 26-28]. Furthermore, anti-IL-23 therapy decreases symptoms of experimental automimmune encephalomyelitis in an animal model of multiple sclerosis[22]. With the administration of anti-IL-23 therapy, not only are IL-17 serum levels reduced, but CNS expression of IFNγ, IL-17, IL-6 and TNFα are also decreased[22]. Interestingly, aside from IL-23, transforming growth factor- beta (TGF-β), which has a modulatory effect on Th1 and Th2 development[29], actually increases IL-17 expression[10, 12].
Recent evidence suggests that Th-17 cells may be involved in development of autoimmune and inflammatory disease and therefore, its potential role in autism warrants investigation. Th-17 cells produce the cytokines IL-6 and TNFα, which have been shown to be increased in subjects with autism[4-6]. Furthermore, in one recent study, mice that were deficient in a Th-17 cell inhibitor, IL-27, developed severe neuroinflammation following infection with Toxoplasma gondii, a finding that correlated with increased IL-17 production[30]. In this study, researchers found that IL-6 and TGF-β induced this IL-17 response. Furthermore, in a study using anti-IL-23p19 antibody to treat autoimmune encephalomyelitis, CNS expression of IFNγ, IL-17, IL-6 and TNFα were decreased[22]. Combining the neuroinflammatory role of Th-17 cells and the apparent abnormal regulation of IL-6, TNFα and IFNγ in autism, cytokines linked to Th-17 cell generation[10, 12, 30], we hypothesized that abnormal Th-17 cell production may play a role in this disorder. In the current study, we examined the presence of secreted IL-17 and IL-23 in the plasma of children with autism.
Materials and Methods
Study subjects
A total of 60 children ranging in age between 2 to 5 years of age were enrolled in the study. The participants for the study were recruited as part of the CHARGE study[31] at the University of California, Davis. The study was reviewed and approved by the institutional review board of UC Davis. Informed consent was obtained from a legal guardian for each subject. Each participant, including general population age-matched control subjects, was evaluated at the UC Davis M.I.N.D. Institute Clinic. Autism diagnosis was confirmed for the case group using the Autism Diagnostic Interview-Revised (ADI-R)[32] and the Autism Diagnostic Observation Schedule (ADOS)[33-36] in order to determine eligibility for the study. The ADI-R is a well standardized interview designed to produce a diagnostic algorithm for autism spectrum disorders using answers provided by the child's caregiver based on a child's developmental and behavioral history. The ADI-R is appropriate for patients with mental ages of two and above and is consistent with the DSM-IV and ICD-10 definitions of autism. The ADOS is a standardized behavioral observation utilizing semi-structured play based interactions designed to elicit communication, social interactive and imaginary play behaviors typically impaired in children with autism spectrum disorders. Plasma cytokine levels were compared in 40 subjects diagnosed with autism (AU) ranging in age from 2 years 1 month to 4y10m (mean 3y5m, 36 males) who met autism cut-off criteria on both the ADI-R and the ADOS, with 20 subjects who were typically developing (TD) ranging from 2y7m to 5y2m (mean 3y8m, 16 males). The AU groups were further subdivided into either early onset or regressive AU, based on the behavioral information provided through the ADI-R. Individuals meeting AU criteria and who did not have a history of loss of developmental skills on the ADI-R were classified as early onset AU (n=20, ranging from 2y4m to 4y10m, mean 3y3m, 17 males) and subjects who attained skills that they later lost were classified as regressive AU (n=20, ranging from 2y1m to 4y10m, mean 3y6m, 19 males). The typically developing controls were screened with the Social Communication Questionnaire (SCQ)[37] for autistic behaviors and assessed for developmental status with the Mullen Scales of Early Learning (MSEL)[38] and the Vineland Adaptive Behavior Scales (VABS)[39]. Children scored below the cut-off on the SCQ and within 2 standard deviations of the mean on both the MSEL and the VABS in order to be included in the control group.
Plasma IL-17 and IL-23 ELISA
Peripheral blood was collected in acid-citrate-dextrose Vacutainer tubes (BD Biosciences, San Jose, CA) and centrifuged for 10 min at 2300 rpm. Plasma was harvested and stored at -80°C until the date of assay. Plasma samples were thawed and analyzed with IL-17 specific and IL-23 specific Ready-SET-Go! Enzyme-Linked Immunosorbent Assay kits (eBioscience, San Diego, CA) using the manufacturer's protocol. Briefly, plates were coated with monoclonal antibody specific to either IL-17 or the p19 subunit of human IL-23. Either 100μl of plasma or standard was added to appropriate wells for 2 hrs at RT. Samples were run in duplicate. After repeated washing, the plates were incubated with biotin-conjugated detector antibody specific to IL-17 or the p40 subunit of IL-23 for 1 h at RT. The plates were then washed a total of 5 times before incubating for 30 min with Avidin-HRP for color development. The reaction was stopped with sulfuric acid per manufacture's protocol and the optical density determined using a Wallac Victor3 multilabel plate reader (PerkinElmer, Boston, MA) at 450 nm. A reference reading obtained at 530 nm was subtracted from the optical densities. Concentration was determined by comparison of sample OD values to a standard curve. Statistical significance was determined using Student's two-tailed t-test for samples with unequal variances. Significance was accepted if p values were less than 0.05.
Results and Discussion
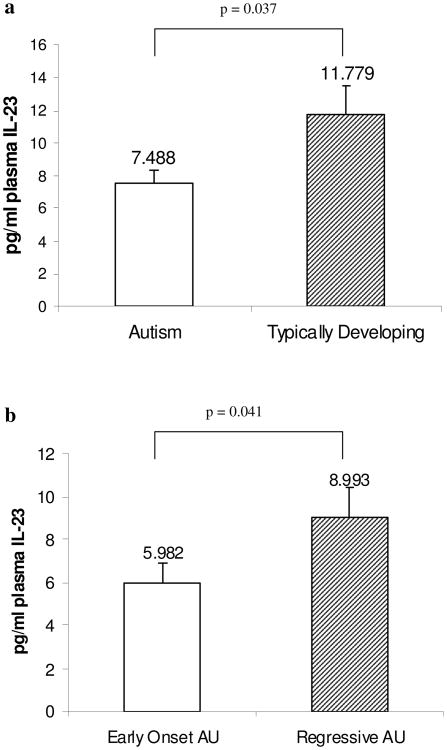
We used ELISA to quantify plasma levels of IL-23 in typically developing children (TD) and children with autism (AU). We were able to detect IL-23 in the ranges of 0.6 pg mL−1 to 30 pg mL−1. AU subjects had decreased plasma levels of IL-23 compared with TD subjects, matched for age (p=0.037, Fig. 1a). For AU subjects, the median with interquartile range (IQR) IL-23 level was 5.05 (3.51-11.16) pg mL−1 and for the TD subjects it was 12.48 (3.79-17.15) pg mL−1. When AU subjects were subdivided based on regression, those subjects with early onset AU had significantly reduced IL-23 compared with TD controls (p=0.002, Table 1) However, AU with regression had similar levels compared with TD controls (p=0.126, Table 1). Consequently, when early onset AU subjects were compared to those with regressive AU, there were lower levels of IL-23 in those with early onset (median values 4.25 pg mL−1 vs. 5.78 pg mL−1, p=0.041, Fig. 1b, Table 1). ELISA analysis of plasma IL-17 levels revealed that there were no statistically significant differences between AU subjects and TD subjects, although there was a trend for decreased IL-17 in early onset AU (Table 1). It is important to note that the measured IL-17 levels were at the low end of the detectable range (from 0.15 to 9.19 pg mL−1) and detection may be slightly impaired by methodological restriction.
Fig. 1.
Levels of IL-23 in human plasma. Quantitative ELISA analysis of plasma IL-23 in (a) children diagnosed with autism (AU, n=40) compared with typically developing age-matched control children (n=20). Mean IL-23 values plus s.e.m. are shown. (b) Decreased IL-23 was present in plasma in children with early onset autism (n=20) compared with those with regression (n=20)
Table 1.
Plasma IL-23 and IL-17 levels from subjects with autism and typically developing subjects. Median values plus inter quartile ranges of IL-23 and IL-17 plasma levels are shown from children diagnosed with Early onset autism (AU), Regressive AU, combined AU subjects, and typically developing (TD) subjects. IL-23 levels were significantly different between Early onset AU and typically developing subjects using Student's t-test
| Early onset AU (n=20) | Regressive AU (n=20) | Combined AU subjects (n=40) | Typically Developing (n=20) | |
|---|---|---|---|---|
| Median IL-23 (pg mL−1) | 4.25** | 5.78 | 5.05* | 12.48 |
| (Q1-Q3) | (2.82-10.33) | (4.01-14.81) | (3.51-11.16) | (3.79-17.15) |
| Median IL-17 pg mL−1 | 1.32 | 1.78 | 1.37 | 1.81 |
| (Q1-Q3) | (1.01- 1.74) | (1.10- 2.56) | (1.06- 2.36) | (1.27- ,2.18) |
p<0.05,
p<0.005
A unique subset of CD4+ T-helper cells, termed Th-17 cells, have recently been identified that produce a similar set of cytokines found to be upregulated in the blood and CNS of some subjects with autism[4-7, 9-13]. Th-17 cells have been associated with several research models of autoimmune and inflammatory diseases, including multiple sclerosis, inflammatory bowel disease and toxoplasma infection[14-22, 30]. We hypothesized that the immune dysregulation seen in children with autism could potentially be due in part to increased frequency of Th-17 cells, as evinced by increased levels of IL-17 and a survival factor for Th-17 cells, namely IL-23, in the blood. To test this hypothesis, we conducted this current initial study to examine IL-17 and IL-23 plasma levels.
We have shown here that IL-23 levels were reduced in children with autism and that there was no difference in IL-17 levels in children with confirmed autism compared with age-matched typically developing control children. However, we cannot conclusively determine from this study that Th-17 cells are not dysregulated in autism subjects, only that the major cytokine produced by these cells was not dysregulated in this study population. The age of the subjects under investigation, 2 to 5 years of age, may be too young to reliably detect elevated levels of plasma IL-17. This may be reflected in the fact that IL-17 levels were consistently near the limit of detection in both cases and controls. A similar finding, in slightly older control children (mean 6 years), was observed in a pediatric burn study that used Luminex analysis to determine IL-17 levels[40]. Moreover, although our initial results indicate that IL-17 levels were comparable to typically developing subjects, this could be an artifact of the age of the study subjects and less to do with the frequency of Th-17 cells in the blood. Interestingly, while there was a strong positive correlation between IL-23 and IL-17 expression in typically developing children, there was no corresponding correlation between IL-23 and IL-17 levels in children with autism (data not shown). This may suggest that IL-23 production may not stimulate the generation and survival of Th-17 cells in individuals with autism. Recent research has revealed a potential neuroprotective and neurodevelopmental role of CNS “self”-reactive T cells[41], which could potentially extend to the Th-17 subset. If this is the case, IL-23 may play an indirect role in the maintenance of neuroprotection, through its generation of Th-17 cells. A major challenge to this study may be that Th-17 cells are present at low frequency in the blood, which may negatively impact the ability to detect differences in secreted IL-17 in the plasma. Therefore, systemic levels may be less pertinent than local responses as well as the actual number and function of Th-17 cells in autism. Further ongoing studies will directly examine IL-17 expression by CD4+ T cells themselves and may yield more conclusive results. Our observation that the level of IL-23 is decreased in the blood of children with autism, in particular in subjects diagnosed with early onset non-regressive autism, may indicate that the generation and survival of Th-17 cells could in fact be altered in some children with autism. However, other cytokines such as TGF-β that act independently of IL-23 could also stimulate the production of Th-17 cells[10].
This initial report does not rule out a possible causative role of Th-17 cells in the pathology of autism but rather raises additional questions. Indeed, the biology of Th-17 is a new field and the consequence of low frequency as well as high frequency of Th-17 has not yet been elucidated. The biological impact of decreased IL-23 in the plasma of children with early onset autism described in our study is intriguing and requires further study. Early onset autism is thought by some clinicians to have a better prognosis than regressive autism[42]. In this case, decreased production of IL-23 in the plasma of children with early onset autism may suggest a regulatory mechanism that could control the extent and severity of proinflammatory and neuroinflammatory responses demonstrated in some autism cases. More definitive research on the precise nature of immune dysregulation in early onset autism and regressive autism is needed. In this study, we have shown for the first time that IL-23 production in autism subjects is decreased, and suggest that further investigation of this altered cytokine production may have important implications in the generation and survival of Th-17 cells, a subset of cells important in neuroinflammatory and autoimmune conditions.
Acknowledgments
We would like to thank the children and families who participated in this study. We also would like to acknowledge the staff of the UC Davis M.I.N.D. Institute and CHARGE study for their technical support and expertise. This work was funded by grants from NIEHS Children's Center grant (1 P01 ES11269-01), US EPA STAR program grant (R829388), the M.I.N.D. Institute, the Cure Autism Now Foundation, the Ted Lindsay Foundation and a generous gift from the Johnson family.
References
- 1.Ashwood P, Wills S, Van De Water J. The immune response in autism: a new frontier for autism research. J Leukocyte Biol. 2006;80:1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- 2.Cohly HHP, Panja A. Immunological findings in autism. Int Rev Neurobiol. 2005;71:317–341. doi: 10.1016/s0074-7742(05)71013-8. [DOI] [PubMed] [Google Scholar]
- 3.Korvatska E, Van de Water J, Anders TF, Gershwin ME. Genetic and immunologic considerations in autism. Neurobiol Dis. 2002;9:107–125. doi: 10.1006/nbdi.2002.0479. [DOI] [PubMed] [Google Scholar]
- 4.Croonenberghs J, Bosmans E, Deboutte D, Kenis G, Maes M. Activation of the inflammatory response system in autism. Neuropsychobiology. 2002;45:1–6. doi: 10.1159/000048665. [DOI] [PubMed] [Google Scholar]
- 5.Jyonouchi H, Sun SN, Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J Neuroimmunol. 2001;120:170–179. doi: 10.1016/s0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- 6.Ashwood P, Wakefield AJ. Immune activation of peripheral blood and mucosal CD3(+) lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms. J Neuroimmunol. 2006;173:126–134. doi: 10.1016/j.jneuroim.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurobiol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 9.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGF beta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4(+) effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 12.Bettelli E, Carrier YJ, Gao WD, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector T(H)17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 13.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 14.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, Mckenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy CA, Langrish CL, Chen Y, Blumenschein C, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro-and Antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chabaud M, Garnero P, Dayer JM, Guerne PA, Fossiez F, Miossec P. Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine. 2000;12:1092–1099. doi: 10.1006/cyto.2000.0681. [DOI] [PubMed] [Google Scholar]
- 20.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 21.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Langrish CL, Mckenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohn TA, Jennings GT, Hernandez M, Grest P, Beck M, Zou Y, Kopf M, Bachmann MF. Vaccination against IL-17 suppresses autoimmune arthritis and encephalomyelitis. Eur J Immunol. 2006;36:2857–2867. doi: 10.1002/eji.200636658. [DOI] [PubMed] [Google Scholar]
- 24.Koenders MI, Lubberts E, Oppers-Walgreen B, van den Bersselaar L, Helsen MM, Di Padova FE, Boots AMH, Gram H, Joosten LAB, van den Berg WB. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am J Pathol. 2005;167:141–149. doi: 10.1016/S0002-9440(10)62961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu MR, Gorman D, Wagner J, Zurawski S, Liu YJ, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 26.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 27.Zhang GX, Gran B, Yu S, Li JF, Siglienti I, Chen XH, Kamoun M, Rostami A. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol. 2003;170:2153–2160. doi: 10.4049/jimmunol.170.4.2153. [DOI] [PubMed] [Google Scholar]
- 28.Gran B, Zhang GX, Yu S, Li JF, Chen XH, Ventura ES, Kamoun M, Rostami A. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: Evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169:7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 29.Li MO, Wan YY, Sanjabi S, Robertson AKL, Flavell RA. Transforming growth factor-beta regulation of immune responses. Ann Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 30.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJM, O'Shea J, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 31.Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, Van de Water J, Pessah IN. The CHARGE study: An epidemiologic investigation of genetic and environmental factors contributing to autism. Env Health Persp. 2006;114:1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lord C, Pickles A, McLennan J, Rutter M, Bregman J, Folstein S, Fombonne E, Leboyer M, Minshew N. Diagnosing autism: Analyses of data from the autism diagnostic interview. J Autism Devel Disord. 1997;27:501–517. doi: 10.1023/a:1025873925661. [DOI] [PubMed] [Google Scholar]
- 33.Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Devel Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 34.Joseph RM, Tager-Flusberg H, Lord C. Cognitive profiles and social-communicative functioning in children with autism spectrum disorder. J Child Psychol Psychiatr Allied Disciplines. 2006;43:807–821. doi: 10.1111/1469-7610.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lord C, Leventhal BL, Cook EH. Quantifying the phenotype in autism spectrum disorders. Am J Med Genet. 2001;105:36–38. [PubMed] [Google Scholar]
- 36.Owley T, McMahon W, Cook EH, Laulhere T, South M, Mays LZ, Shernoff ES, Lainhart J, Modahl CB, Corsello C, Ozonoff S, Risi S, Lord C, Leventhal BL, Filipek PA. Multisite, double-blind, placebo-controlled trial of porcine secretin in autism. J Am Acad Child Adolesc Phychiatr. 2001;40:1293–1299. doi: 10.1097/00004583-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: Diagnostic validity. Brit J Psychol. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- 38.Mullen EM. Mullen scales of early learning. Circle Pines, MN: American Guidance Services, Inc; 1995. [Google Scholar]
- 39.Sparrow SS, Balla DA, Cicchetti DV. Vineland adaptive behavior scales interview edition expanded form manual. Circle Pines, MN: American Guidance Services, Inc; 1984. [Google Scholar]
- 40.Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, Queiroz DMM, Rocha AMC, Jeschke MG. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13–19. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- 41.Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 42.Rogers SJ. Developmental regression in autism spectrum disorders. Mental Retard Devel Disabilities Res Rev. 2004;10:139–143. doi: 10.1002/mrdd.20027. [DOI] [PubMed] [Google Scholar]