Abstract
Polygonum multiflorum Thunb., which is known as Heshouwu in China, is a Taoist medicine sourced from the Wudang mountain area. At present, the quality of the Heshouwu sourced from this region is unknown. The present study aimed to evaluate the quality of wild Heshouwu collected from the Wudang mountain area, particularly the 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside (TSG) and combined anthraquinone (CAQ) content, compared with that of commercially available Heshouwu. Furthermore, the potential quantities of organic pesticide residues were determined. High performance liquid chromatography with a diode array detector was used to quantify TSG and CAQ content, whereas gas chromatography (GC), performed using a temperature gradient, was used to detect the presence of organochlorine, pyrethroid and organophosphorus pesticides. The average TSG content present in the wild Heshouwu from the Wudang mountain area and in the commercially available Heshouwu was 2.39 and 1.10%, respectively. In addition, the average content of CAQ in these was 1.41 and 3.46%, respectively. GC did not detect residues of organic pesticides in the wild Heshouwu, thus this plant met the criterion of the Chinese Pharmacopeia (2010 edition). The results of the present study indicated that wild Heshouwu from the Wudang mountain area may be suitable for use as a Chinese medicine across China.
Keywords: Wudang mountain area; Radix Polygoni multiflori; 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside; combined anthraquinones; pesticide residues; high performance liquid chromatography; gas chromatography
Introduction
Taoism is a religion native to China that has existed for ~2,000 years, since the Han Dynasty. The core value of Taoist philosophy is to promote longevity and immortality by oral administration of certain Chinese herbs; thus, Taoist medicine has been well developed and may be considered the origin of Traditional Chinese Medicine (1). As the leading center of Taoism, the Wudang mountain area in the Hubei province of central China has emerged as the center of Taoist training and Taoist medicine development since the Ming Dynasty ~600 years ago (2). At present, 59.1% of patients with chronic diseases in the Wudang mountain area prefer traditional medicine or integrated medicine compared with Western medicine (3). Therefore, investigation of the role of Taoist medicine in the Chinese medicine system is crucial.
Radix Polygoni multiflori, which is known as Heshouwu in China, is the dried root form of Polygonum multiflorum Thunb. The key places of Heshouwu production in China are the central and southern provinces, including the Shaanxi, Gansu, Jiangsu, Zhejiang, Anhui, Fujian, Jiangxi, Henan, Hunan, Guangdong, Guangxi, Hainan, Guizhou, Chongqing, Sichuan and Yunnan provinces (4). However, Heshouwu is distributed across the entire Wudang mountain area (5), which is localized to the north-west district of Hubei province (Fig. 1).
Figure 1.
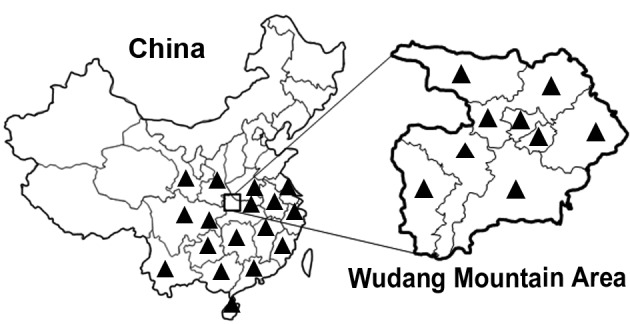
Distribution of Heshouwu across the Wudang Mountain Area. Black triangles correspond to the production locations of Heshouwu in China.
Heshouwu is one of the major components of Taoist formulations used in tonics, for dyeing hair, to slow aging (6) and for the treatment of gynecological diseases such as metrorrhagia (7). Although Heshouwu is commercially available and has been documented in the Chinese Pharmacopoeia (4), Taoists and the residents of the Wudang mountain area collect fresh Heshouwu for preparation of the formulations themselves. However, at present, there has been no data assessing the quality of wild Heshouwu in the Wudang mountain area. The main compounds in Heshouwu are phospholipids, anthraquinones (chrysophanol, chrysophanol anthrone, emodin, physcion and rhein) and glucosides, among which the main bioactive components are combined anthraquinones (CAQ) and 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside (TSG) (8). As CAQ and TSG content are indicators of the quality of Heshouwu, according to the criteria of the Chinese Pharmacopeia (2010 version) (4), the present study collected wild Heshouwu roots from the Wudang mountain area and analyzed their predominant components, in addition to the presence of any organic pesticide residues.
Materials and methods
Reagents
TSG (batch no. 110844-201109), emodin (batch no. 110756-200110) and physcion (batch no. 110758-200006) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). The following compounds were purchased from the Agro-Environmental Protection Institute, Ministry of Agriculture (Tianjin, China) for use as standards in high performance liquid chromatography (HPLC) and gas chromatography (GC): Organochlorines, including α-benzene hexachloride (α-BHC; batch no. GSB05-2276-2008), β-BHC (batch no. GSB05-2277-2008), γ-BHC (batch no. GSB05-2278-2008), δ-BHC (batch no. GSB05-2279-2008), p,p'-dichlorodiphenyltrichloroethane (p,p'-DDT; batch no. GSB05-2283-2008), p,p'-dichlorodiphenyldichloroethylene (DDE; batch no. GSB05-2280-2008), p,p'-dichlorodiphenyldichloroethane (p,p'-DDDE; batch no. GSB05-2282-2008), o,p'-DDT (batch no. GSB05-2281-2008) and pentachloronitrobenzene (PCNB; batch no. GSB05-1845-2008); pyrethroids, including cypermethrin (batch no. GSB05-2308-2008), fenvalerate (batch no. GSB05-2307-2008) and deltamethrin (batch no. GSB05-2310-2008); and organophosphorus compounds, including malathion (batch no. GSB05-2293-2008), parathion (batch no. GSB05-2284-2008), ethion (batch no. GSB05-2292-2008), methidathion E.C. (batch no. GSB05-2295-2008), omethoate (batch no. GSB05-2288-2008), parathion-methyl (batch no. GSB05-2285-2008), diazinon (batch no. GSB05-2291-2008), dimethoate (batch no. GSB05-2286-2008), acephate (batch no. GSB05-2322-2008) and o,o-dimethyl-O-2,2-dichlorovinyl phosphate (batch no. GSB05-2398-2008). The water used was double distilled and all other solvents were HPLC-grade.
Sample collection
The fresh roots of wild Heshouwu were collected from across the Wudang mountain area (Shiyan, Hubei, China) between June 2013 and July 2014, and were identified by Dr Xuanbin Wang. The plant specimens were nos. 20130614-20140719 (Fig. 2; Table I), according to a YY/MM/DD system used in our lab. Commercial Heshouwu was provided by Hubei Shennong Chinese Materia Medica, Co., Ltd. (Shiyan, China), Wuhan Liyuan Pharmaceutical, Co., Ltd. (Wuhan, China) and Bozhou Hongyu Chinese Materia Medica, Co., Ltd. (Bozhou, China). All samples were ground down into powder, passed through a 40-mesh filter and stored at 4°C until use.
Figure 2.

Wild Heshouwu in the Wudang Mountain Area. (A) The leaves of the Heshouwu plant. (B) The root slices of Heshouwu, used in Wudang Taoist Medicine.
Table I.
Information of the collected HSW samples.
| Plant specimen | Sample | Source | Date of collection |
|---|---|---|---|
| 20130614 | HSW-1 | Dachuan, Shiyan | June 14, 2013 |
| 20130914 | HSW-2 | Liuliping, Danjiangkouy | September 14, 2013 |
| 20140705 | HSW-3 | Longtanhe, Yunxi | July 5, 2014 |
| 20140329 | HSW-4 | Yanhu Park, Shiyan | March 29, 2014 |
| 20140719 | HSW-5 | Shuitianfan, Zhushan | July 19, 2014 |
The plant specimen numbers are given as YY/MM/DD, according to the system used in our laboratory. HSW, Heshouwu.
Reversed phase (RP)-HPLC analysis
TSG and CAQ in the Heshouwu samples were analyzed using a LC-20A HPLC system (Shimadzu Corporation, Kyoto, Japan), equipped with a diode array detector. The methodology of HPLC was consistent with the criteria of the Chinese Pharmacopoeia (2010 edition) (4). Briefly, for TSG, HPLC was performed using acetonitrile water (25:75 v/v) as the mobile phase on a SinoChrom ODS BP C18 RP column (250×4.6 mm, 5 µm), with a flow rate of 0.9 ml/min. The detection wavelength was set at 320 nm with the column temperature at 30°C. For CAQ, HPLC was performed using methanol in 0.1% phosphoric acid water (80:20 v/v) as the mobile phase, with a flow rate of 1.0 ml/min. The detection wavelength was set at 254 nm with the column temperature at 30°C.”
GC analysis
Organochlorine, pyrethroid and organophosphorus residues in the Heshouwu samples were analyzed using an Agilent 7890A GC system (Agilent Technologies, Inc., Santa Clara, CA, USA), equipped with a micro-electron capture detector (µECD). The methodology of GC was consistent with the criteria of the Chinese Pharmacopoeia (2010 edition) (9). Briefly, for detection of organochlorine residues, GC was performed using nitrogen (purity, 99.9999%) as the mobile phase on an Agilent HP-5 elastic quartz capillary GC column (30×0.32 mm, 0.25 µm; Agilent Technologies, Inc.), with a column flow rate of 0.9 ml/min. The inlet gas flow rate was 24 ml/min at 225°C. The initial temperature of the column was 100°C, after which the column was programmed to increase by 6°C/min up to 220°C, followed by 7°C/min up to 250°C, and finally maintained at 250°C for 6 min. The temperature of the µECD was 300°C, and 1 µl samples were injected using the split injection technique (9). The flow rate of nitrogen was 25 ml/min.
For detection of pyrethroid residues, GC was performed using nitrogen as the mobile phase (99.9999%) on an Agilent HP-5 elastic quartz capillary GC column (30×0.32 mm, 0.25 µm), with a column flow rate of 1.0 ml/min. The inlet gas flow rate was 24 ml/min at 200°C. The temperature of the column was programmed as follows: Holding at 150°C for 2 min, increases of 6°C/min from 150–270°C, holding at 270°C for 23 min, increases of 7°C/min from 220–250°C, followed by holding at 250°C for 6 min. The temperature of the µECD was 320°C and 1 µl samples were injected using the split injection technique (9). The flow rate of nitrogen was 25 ml/min.
For organophosphorus residue detection, GC was performed using nitrogen as the mobile phase (99.9999%) on an Agilent HP-5 elastic quartz capillary GC column (30mx0.25 mm, 0.25 µm), with a column flow rate of 1.0 ml/min. The inlet gas flow rate was 24 ml/min at 230°C. The temperature of the column was programmed as follows: Increases of 10°C/min from 120–220°C, followed by holding at 220°C for 2 min, increases of 20°C/min from 240–270°C, and finally holding at 270°C for 30 sec. The temperature of the µECD was 300°C and 1 µl samples were injected without the split.
Results
RP-HPLC to quantify TSG in Heshouwu from the Wudang mountain area
There was a reliable linear relationship at a range of 1.8–144 µg/l. The calibration curve was Y=5.7645649×104−1.63377×105 (r2=0.998). The limit of detection (LOD) and limit of quantification (LOQ) were 0.0092 and 0.0279 µg/ml, respectively. The average percentage of TSG in Heshouwu sourced from the Wudang mountain area and in the commercial product were 2.39 and 1.10%, respectively (Fig. 3; Table II). The criterion of TSG in the Chinese Pharmacopoeia (2010 edition) is ≥1.0% (4); thus the content of TSG in the wild Heshouwu from the Wudang Mountain Area exceeded the criterion of the Chinese Pharmacopoeia, in addition to that of Heshouwu available commercially.
Figure 3.
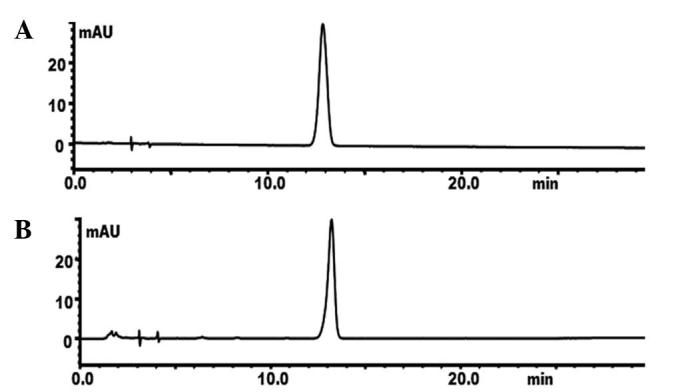
Reversed phase-HPLC of the (A) standard and (B) sample demonstrating 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside, in Heshouwu sourced from the Wudang mountain area. mAU, milli absorbance unit; HPLC, high-pressure liquid chromatography.
Table II.
TSG, emodin, physcion and combined anthraquinone content determination in the wild roots of HSW.
| Sample | TSG (%) | Emodin (%) | Physcion (%) | Combined anthraquinone (%) |
|---|---|---|---|---|
| HSW-1 | 2.43 | 2.32 | 0.245 | 2.57 |
| HSW-2 | 2.70 | 0.67 | 0.10 | 0.78 |
| HSW-3 | 2.44 | 1.52 | 0.13 | 1.63 |
| HSW-4 | 2.02 | 1.17 | 0.14 | 1.32 |
| HSW-5 | 2.33 | 0.66 | 0.08 | 0.74 |
| HSW average content in WMA | 2.39 | 1.27 | 0.14 | 1.41 |
| HSW average content in commercial product | 1.10 | 3.25 | 0.21 | 3.46 |
TSG, 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside; HSW, Heshouwu; WMA, Wudang Mountain Area.
RP-HPLC to quantify CAQ in Heshouwu from the Wudang mountain area
The data for emodin and physcion showed a reliable linear relationship at a range of 0.17–170 and 0.23–46 µg/ml, respectively. The calibration curves were Y=2626.071X+205.3 (r2=0.999) and Y=8554.358X+2629.933 (r2=0.998), respectively. The LOD and LOQ for emodin were 0.086 and 0.260 µg/ml, respectively, whereas LOD and LOQ for physcion were 0.023 and 0.070 µg/ml, respectively. The percentage of total CAQ (calculated as the sum of emodin and physcion) in the Heshouwu from the Wudang Mountain Area and in the commercial product was 1.41 and 3.46%, respectively (Fig. 4; Table II). According to the Chinese Pharmacopoeia (2010 edition), the content of CAQ should be >0.05%; thus, the content of CAQ in the wild Heshouwu sourced from the Wudang mountain area was consistent with the criterion of the Chinese Pharmacopoeia (9).
Figure 4.
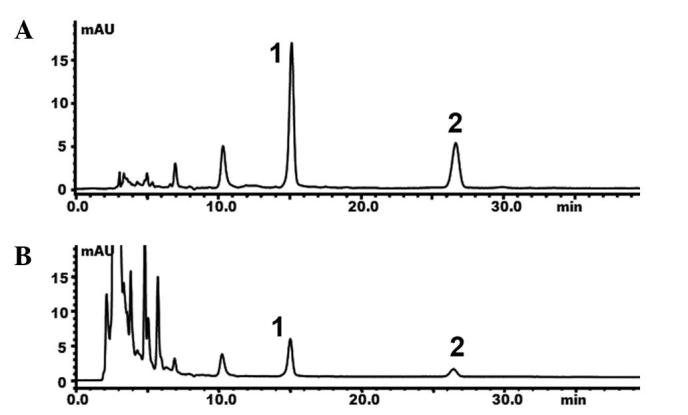
Reversed phase-HPLC of the (A) standard and (B) sample demonstrating combined anthraquinones, in Heshouwu sourced from the Wudang Mountain Area. 1, emodin; 2, physcion; mAU, milli absorbance unit; HPLC, high pressure liquid chromatography.
GC to detect organochlorine pesticides in Heshouwu samples from the Wudang mountain area
As organochlorine pesticide residues have previously been reported in agriculture in other parts of China (10), the present study also determined whether the wild Heshouwu from the in Wudang mountain area was contaminated with pesticides. GC was performed to quantify organochlorine pesticide residues in these samples. The calibration curves, LOD and LOQ for α-BHC, PCNB, γ-BHC, β-BHC, p,p'-DDE, δ-BHC, p,p'-DDDE, p,p'-DDT and o,p'-DDT are presented in Table III. Organochlorine residues were not detected in the samples (Fig. 5), suggesting that Heshouwu from the Wudang Mountain Area and the commercial products are not contaminated with organochlorine pesticide residues.
Table III.
Calibration curve, LOD and LOQ of organochlorine pesticides in Heshouwu derived from the Wudang Mountain Area, as determined by gas chromatography.
| Compoundsa | Regression equation | Correlation coefficient | LOD (ng/ml) | LOQ (ng/ml) |
|---|---|---|---|---|
| α-BHC | Y=2.725X+3.482 | 0.9998 | 1.05 | 3.50 |
| PCNB | Y=1.963X+4.186 | 0.9996 | 0.95 | 3.16 |
| γ-BHC | Y=2.083X+10.650 | 0.998 | 0.84 | 2.80 |
| β-BHC | Y=2.805X+16.196 | 0.996 | 0.75 | 2.50 |
| p,p'-DDE | Y=1.049X+4.436 | 0.997 | 0.61 | 2.03 |
| δ-BHC | Y=1.751X+1.212 | 0.99995 | 0.97 | 3.23 |
| p,p'-DDDE | Y=1.796X-0.197 | 0.99990 | 1.03 | 3.43 |
| p,p'-DDT | Y=1.650X+1.975 | 0.9997 | 1.08 | 3.60 |
| o,p'-DDT | Y=1.965X+1.535 | 0.9998 | 0.98 | 3.27 |
Concentration range, 4–250 ng/ml. LOD, limit of detection; LOQ, limit of quantification; BHC, benzenehexachloride; PCNB, pentachloronitrobenzene; DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; DDDE, dichlorodiphenyldichloroethane.
Figure 5.
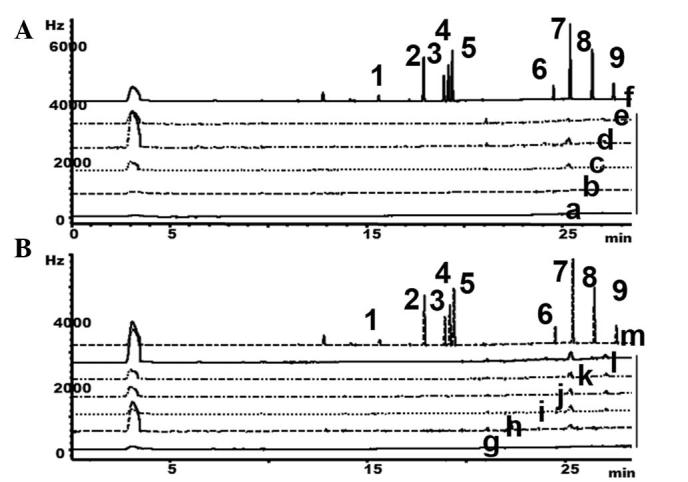
GC was performed to detect organochlorine residues in HSW sourced from (A) the Wudang mountain area and (B) the commercial products. 1, α-benzenehexachloride (α-BHC); 2, pentachloronitrobenzene; 3, γ-BHC; 4, β-BHC; 5, p,p'-dichlorodiphenyldichloroethylene; 6, δ-BHC; 7, p,p'-dichlorodiphenyldichloroethane; 8, p,p'-dichlorodiphenyltrichloroethane (p,p'-DDT); 9, o,p'-DDT; a, HSW1; b, HSW2; c, HSW3; d, HSW4; e, HSW5; f, standard; g, Sichuan; h, Yunan; i, Hubei; j, Hunan; k, Henan; l, Guangdong; m, standard; Hz, hertz; GC, gas chromatography; HSW, Heshouwu.
GC to detect pyrethroids in Heshouwu samples from the Wudang mountain area
GC was used to detect pyrethroid residues, including cypermethrin, fenvalerate and deltamethrin, in the collected Heshouwu (Table IV). The results suggested that there were no pyrethroids detected in the samples (Fig. 6).
Table IV.
Calibration curve, LOD and LOQ of pyrethroids in Heshouwu sourced from the Wudang mountain area, as determined by gas chromatography.
| Compoundsa | Regression equation | Correlation coefficient | LOD (ng/ml) | LOQ (ng/ml) |
|---|---|---|---|---|
| Cypermethrin | Y=6.142X-65.528 | 0.97 | 0.99 | 3.30 |
| Fenvalerate | Y=10.990X-116.270 | 0.97 | 1.08 | 3.50 |
| Deltamethrin | Y=15.717X-165.620 | 0.97 | 1.05 | 3.30 |
Concentration range, 4–200 ng/ml. LOD, limit of detection; LOQ, limit of quantification.
Figure 6.
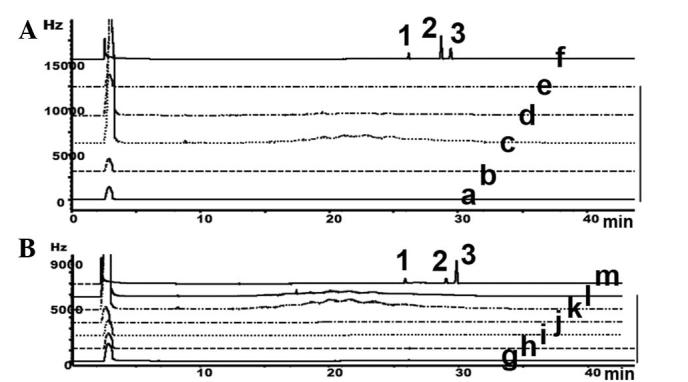
GC was performed to detect pyrethroid residues in HSW derived from (A) the Wudang mountain area and (B) the commercial products. 1, cypermethrin; 2, envalerate; 3, deltamethrin; a, HSW1; b, HSW2; c, HSW3; d, HSW4; e, HSW5; f, standard; g, Sichuan; h, Yunan; i, Hubei; j, Hunan; k, Henan; l, Guangdong; m, standard; Hz, hertz; GC, gas chromatography; HSW, Heshouwu.
GC to detect organophosphorus pesticides in Heshouwu samples from the Wudang mountain area
As organophosphorus pesticides have been the most widely used pesticides in China, and have previously been demonstrated to contaminate water and food (11–17), the present study aimed to detect the presence of 10 organophosphorus pesticides using GC, according to the criteria of the Chinese Pharmacopoeia (9). The results suggested that there were no organophosphorus pesticide residues in the samples (Table V; Fig. 7).
Table V.
Calibration curve, LOD and LOQ of organophosphorus pesticides in Heshouwu sourced from the Wudang mountain area, as determined by gas chromatography.
| Compounds | Regression equation | Correlation coefficient | LOD (ng/ml) | LOQ (ng/ml) |
|---|---|---|---|---|
| Malathion | Y=9763.1X-5009.5 | 0.92 | 0.75 | 2.50 |
| Parathion | Y=9763.1X-5009.5 | 0.97 | 0.64 | 2.13 |
| Ethiofos | Y=43629.0X-22022.0 | 0.96 | 0.45 | 1.50 |
| Methidathion | Y=20295.0X-10986.0 | 0.95 | 0.60 | 2.00 |
| Omethoate | Y=42666.0X-19621.0 | 0.97 | 0.92 | 3.07 |
| Methyl parathion | Y=48727.0X-24117.0 | 0.96 | 1.20 | 4.03 |
| Diazinon | Y=10560.0X-4395.5 | 0.97 | 0.98 | 3.27 |
| Dimethoate | Y=21569.0X-12247.0 | 0.95 | 1.09 | 3.63 |
| Acephate | Y=11168.0X-6745.0 | 0.93 | 0.35 | 1.17 |
| Dichlorvos | Y=7423.1X+869.0 | 0.992 | 0.56 | 1.86 |
Concentration range, 4–200 ng/ml. LOD, limit of detection; LOQ, limit of quantification.
Figure 7.
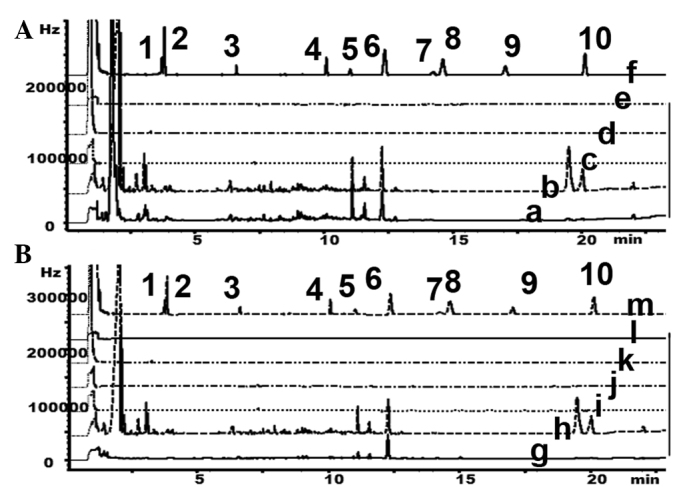
GC was performed to detect organophosphorus residues in HSW derived from (A) the Wudang mountain area and (B) the commercial products. 1, o,o-dimethyl-O-2,2-dichlorovinylphosphate; 2, omethoate; 3, acephate; 4, dimethoate; 5, diazinon; 6, parathion-methyl; 7, malathion; 8, parathion; 9, methidathion E.C; 10, ethion; a, HSW1; b, HSW2; c, HSW3; d, HSW4; e, HSW5; f, standard; g, Sichuan; h, Yunan; i, Hubei; j, Hunan; k, Henan; l, Guangdong; m, standard; Hz, hertz; GC, gas chromatography; HSW, Heshouwu.
Discussion
Taoism is a religion native to China, which has pursued longevity and immortalization since the Han Dynasty (18). According to Taoism, aging may be slowed by performing religious activities, Tai Chi Kung Fu and Ba Duan Jin (a Taoist physical exercise) and applying Taoist medical theories using medicines listed in the Materia medica (1,19,20). Heshouwu has been described as a dietary herbal medicine, rather than a therapeutic drug in the Wudang Taoist medicine system (6,7). Previous studies have demonstrated various biological effects for Heshouwu, including anti-aging, immunomodulatory, antihyperlipidemic, hepatoprotective, anticancer and anti-inflammatory effects, in addition to an ability to improve learning and memory (21–28). At present, residents of the Wudang Mountain Area prefer to collect wild Heshouwu for their dietary and medicinal requirements, instead of purchasing it commercially (3,6,7). Therefore, it was important to determine the quality of the wild Heshouwu in the Wudang mountain area, as its quality is currently unclear.
In the present study, fresh Heshouwu roots from across the Wudang mountain area and the commercial products were evaluated, in order to assess the concentrations of CAQ and TSG. Furthermore, due to concerns of environmental pollution, organic pesticide residues were also quantified. In the present study, the concentration of TSG in the Wudang mountain area exceeded the criterion of the Chinese Pharmacopoeia (4), in addition to that of commercially available Heshouwu, and that concentrations of CAQ were consistent with the criteria of the Chinese Pharmacopoeia (4). These results suggested that the wild Heshouwu from the Wudang mountain area met the criteria of the Chinese Pharmacopoeia, and exceeded those in the commercial product with regard to TSG content. Furthermore, GC data demonstrated that there was no organic pesticide contamination in wild Heshouwu, indicating its safety for consumption.
In conclusion, the present study demonstrated that the wild Heshouwu from the Wudang mountain area met the criteria of the Chinese Pharmacopeia (2010 edition), and that there were no pesticide residues in the wild Heshouwu. These results indicated that wild Heshouwu from the Wudang mountain area may safely be used as a medicine.
Acknowledgements
The present study was supported by the Young Scientist Innovation Team Project of Hubei Colleges (grant no. T201510); the 2013 Innovation & Investment Projects for Undergraduates of State (grant no. 201310929008); the Scientific Research Project of Health and Family Planning Commission of Hubei Province (grant no. WJ2015Z113); the 2011 Project of Hubei Province (no. 4); the Natural Science Foundation of Hubei Province of China (grant no. 2010CDZ066, 2014CFB642); the Foundation for Innovative Research Team of Hubei University of Medicine and the Key Discipline Project of Hubei University of Medicine (grant no. 2014CXG03); and the Open Project of Hubei Key Laboratory of Wudang Local Chinese Medicine Research (Hubei University of Medicine; grant no. WDCM001).
References
- 1.Wen MX. The formation and development of complementary medicine in Wudang Taoism. Hu Nan Zhong Yi Xue Yuan Xue Bao. 2006;26:14–15. (In Chinese) [Google Scholar]
- 2.Xu D, Wang D, Hu J, Xiong T, Zhu Y. Study on development history of Taoist medicine in Wudang. Zhonghua Yi Shi Za Zhi. 2000;30:225–227. (In Chinese) [PubMed] [Google Scholar]
- 3.Chai Y, Xu Z, Wang B, Mao Z. Studies on rural residents' utilization and tendency of traditional Chinese drugs in Wudang Town. Shaanxi Zhong Yi Xue Yuan Xue Bao. 2005;28:67–68. (In Chinese) [Google Scholar]
- 4.Commission of Chinese Pharmacopoeia: Heshouwu, corp-author. Pharmacopoeia of the People's Republic of China. 1st. Vol. 1. China Medico-Pharmaceutical Science & Technology Publishing House; Beijing: 2010. pp. 164–165. (In Chinese) [Google Scholar]
- 5.Chen JY, Tu HJ, Tu ZL, Li GF. Materia Medica in Wudang, China. Hubei Ke Xue Chu Ban She; Wuhan, China: 2009. Heshouwu; pp. 200–207. (In Chinese) [Google Scholar]
- 6.Shang R. Kan Gua formula in Taoist medicine. Wu Dang. 2004:55–56. (In Chinese) [Google Scholar]
- 7.Shang R. Introduction of plasters of Wudang Taoist medicine for gynaecology. Wu Dang. 2006:52–53. (In Chinese) [Google Scholar]
- 8.Hou JY, Fang TH. Pharmacology of Chinese Materia Medica. 2nd. Zhong Guo Zhong Yi Yao Chu Ban She; Beijing, China: 2007. pp. 230–232. (In Chinese) [Google Scholar]
- 9.Pharmacopoeia of the People's Republic of China. 1st. Vol. 1. China Medico-Pharmaceutical Science & Technology Publishing House; Beijing: 2010. Appendum, Methods of residues of pesticides. Commission of Chinese Pharmacopoeia. appendum. (In Chinese) [Google Scholar]
- 10.Da C, Liu G, Tang Q, Liu J. Distribution, sources, and ecological risks of organochlorine pesticides in surface sediments from the Yellow River Estuary, China. Environ Sci Process Impacts. 2013;15:2288–2296. doi: 10.1039/c3em00369h. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Asiri AM, Du D, Lin Y. Acetylcholinesterase biosensor based on a gold nanoparticle-polypyrrole-reduced graphene oxide nanocomposite modified electrode for the amperometric detection of organophosphorus pesticides. Analyst (Lond) 2014;139:3055–3060. doi: 10.1039/c4an00068d. [DOI] [PubMed] [Google Scholar]
- 12.Hu X, Shi W, Yu N, Jiang X, Wang S, Giesy JP, Zhang X, Wei S, Yu H. Bioassay-directed identification of organic toxicants in water and sediment of Tai Lake, China. Water Res. 2015;73:231–241. doi: 10.1016/j.watres.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 13.Tang X, Liang B, Yi T, Manco G, Palchetti I, Liu A. Cell surface display of organophosphorus hydrolase for sensitive spectrophotometric detection of p-nitrophenol substituted organophosphates. Enzyme Microb Technol. 2014;55:107–112. doi: 10.1016/j.enzmictec.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Wu L, Yao J, Trebse P, Zhang N, Richnow HH. Compound specific isotope analysis of organophosphorus pesticides. Chemosphere. 2014;111:458–463. doi: 10.1016/j.chemosphere.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 15.Yu G, Wu W, Zhao Q, Wei X, Lu Q. Efficient immobilization of acetylcholinesterase onto amino functionalized carbon nanotubes for the fabrication of high sensitive organophosphorus pesticides biosensors. Biosens Bioelectron. 2015;68:288–294. doi: 10.1016/j.bios.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Li D, Li J, Rose G, Marriott PJ. Organophosphorus pesticide and ester analysis by using comprehensive two-dimensional gas chromatography with flame photometric detection. J Hazard Mater. 2013;263:761–767. doi: 10.1016/j.jhazmat.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 17.Yuan Z, Yao J, Liu H, Han J, Trebše P. Photodegradation of organophosphorus pesticides in honey medium. Ecotoxicol Environ Saf. 2014;108:84–88. doi: 10.1016/j.ecoenv.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 18.Wen MX. History and Characters of Wudang Taoist Medicine. Nei Meng Gu Zhong Yi Yao. 2013;32:118–119. (In Chinese) [Google Scholar]
- 19.Shang R. Properties of Taoist medicine in Wudang. Wudang. 2011:37–39. (In Chinese) [Google Scholar]
- 20.Xu D. Nature of Wudang Taoist Medicine. Wu Dang. 2006:49–50. [Google Scholar]
- 21.Lin L, Ni B, Lin H, Zhang M, Li X, Yin X, Qu C, Ni J. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: A review. J Ethnopharmacol. 2015;159:158–183. doi: 10.1016/j.jep.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou XX, Yang Q, Xie YH, Sun JY, Qiu PC, Cao W, Wang SW. Protective effect of tetrahydroxystilbene glucoside against D-galactose induced aging process in mice. Phytochem Lett. 2013;6:372–378. doi: 10.1016/j.phytol.2013.05.002. [DOI] [Google Scholar]
- 23.Chen Q, Zhang SZ, Ying HZ, Dai XY, Li XX, Yu CH, Yu CH. Chemical characterization and immunostimulatory effects of a polysaccharide from Polygoni Multiflori Radix Praeparata in cyclophosphamide-induced anemic mice. Carbohyd Polym. 2012;88:1476–1482. doi: 10.1016/j.carbpol.2012.02.055. [DOI] [Google Scholar]
- 24.Wang W, He Y, Lin P, Li Y, Sun R, Gu W, Yu J, Zhao R. In vitro effects of active components of Polygonum Multiflorum Radix on enzymes involved in the lipid metabolism. J Ethnopharmacol. 2014;153:763–770. doi: 10.1016/j.jep.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 25.Wang M, Zhao R, Wang W, Mao X, Yu J. Lipid regulation effects of Polygoni Multiflori Radix, its processed products and its major substances on steatosis human liver cell line L02. J Ethnopharmacol. 2012;139:287–293. doi: 10.1016/j.jep.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 26.Lin P, He YR, Lu JM, Li N, Wang WG, Gu W, Yu J, Zhao RH. In vivo lipid regulation mechanism of polygoni multiflori radix in high-fat diet fed rats. Evid Based Complement Alternat Med. 2014;2014:642058. doi: 10.1155/2014/642058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee BH, Huang YY, Duh PD, Wu SC. Hepatoprotection of emodin and Polygonum multiflorum against CCl(4)-induced liver injury. Pharm Biol. 2012;50:351–359. doi: 10.3109/13880209.2011.604335. [DOI] [PubMed] [Google Scholar]
- 28.Jin W. Studies of Zhiheshouwu on CCl4-induced liver fibrosis in rats. Dalian: 2004. (In Chinese) [Google Scholar]


