Abstract
In the present study, the effects and molecular mechanisms of thymoquinone (TQ) on colon cancer cells were investigated. Cell viability was determined using a Cell Counting Kit-8 assay, and the results revealed that treatment with TQ significantly decreased cell viability in COLO205 and HCT116 cells in a dose-dependent manner. TQ treatment additionally sensitized COLO205 and HCT116 cells to cisplatin therapy in a concentration-dependent manner. To investigate the molecular mechanisms of TQ action, western blot analysis was used to determine the levels of phosphorylated p65 and nuclear factor-κB (NF-κB)-regulated gene products vascular endothelial growth factor (VEGF), c-Myc and B-cell lymphoma 2 (Bcl-2). The results indicated that TQ treatment significantly decreased the level of phosphorylated p65 in the nucleus, which indicated the inhibition of NF-κB activation by TQ treatment. Treatment with TQ also decreased the expression levels of VEGF, c-Myc and Bcl-2. In addition, the inhibition of NF-κB activation with a specific inhibitor, pyrrolidine dithiocarbamate, potentiated the induction of cell death and caused a chemosensitization effect of TQ in colon cancer cells. Overall, the results of the present study suggested that TQ induced cell death and chemosensitized colon cancer cells by inhibiting NF-κB signaling.
Keywords: thymoquinone, chemosensitization, cell death, nuclear factor-κB, colon cancer
Introduction
Cancer is a significant public health problem worldwide. According to the World Cancer Research Fund International, ~12.7 million cancer-associated mortalities (13% of all mortalities) occurred worldwide in 2008, with males accounting for 6.6 million mortalities and females accounting for 6 million (1). In developed countries, colorectal cancer is one of the most commonly observed types of cancer, ranking 2nd and 3rd in women and men, respectively (1). The lifetime risk for colorectal cancer development in the general population is ~6%, and colorectal cancer is responsible for ~8% of all cancer-associated mortalities worldwide (1). Among the Chinese population, the incidence of colorectal cancer is increasing (2). At present, no optimal adjuvant chemotherapy exists for clinical use; therefore, developing rationally designed, novel adjuvant therapeutic tools for the treatment of colon cancer is a constant requirement (2). Previously, the use of natural substances, including curcumin, eicosapentaenoic acid, apple polyphenols, capsaicin and thymoquinone (TQ), for cancer chemoprevention has been investigated (3–5). TQ is the primary active ingredient of volatile Nigella sativa (black cumin) seed oil, which is used as a spice in countries with a low incidence of colorectal cancer, including Egypt, Pakistan and India (6). Traditional medicine has taken advantage of the anti-inflammatory, antioxidant and anticarcinogenic properties associated with TQ, which supports the hypothesis of TQ being a promising dietary chemopreventive agent (6). In the previous decade, the antitumor activity of TQ has been investigated in a number of studies (6–8). TQ was observed to induce antitumor effects in several types of cancer, including breast (9), lung (10), multiple myeloma (11), pancreatic (12), cervical (13), colon (14) and prostate cancer (15), as well as squamous (16) and hepatocellular carcinoma (17), acute lymphoblastic leukemia (18), glioblastoma (19), osteosarcoma (20), neuroblastoma (21), bladder (22), gastric (23) and ovarian cancer (24). Although the effect of TQ has been investigated in numerous types of cancer, the molecular mechanisms underlying its action remain to be elucidated. The present study investigated the effect of TQ on colon cancer cell growth and the underlying molecular mechanisms. In addition, the present study identified the effect and molecular mechanism of TQ action on the chemosensitivity of colon cancer cells to cisplatin (CisPt).
Materials and methods
Cell culture and materials
TQ, PDTC, Tris, glycine, NaCl, sodium dodecyl sulfate (SDS), bovine serum albumin (BSA) and β-actin antibody were obtained from Sigma-Aldrich (St. Louis, MO, USA). TQ was dissolved in dimethyl sulfoxide (DMSO; Merck Millipore, Darmstadt, Germany) to make a 50 mM stock solution and stored at −20°C until use in subsequent experiments. Additional dilutions were performed in cell culture medium (RPMI-1640; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) so that the final DMSO concentration was <0.1%. Primary antibodies, including rabbit polyclonal anti-human p65 (catalog no., sc-101749; 1:500), rabbit polyclonal anti-human B-cell lymphoma 2 (Bcl-2; catalog no., sc-492; 1:2,000), rabbit polyclonal anti-human vascular endothelial growth factor (VEGF; catalog no., sc-507; 1:1,000), mouse polyclonal anti-human c-Myc (catalog no., sc-40: 1:500), and secondary antibodies, including goat anti-rabbit horseradish peroxidase (HRP)-conjugated (catalog no., sc-2054: 1:10,000) and goat anti-mouse HRP-conjugated (catalog no., sc-2005 1:10,000) were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Primary rabbit polyclonal anti-human β-actin (catalog no., sc-7210: 1:200) antibody was purchased from Sigma-Aldrich. The COLO205 and HCT116 colon cancer cell lines were purchased from American Type Culture Collection (Manassas, VA, USA). The cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.), supplemented with 10% heat-inactivated fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 2% penicillin/streptomycin (6 mg/ml penicillin, 10 mg/ml streptomycin; Sigma-Aldrich). The cells were cultured in tissue culture flasks (75 cm2; Corning Incorporated, New York, NY, USA) and incubated at 37°C in a humidified chamber containing 5% CO2.
Cell viability assay
Cell viability was determined using a Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan). Briefly, 2×104 cells per well were seeded into a 96-well plate (Corning Incorporated). Subsequent to 24 h culturing, cells were treated with cisplatin and TQ for 24 h or TQ alone for 48 h. Finally, 20 µl CCK-8 solution was added to each well, and was incubated for 2 h at 37°C. The optical density (OD) of each well at 450 nm was measured using a VICTOR™ X multi-label reader (PerkinElmer, Inc., Waltham, MA, USA). The percentage cell viability was calculated as follows: (ODdrug / ODcontrol) × 100. To analyze the role of nuclear factor-κB (NF-κB) in TQ activity, cells (2×104 cells per well) were treated with 50 µm pyrrolidine dithiocarbamate (PDTC) in combination with TQ for 12, 24 and 48 h. The percentage cell growth inhibition was calculated as follows: (ODcontrol-ODdrug) × 100.
Preparation of nuclear extract
Nuclear extracts were prepared as previously described (25). Briefly, cells were harvested, washed twice with ice-cold phosphate buffered saline (PBS; Hyclone, Beijing, China) for 1 min and resuspended in 1 ml of ice-cold PBS. Cells were pelleted by centrifugation at 12,000 × g for 5 min, suspended in ice-cold buffer [10 mmol/l 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 1.5 mmol/l MgCl2, 0.2 mmol/l KCl, 0.2 mmol/l phenylmethylsulphonyl fluoride, 0.5 mmol/l dithothreitol], mixed by vortexing for 10 sec and centrifuged at 12,000 × g for 5 min. The nuclear pellet was washed in 1 ml buffer (20 mmol/l HEPES, 25% glycerol, 0.42 mol/l NaCl, 1.5 mmol/l MgCl2, 0.2 mmol/l ethylenediaminetetraacetic acid), resuspended in 30 ml buffer, mixed by rotation for 30 min at 4°C and centrifuged at 12,000 × g for 20 min. Finally, the supernatants were used as nuclear extracts.
Western blot analysis
Using 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), whole cell lysates or nuclear extracts were electrophoresed, as previously described (26). The proteins were transferred onto a 0.4-µm polyvinylidene difluoride membrane (EMD Millipore, Bedford, MA, USA) in transfer buffer (25 mM Tris, pH 8.5, 0.2 M glycine and 20% methanol). The membranes were blocked by 5% BSA in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 h, washed twice with TBST for 10 min each and incubated with primary β-actin, p65, Bcl-2, VEGF and c-Myc antibodies at 4°C overnight. Subsequent to three washes with TBST for 10 min each, the membranes were probed with secondary peroxidase-conjugated antibody (dilution, 1:10,000; Sigma-Aldrich). Finally, the immunoreactive bands were visualized using an enhanced chemiluminescence detection kit (Immobilon WBKLS0500; Merck Millipore). β-actin was used as an internal control for all western blot analyses.
Statistical analysis
SPSS version 17.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Values were expressed as the mean ± standard deviation. Numeric variables were compared by one-way analysis of variance. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of TQ on the viability of colon cancer cells
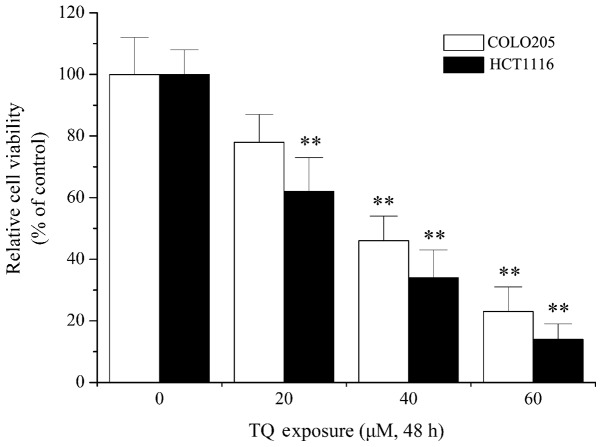
Previous studies have shown the antitumor and anticarcinogenic activities of TQ in numerous types of cancer, including breast cancer, glioblastoma and lymphoma (27–29). In order to elucidate the molecular mechanisms underlying the function of TQ, the effect of TQ on colon cancer cells was examined in the present study. As shown in Fig. 1, 20 µM TQ treatment resulted in a significant decrease in cell viability in HCT1116 cells when compared with the control (P=0.013). Furthermore, 40 µM TQ treatment significantly decreased call viability in COLO205 cells compared with the control (P=0.007). Cytotoxicity assays indicated that TQ dose-dependently decreased cell viability of the COLO205 and HCT116 colon cancer cell lines (Fig. 1), which confirmed the antitumor activity of TQ, as previously reported (30,31).
Figure 1.
TQ induced death of colon cancer cells. Cultured cells (2×104 cells per well) were treated with various concentrations (0, 20, 40 and 60 µM) of TQ for 48 h. Cell viability was measured using Cell Counting Kit-8 and presented as a percentage of the control. Data are presented as the mean ± standard deviation of five experiments. **P<0.05 vs. the respective control group. TQ, thymoquinone.
TQ chemosensitizes colon cancer cells
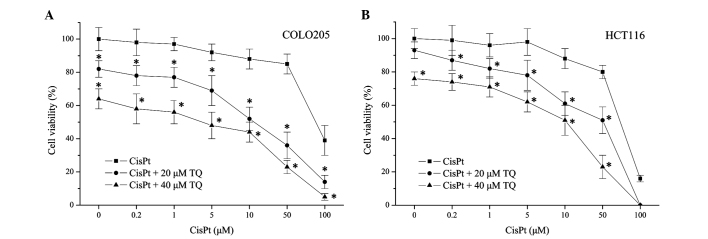
The present study aimed to investigate whether TQ affected the sensitivity of colon cancer cells to chemotherapy. Therefore, TQ and CisPt were combined to treat COLO205 and HCT116 colon cancer cell lines. As shown in Fig. 2, treatment with cisplatin alone induced COLO205 and HCT1116 cell death in a dose-dependent manner. Combined treatment with cisplatin (0.2 µM) and TQ (20 or 40 µM) significantly decreased the cell viability of COLO205 (P=0.021, 20 µM TQ; P=0.003, 40 µM TQ) and HCT116 cells (P=0.038, 20 µM TQ; P=0.004, 40 µM TQ) when compared with 0.2 µM cisplatin treatment alone. These results revealed that cell death induced by CisPt was enhanced by TQ in a concentration-dependent manner (Fig. 2), which indicated that TQ may potentiate the chemosensitivity of colon cancer cells.
Figure 2.
TQ increases the cytotoxicity of CisPt in colon cancer cells. (A) COLO205 cell line; (B) HCT116 cell line. Colon cancer cells were treated with 0.2, 1, 5, 10, 50 and 100 µM CisPt, with or without 20 or 40 µM TQ for 24 h. Cell viability was measured by a Cell Counting Kit-8 and is presented as a percentage of the control. Values are expressed as the mean ± standard deviation of five experiments. *P<0.05 vs. cisplatin alone. TQ, thymoquinone; CisPt, cisplatin.
Role of NF-κB in TQ activity
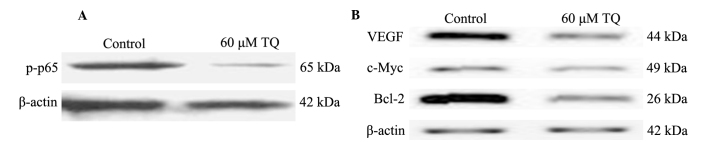
As demonstrated in the present results, TQ treatment may result in cell death and chemosensitizing of colon cancer cells. Therefore, the present study aimed to investigate the underlying mechanisms of TQ action, including the role of NF-κB in the process. Western blot analysis was used to determine the effect of TQ on NF-κB activation. The results demonstrated that 60 µM TQ significantly inhibited the phosphorylation of p65 protein, subunit of NF-κB (Fig. 3A). Furthermore, the expression levels of NF-κB-regulated genes that are involved in tumor angiogenesis, survival and apoptosis were measured. As demonstrated in Fig. 3B, protein levels of VEGF, c-Myc and Bcl-2 were markedly downregulated following 60 µM TQ treatment.
Figure 3.
TQ inhibits the activation of NF-κB and downregulates its downstream gene expression. (A) TQ inhibited phosphorylation of the subunit of NF-κB, p65. COLO205 cells were treated with 60 µM TQ for 18 h. Cells were harvested and the expression of phosphorylated p65 in the nucleus was evaluated by western blot analysis. β-actin was used as an internal control. A representative blot of three experiments with similar results is shown. (B) TQ downregulated NF-κB downstream gene products in COLO205 cells. Following 18 h of incubation, the levels of VEGF, c-Myc and Bcl-2 were determined by western blot analysis. β-actin was used as an internal control. A representative blot of three experiments with similar results is shown. TQ, thymoquinone; NF-κB, nuclear factor-κB; VEGF, vascular endothelial growth factor; Bcl-2, B-cell lymphoma 2.
NF-κB inhibitor, PDTC, potentiates the activity of TQ
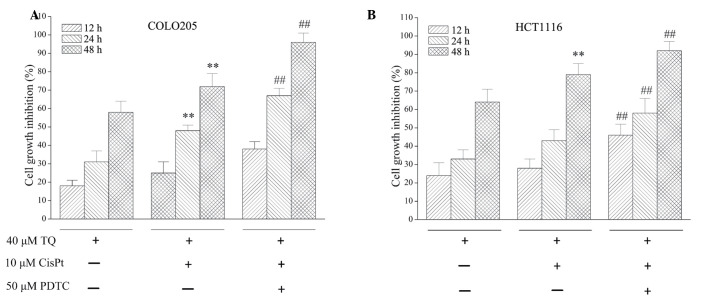
As TQ inhibited NF-κB activation, the effect of NF-κB on TQ function was assessed. The NF-κB inhibitor, PDTC, was used to investigate the activity of TQ. As demonstrated in Fig. 4, combined treatment with 10 µM cisplatin and 40 µM TQ treatment for 24 or 48 h significantly inhibited cell growth inhibition when compared with 40 µM TQ treatment alone (P=0.018 and P=0.009, respectively) in COLO205 cells. Treatment with 50 µM PDTC further potentiated the growth inhibition observed following 24 h (P=0.002) and 48 h (P=0.031) treatment when compared with 10 µM cisplatin and 40 µM TQ combined treatment. For HCT1116 cells, following 12, 24 and 48 h treatment with 50 µM PDTC, 10 µM cisplatin and 40 µM TQ significantly inhibited cell growth inhibition (P=0.014, P=0.043 and P=0.027, respectively) when compared with combined 10 µM cisplatin and 40 µM TQ treatment. Furthermore, a chemosensitization effect of TQ on colon cancer cells was enhanced by 50 µM PDTC treatment, which was demonstrated by increased cell growth inhibition (Fig. 4).
Figure 4.
PDTC, a nuclear factor-κB inhibitor, potentiated the death-inducing effect of TQ on colon cancer cells. (A) COLO205 cell line; (B) HCT116 cell line. Cultured cells were seeded in a 96-well plate at a density of 2×104 cells per well and treated with 40 µM TQ, with or without 50 µM PDTC. Following 12, 24 or 48 h incubation, cell viability was determined with a Cell Counting Kit-8. Cell viability is presented as a percentage of the control. Results are presented as the mean ± standard deviation of at least three independent experiments. **P<0.05 vs. TQ treatment; ##P<0.05 vs. TQ+CisPt treatment. TQ, thymoquinone; PDTC, pyrrolidine dithiocarbamate; CisPt, cisplatin.
Discussion
Nigella sativa is an annual herbaceous plant belonging to the Ranunculaceae family, which has been commonly used in traditional Middle Eastern folk medicine as a natural remedy for various ailments for >2,000 years (8). Nigella sativa is additionally used as a food additive and flavoring in numerous countries (8). TQ, or 2-isopropyl-5-methyl-1,4-benzoquinone, is a Nigella sativa essential oil that is known to be the principal active compound of the seed, and is responsible for a number of its antioxidant and anti-inflammatory effects (6,8).
In 2003, Shoieb et al (32) reported in vitro experimental results that revealed TQ was able to inhibit growth and induce apoptosis in cancer cell lines. Since then, increasing numbers of studies have focused on TQ in cancer therapy. Numerous in vitro and in vivo studies have investigated the antitumor activity of TQ in several types of cancer (6,14,19,27–29,33). In tumor protein p53-null myeloblastic leukemia HL-60 cells, TQ induced apoptosis through an intrinsic signaling pathway (34). In human multiple myeloma cells, TQ inhibits proliferation, induces apoptosis and exerts a chemosensitization effect through suppressing signal transducer and activator of transcription 3 (acute-phase response factor) activation (35), and decreases F-actin polymerization and Bcl-2/Bcl-2 like 1 expression (36). TQ-induced apoptosis has been indicated to be mediated by reactive oxygen species generation (29,33,37) and the mitogen-activated protein kinase 14 signaling pathway (33). In 2004, Gali-Muhtasib et al (38) reported that TQ triggered apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism, and in 2008, Gali-Muhtasib et al (31) demonstrated that TQ triggered inactivation of the stress response pathway sensor checkpoint kinase 1 and contributed to apoptosis in colorectal cancer cells. Gali-Muhtasib et al (30) additionally indicated that TQ may inhibit colon tumor cell invasion (30), which was confirmed by additional studies (39–41).
A recent study reported that TQ exerted an antitumor effect through the interruption of pro-survival mitogen-activated protein kinase kinase 7-mitogen-activated protein kinase 1 signaling in colorectal cancer (14). TQ exerted a direct antitumor effect, and also sensitized cancer cells to other therapies (23,42–44). In general, cancer cells may be initially susceptible to chemotherapy; however, over time they may develop resistance through certain mechanisms, including DNA mutations and metabolic changes that promote drug inhibition and degradation. Drug resistance has been a challenge for clinical cancer treatment (45). Velho-Pereira et al (42) reported that TQ may radiosensitize human breast carcinoma cells. Jafri et al (43) indicated that in lung cancer, TQ treatment may overcome resistance and sensitize lung cancer cells to CisPt. Previous studies have revealed the chemosensitization and radiosensitization effect of TQ in pancreatic (44), lung (43), gastric (23) and breast cancer (42). However, the mechanism by which TQ affects the sensitivity of colon cancer to chemotherapy has not been investigated. In the present study, the results confirmed the antitumor activity of TQ in colon cancer cells and provided novel evidence that TQ sensitizes colon cancer cells to CisPt by suppressing NF-κB activation.
NF-κB is an ubiquitous transcription factor, consisting of p50, p65 and nuclear factor-κB inhibitor α (IκBα), which is present in the cytoplasm and is activated in response to certain inflammatory stimuli, environmental pollutants, prooxidants, carcinogens, stress and growth factors (46). Following activation, NF-κB translocates from the cytoplasm to the nucleus, binds DNA and induces gene transcription. A number of kinases have been associated with the activation of NF-κB, including IκBα kinase. This activation has been shown to result in the expression of a number of gene products that regulate apoptosis, proliferation, chemoresistance, radioresistance, invasion, angiogenesis, metastasis and inflammation (47,48). In numerous human cancers NF-κB is constitutively activated (49–51). NF-κB activation has been associated with various aspects of oncogenesis, including control of apoptosis, cell cycle, differentiation and cell migration (52,53). In addition, the activation of NF-κB in cancer cells by chemotherapy or radiation may hinder the ability of cancer therapy to induce cell death; therefore, NF-κB has been used as a target for tumor therapies (52,53). In colon cancer, via the regulation of numerous genes differentially expressed and implicated in tumorigenesis, NF-κB activation participates in the promotion and progression steps of colon cancer (54). The present study investigated the effect of TQ on NF-κB activation, and additionally examined the effect of TQ on NF-κB-regulated gene products. The results of the present study revealed that TQ treatment inhibited the phosphorylation of p65 protein in the nucleus of colon cancer cells and decreased the expression of NF-κB-regulated genes, including VEGF, c-Myc and Bcl-2. In addition, the inhibition of NF-κB with a specific inhibitor, PDTC, may potentiate the cell death induction and chemosensitization effect of TQ in colon cancer cells.
In conclusion, the present study demonstrated that TQ may result in cell death in colon cancer cells and sensitize colon cancer cells to CisPt therapy by suppressing NF-κB activation. TQ may be a positive option for adjuvant chemotherapy in the treatment of colon cancer.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jin P, Wu ZT, Li SR, Li SJ, Wang JH, Wang ZH, Lu JG, Cui XJ, Han Y, Rao J, Sheng JQ. Colorectal cancer screening with fecal occult blood test: A 22-year cohort study. Oncol Lett. 2013;6:576–582. doi: 10.3892/ol.2013.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim B, Giardiello FM. Chemoprevention in familial adenomatous polyposis. Best Pract Res Clin Gastroenterol. 2011;25:607–622. doi: 10.1016/j.bpg.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fini L, Piazzi G, Daoud Y, Selgrad M, Maegawa S, Garcia M, Fogliano V, Romano M, Graziani G, Vitaglione P, et al. Chemoprevention of intestinal polyps in ApcMin/+ mice fed with western or balanced diets by drinking annurca apple polyphenol extract. Cancer Prev Res (Phila) 2011;4:907–915. doi: 10.1158/1940-6207.CAPR-10-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajput S, Mandal M. Antitumor promoting potential of selected phytochemicals derived from spices: A review. Eur J Cancer Prev. 2012;21:205–215. doi: 10.1097/CEJ.0b013e32834a7f0c. [DOI] [PubMed] [Google Scholar]
- 6.Woo CC, Kumar AP, Sethi G, Tan KH. Thymoquinone: Potential cure for inflammatory disorders and cancer. Biochem Pharmacol. 2012;83:443–451. doi: 10.1016/j.bcp.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 7.Abukhader MM. Thymoquinone in the clinical treatment of cancer: Fact or fiction? Pharmacogn Rev. 2013;7:117–120. doi: 10.4103/0973-7847.120509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider-Stock R, Fakhoury IH, Zaki AM, El-Baba CO, Gali-Muhtasib HU. Thymoquinone: Fifty years of success in the battle against cancer models. Drug Discov Today. 2014;19:18–30. doi: 10.1016/j.drudis.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Sutton KM, Greenshields AL, Hoskin DW. Thymoquinone, a bioactive component of black caraway seeds, causes G1 phase cell cycle arrest and apoptosis in triple-negative breast cancer cells with mutant p53. Nutr Cancer. 2014;66:408–418. doi: 10.1080/01635581.2013.878739. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Kuang XR, Lv PT, Yan XX. Thymoquinone inhibits proliferation and invasion of human nonsmall-cell lung cancer cells via ERK pathway. Tumour Biol. 2015;36:259–269. doi: 10.1007/s13277-014-2628-z. [DOI] [PubMed] [Google Scholar]
- 11.Siveen KS, Mustafa N, Li F, Kannaiyan R, Ahn KS, Kumar AP, Chng WJ, Sethi G. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-κB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget. 2014;5:634–648. doi: 10.18632/oncotarget.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mu GG, Zhang LL, Li HY, Liao Y, Yu HG. Thymoquinone pretreatment overcomes the insensitivity and potentiates the antitumor effect of gemcitabine through abrogation of Notch1, PI3K/Akt/mTOR regulated signaling pathways in pancreatic cancer. Dig Dis Sci. 2015;60:1067–1080. doi: 10.1007/s10620-014-3394-x. [DOI] [PubMed] [Google Scholar]
- 13.Ichwan SJ, Al-Ani IM, Bilal HG, Suriyah WH, Taher M, Ikeda MA. Apoptotic activities of thymoquinone, an active ingredient of black seed (Nigella sativa), in cervical cancer cell lines. Chin J Physiol. 2014;57:249–255. doi: 10.4077/CJP.2014.BAB190. [DOI] [PubMed] [Google Scholar]
- 14.El-Baba C, Mahadevan V, Fahlbusch FB, S SM, Rau TT, Gali-Muhtasib H, Schneider-Stock R. Thymoquinone-induced conformational changes of PAK1 interrupt prosurvival MEK-ERK signaling in colorectal cancer. Mol Cancer. 2014;13:201. doi: 10.1186/1476-4598-13-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dirican A, Atmaca H, Bozkurt E, Erten C, Karaca B, Uslu R. Novel combination of docetaxel and thymoquinone induces synergistic cytotoxicity and apoptosis in DU-145 human prostate cancer cells by modulating PI3K-AKT pathway. Clin Transl Oncol. 2015;17:145–151. doi: 10.1007/s12094-014-1206-6. [DOI] [PubMed] [Google Scholar]
- 16.Chu SC, Hsieh YS, Yu CC, Lai YY, Chen PN. Thymoquinone induces cell death in human squamous carcinoma cells via caspase activation-dependent apoptosis and LC3-II activation-dependent autophagy. PLoS One. 2014;9:e101579. doi: 10.1371/journal.pone.0101579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashour AE, Abd-Allah AR, Korashy HM, Attia SM, Alzahrani AZ, Saquib Q, Bakheet SA, Abdel-Hamied HE, Jamal S, Rishi AK. Thymoquinone suppression of the human hepatocellular carcinoma cell growth involves inhibition of IL-8 expression, elevated levels of TRAIL receptors, oxidative stress and apoptosis. Mol Cell Biochem. 2014;389:85–98. doi: 10.1007/s11010-013-1930-1. [DOI] [PubMed] [Google Scholar]
- 18.Salim LZ, Mohan S, Othman R, Abdelwahab SI, Kamalidehghan B, Sheikh BY, Ibrahim MY. Thymoquinone induces mitochondria-mediated apoptosis in acute lymphoblastic leukaemia in vitro. Molecules. 2013;18:11219–11240. doi: 10.3390/molecules180911219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Racoma IO, Meisen WH, Wang QE, Kaur B, Wani AA. Thymoquinone inhibits autophagy and induces cathepsin-mediated, caspase-independent cell death in glioblastoma cells. PLoS One. 2013;8:e72882. doi: 10.1371/journal.pone.0072882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng L, Liu A, Shen Y, Xu HZ, Yang SZ, Ying XZ, Liao W, Liu HX, Lin ZQ, Chen QY, et al. Antitumor and anti-angiogenesis effects of thymoquinone on osteosarcoma through the NF-κB pathway. Oncol Rep. 2013;29:571–578. doi: 10.3892/or.2012.2165. [DOI] [PubMed] [Google Scholar]
- 21.Paramasivam A, Sambantham S, Shabnam J, et al. Anti-cancer effects of thymoquinone in mouse neuroblastoma (Neuro-2a) cells through caspase-3 activation with down-regulation of XIAP. Toxicol Lett. 2012;213:151–159. doi: 10.1016/j.toxlet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Mu HQ, Yang S, Wang YJ, Chen YH. Role of NF-κB in the anti-tumor effect of thymoquinone on bladder cancer. Zhonghua Yi Xue Za Zhi. 2012;92:392–396. (In Chinese) [PubMed] [Google Scholar]
- 23.Lei X, Lv X, Liu M, Yang Z, Ji M, Guo X, Dong W. Thymoquinone inhibits growth and augments 5-fluorouracil-induced apoptosis in gastric cancer cells both in vitro and in vivo. Biochem Biophys Res Commun. 2012;417:864–868. doi: 10.1016/j.bbrc.2011.12.063. [DOI] [PubMed] [Google Scholar]
- 24.Nessa MU, Beale P, Chan C, Yu JQ, Huq F. Synergism from combinations of cisplatin and oxaliplatin with quercetin and thymoquinone in human ovarian tumour models. Anticancer Res. 2011;31:3789–3797. [PubMed] [Google Scholar]
- 25.Luo P, Tan Z, Zhang Z, Li H, Mo Z. Inhibitory effects of salvianolic acid B on the high glucose-induced mesangial proliferation via NF-κB-dependent pathway. Biol Pharm Bull. 2008;31:1381–1386. doi: 10.1248/bpb.31.1381. [DOI] [PubMed] [Google Scholar]
- 26.Lui VW, Boehm AL, Koppikar P, Leeman RJ, Johnson D, Ogagan M, Childs E, Freilino M, Grandis JR. Antiproliferative mechanisms of a transcription factor decoy targeting signal transducer and activator of transcription (STAT) 3: The role of STAT1. Mol Pharmacol. 2007;71:1435–1443. doi: 10.1124/mol.106.032284. [DOI] [PubMed] [Google Scholar]
- 27.Rajput S, Kumar BN, Sarkar S, Das S, Azab B, Santhekadur PK, Das SK, Emdad L, Sarkar D, Fisher PB, Mandal M. Targeted apoptotic effects of thymoquinone and tamoxifen on XIAP mediated Akt regulation in breast cancer. PLoS One. 2013;8:e61342. doi: 10.1371/journal.pone.0061342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolli-Bouhafs K, Boukhari A, Abusnina A, Velot E, Gies JP, Lugnier C, Rondé P. Thymoquinone reduces migration and invasion of human glioblastoma cells associated with FAK, MMP-2 and MMP-9 down-regulation. Invest New Drugs. 2012;30:2121–2131. doi: 10.1007/s10637-011-9777-3. [DOI] [PubMed] [Google Scholar]
- 29.Hussain AR, Ahmed M, Ahmed S, Manogaran P, Platanias LC, Alvi SN, Al-Kuraya KS, Uddin S. Thymoquinone suppresses growth and induces apoptosis via generation of reactive oxygen species in primary effusion lymphoma. Free Radic Biol Med. 2011;50:978–987. doi: 10.1016/j.freeradbiomed.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 30.Gali-Muhtasib H, Ocker M, Kuester D, Krueger S, El-Hajj Z, Diestel A, Evert M, El-Najjar N, Peters B, Jurjus A, et al. Thymoquinone reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. J Cell Mol Med. 2008;12:330–342. doi: 10.1111/j.1582-4934.2007.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gali-Muhtasib H, Kuester D, Mawrin C, Bajbouj K, Diestel A, Ocker M, Habold C, Foltzer-Jourdainne C, Schoenfeld P, Peters B, et al. Thymoquinone triggers inactivation of the stress response pathway sensor CHEK1 and contributes to apoptosis in colorectal cancer cells. Cancer Res. 2008;68:5609–5618. doi: 10.1158/0008-5472.CAN-08-0884. [DOI] [PubMed] [Google Scholar]
- 32.Shoieb AM, Elgayyar M, Dudrick PS, Bell JL, Tithof PK. In vitro inhibition of growth and induction of apoptosis in cancer cell lines by thymoquinone. Int J Oncol. 2003;22:107–113. [PubMed] [Google Scholar]
- 33.Woo CC, Hsu A, Kumar AP, Sethi G, Tan KH. Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: The role of p38 MAPK and ROS. PLoS One. 2013;8:e75356. doi: 10.1371/journal.pone.0075356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Mahdy MA, Zhu Q, Wang QE, Wani G, Wani AA. Thymoquinone induces apoptosis through activation of caspase-8 and mitochondrial events in p53-null myeloblastic leukemia HL-60 cells. Int J Cancer. 2005;117:409–417. doi: 10.1002/ijc.21205. [DOI] [PubMed] [Google Scholar]
- 35.Li F, Rajendran P, Sethi G. Thymoquinone inhibits proliferation, induces apoptosis and chemosensitizes human multiple myeloma cells through suppression of signal transducer and activator of transcription 3 activation pathway. Br J Pharmacol. 2010;161:541–554. doi: 10.1111/j.1476-5381.2010.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badr G, Mohany M, Abu-Tarboush F. Thymoquinone decreases F-actin polymerization and the proliferation of human multiple myeloma cells by suppressing STAT3 phosphorylation and Bcl2/Bcl-XL expression. Lipids Health Dis. 2011;10:236. doi: 10.1186/1476-511X-10-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Najjar N, Chatila M, Moukadem H, Vuorela H, Ocker M, Gandesiri M, Schneider-Stock R, Gali-Muhtasib H. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis. 2010;15:183–195. doi: 10.1007/s10495-009-0421-z. [DOI] [PubMed] [Google Scholar]
- 38.Gali-Muhtasib H, Diab-Assaf M, Boltze C, Al-Hmaira J, Hartig R, Roessner A, Schneider-Stock R. Thymoquinone extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism. Int J Oncol. 2004;25:857–866. [PubMed] [Google Scholar]
- 39.Lang M, Borgmann M, Oberhuber G, Evstatiev R, Jimenez K, Dammann KW, Jambrich M, Khare V, Campregher C, Ristl R, Gasche C. Thymoquinone attenuates tumor growth in ApcMin mice by interference with Wnt-signaling. Mol Cancer. 2013;12:41. doi: 10.1186/1476-4598-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jrah-Harzallah H, Ben-Hadj-Khalifa S, Almawi WY, Maaloul A, Houas Z, Mahjoub T. Effect of Thymoquinone on 1,2-dimethyl-hydrazine-induced oxidative stress during initiation and promotion of colon carcinogenesis. Eur J Cancer. 2013;49:1127–1135. doi: 10.1016/j.ejca.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Asfour W, Almadi S, Haffar L. Thymoquinone suppresses cellular proliferation, inhibits VEGF production and obstructs tumor progression and invasion in the rat model of DMH-induced colon carcinogenesis. Pharmacol Pharm. 2013;4:7–17. doi: 10.4236/pp.2013.41002. [DOI] [Google Scholar]
- 42.Velho-Pereira R, Kumar A, Pandey BN, Jagtap AG, Mishra KP. Radiosensitization in human breast carcinoma cells by thymoquinone: Role of cell cycle and apoptosis. Cell Biol Int. 2011;35:1025–1029. doi: 10.1042/CBI20100701. [DOI] [PubMed] [Google Scholar]
- 43.Jafri SH, Glass J, Shi R, Zhang S, Prince M, Kleiner-Hancock H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: In vitro and in vivo. J Exp Clin Cancer Res. 2010;29:87. doi: 10.1186/1756-9966-29-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banerjee S, Kaseb AO, Wang Z, Kong D, Mohammad M, Padhye S, Sarkar FH, Mohammad RM. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res. 2009;69:5575–5583. doi: 10.1158/0008-5472.CAN-08-4235. [DOI] [PubMed] [Google Scholar]
- 45.Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, Sarkar S. Drug resistance in cancer: An overview. Cancers (Basel) 2014;6:1769–1792. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aggarwal BB. Nuclear factor-kappaB: The enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Shishodia S, Aggarwal BB. Nuclear factor-kappaB activation mediates cellular transformation, proliferation, invasion angiogenesis and metastasis of cancer. Cancer Treat Res. 2004;119:139–173. doi: 10.1007/1-4020-7847-1_8. [DOI] [PubMed] [Google Scholar]
- 48.Ahn KS, Aggarwal BB. Transcription factor NF-kappaB: A sensor for smoke and stress signals. Ann NY Acad Sci. 2005;1056:218–233. doi: 10.1196/annals.1352.026. [DOI] [PubMed] [Google Scholar]
- 49.Nair A, Venkatraman M, Maliekal TT, Nair B, Karunagaran D. NF-kappaB is constitutively activated in high-grade squamous intraepithelial lesions and squamous cell carcinomas of the human uterine cervix. Oncogene. 2003;22:50–58. doi: 10.1038/sj.onc.1206043. [DOI] [PubMed] [Google Scholar]
- 50.Li W, Tan D, Zenali MJ, Brown RE. Constitutive activation of nuclear factor-kappaB (NF-κB) signaling pathway in fibrolamellar hepatocellular carcinoma. Int J Clin Exp Pathol. 2009;3:238–243. [PMC free article] [PubMed] [Google Scholar]
- 51.Nagel D, Vincendeau M, Eitelhuber AC, Krappmann D. Mechanisms and consequences of constitutive NF-κB activation in B-cell lymphoid malignancies. Oncogene. 2014;33:5655–5665. doi: 10.1038/onc.2013.565. [DOI] [PubMed] [Google Scholar]
- 52.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen HM, Tergaonkar V. NFkappaB signaling in carcinogenesis and as a potential molecular target for cancer therapy. Apoptosis. 2009;14:348–363. doi: 10.1007/s10495-009-0315-0. [DOI] [PubMed] [Google Scholar]
- 54.Wang S, Liu Z, Wang L, Zhang X. NF-kappaB signaling pathway, inflammation and colorectal cancer. Cell Mol Immunol. 2009;6:327–334. doi: 10.1038/cmi.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]