Abstract
The aim of the present study was to summarize the clinical manifestations, diagnosis, treatment, and prognosis of solitary fibrous tumor (SFT). In total, 47 cases of SFTs diagnosed by postoperative pathology between January 2002 and September 2014 were retrospectively reviewed, and the general information, clinical manifestations, imaging techniques, treatment, pathology and follow-up findings were analyzed. Of the 47 patients, clinical characteristics were collected in 37 cases (18 men and 19 women; mean age, 44.1 years; age range, 13–72 years). The maximum diameters of the tumors were 1.5–25 cm, with a mean diameter of 8.8 cm. The symptoms were various and non-specific. Imaging examinations following iodinated contrast administration showed the SFTs to be well-defined, cystic or solid mass and enhanced. On color Doppler ultrasound, SFTs were described as hypoechoic, clear, irregular masses. All patients underwent surgical resection, and SFT was diagnosed by postoperative pathological and immunohistochemical examination. Of the 47 patients, 25 received complete follow-up of 5–130 months, with a median follow-up period of 35.2 months, that included a color Doppler ultrasound or computed tomography (CT) scan every 6–12 months. At the end of the follow-up period all patients were alive and healthy, with the exception of one patient, who presented with recurrence 15 months after surgery. The findings of the present study showed SFT to be a rare systemic disease with no particular clinical manifestations. In the cases reviewed in the present study, CT, magnetic resonance imaging scans and color Doppler ultrasound were important for the diagnosis of SFT, while the definitive diagnosis relied on pathological and immunohistochemical examinations. Surgery, the primary treatment for SFT, was performed, and, following complete removal of the tumor, the prognosis was favorable.
Keywords: solitary fibrous tumor, clinical analysis, pleura
Introduction
Solitary fibrous tumor (SFT), also known as hemangiopericytoma, is a fibroblastic mesenchymal tumor that was first described by Klemperer and Rabin in 1992 (1). SFTs are rare entities accounting for <2% of all soft tissue sarcomas (2). Although SFTs are commonly found in the pleura, according to the literature, 50–70% of these tumors are extrapleural and can manifest in the head and neck, abdomen and pelvis (3). This condition is rare and only limited cases of SFT have been reported. Due to the rarity of this tumor, preoperative diagnosis is challenging and no consensus has been reached on standard treatment. The present study summarizes the diagnosis, treatment and prognosis of 47 cases of SFTs from the literature, in order to determine the clinicopathological profile of SFT. To the best of our knowledge, the present retrospective study on SFT is the largest reported to date.
Patients and methods
Patients
Patient files from the Department of Pathology of the Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, (Nanjing, China) from the period between January 2002 and September 2014 were retrospectively reviewed, and 47 cases of SFT were identified, including 39 inpatients and 8 outpatients. The present study was approved by the Institutional Review Board of Nanjing Drum Tower Hospital. Informed consent for participation in the study was obtained from all patients. All patients had been diagnosed with SFT by a pathological examination. Patient information, including age and gender, details of the lesion, clinical symptoms at the time of diagnosis, auxiliary and pathological examination findings, treatment and follow-up, was recorded.
Auxiliary examination types and findings
The findings of different types of auxiliary examination, including computed tomography (CT), magnetic resonance imaging (MRI) scans and color Doppler ultrasound, were reviewed. A histopathological examination of paraffin-embedded surgical specimens was performed to confirm the diagnosis using standard hematoxylin and eosin staining and immunohistochemical techniques. Immunohistochemical staining was performed using a Dako EnVision System (Dako, Glostrup, Denmark), according to the manufacturer's instructions. The primary antibodies included rabbit polyclonal antibodies against vimentin (sc-5565; 1:200), cluster of differentiation (CD)34 (sc-9095; 1:100), actin (sc-7210; 1:200), desmin (sc-14026; 1:200), cytokeratin (CK; sc-134493; 1:100), S100 (sc-7849-R; 1:100), B-cell lymphoma 2 (bcl-2; sc-783; 1:200), CD99 (sc-241355; 1:200), CD117 (sc-3936; 1:200), epithelial membrane antigen (EMA; sc-6826; 1:200) and smooth muscle actin (SMA; sc-92040; 1:200; all Santa Cruz Biotechnology Inc., Dallas, TX, USA). 3,3′-diaminobenzidine was used as the chromogen.
Results
Baseline characteristics
Out of the 47 screened patients, full clinical characteristics were collected from 37 (18 men and 19 women; age range, 13–72 years; mean age, 44.1 years) and are summarized in Table I. The maximum diameter of the tumors was 1.5–25 cm, with a mean diameter of 8.8 cm. The patients' symptoms were various and non-specific, and included pain, cough, inhibited defecation and frequent micturition. In total, 23 of the 37 patients (62.2%) had no symptoms at all, and the tumor was discovered incidentally during physical examination. Common lesion locations included the lungs, pleura, chest wall and pelvic cavity, while certain rare sites, including the thyroid, groin, eyebrow bow, bladder, kidney, eye, mediastinum and sigmoid mesocolon, were discovered.
Table I.
Clinical data of 37 patients with solitary fibrous tumor.
| Case no./gender/agea | Symptom | Location | Treatment | Margin status | Diameter | Recurrence | Metastasis | Follow-up, months |
|---|---|---|---|---|---|---|---|---|
| 1/F/48 | No | Chest wall | SE | Negative | 3.0 | No | No | NED, 130 |
| 2/M/35 | No | Lung | SE | Negative | 6.0 | NA | NA | NA |
| 3/F/48 | No | Thoracic cavity | SE | Negative | 21.0 | NA | NA | NA |
| 4/M/49 | Pharyngeal dryness | Pharynx side clearance | SE | Negative | 4.0 | NA | NA | NA |
| 5/F/41 | Pain | Infratemporal fossa | SE | Negative | 5.7 | NA | NA | NA |
| 6/F/44 | No | Diaphragm | SE | Negative | 8.0 | NA | NA | NA |
| 7/F/53 | Abdominal pain | Mesentery of small intestine | SE | Negative | 7.5 | NA | NA | NA |
| 8/M/55 | Chest pain | Posterior superior iliac spine | SE | Negative | NA | No | No | NED, 40 |
| 9/F/44 | No | Kidney | SE | Negative | 11.0 | NA | NA | NA |
| 10/M/47 | No | Forearm | SE | Negative | 10.0 | NA | NA | NA |
| 11/F/62 | No | Chest wall | SE | Negative | 8.0 | No | No | NED, 68 |
| 12/F/13 | Knees ache | Knee | SE | Negative | 4.0 | NA | NA | NA |
| 13/M/23 | No | Nasal cavity | SE | Negative | 5.0 | No | No | NED, 65 |
| 14/M/62 | No | Pelvic cavity | SE | Negative | 9.0 | NA | NA | NA |
| 15/F/51 | Neck Pain | Cervical vertebra | SE | Negative | 2.0 | No | No | NED, 57 |
| 16/M/53 | No | Lung | SE | Negative | 3.0 | No | No | NED, 55 |
| 17/M/49 | Abdominal distension | Abdomen | SE | Negative | 18.0 | NA | NA | NA |
| 18/M/47 | No | Thoracic cavity | SE | Negative | 8.0 | No | No | NED, 50 |
| 19/M/53 | No | Hip joint | SE | Negative | 10.0 | No | No | NED, 45 |
| 20/M/55 | Frequency of urination | Pelvic cavity | SE | Negative | 14.0 | No | No | NED, 44 |
| 21/F/13 | Cough | Lung | SE | Negative | 5.0 | No | No | NED, 39 |
| 22/M/27 | Pain | Hip | SE | Negative | 16.0 | NA | NA | NA |
| 23/F/48 | Headache, tinnitus | Temporal lobe | SE | Negative | 6.0 | No | No | NED, 35 |
| 24/F/39 | Chest pain | Mediastinum | SE | Negative | 11.0 | No | No | NED, 33 |
| 25/M/61 | No | Gastrocolic ligament | SE | Negative | 5.0 | No | No | NED, 33 |
| 26/F/58 | No | Kidney | SE | Negative | 8.0 | Yes | No | NED, 15 after 2nd surgery |
| 27/F/40 | No | Bladder | SE | Negative | 5.0 | No | No | NED, 30 |
| 28/F/64 | No | Lung | SE | Negative | NA | No | No | NED, 18 |
| 29/M/49 | No | Pleura | SE | Negative | 3.5 | No | No | NED, 18 |
| 30/M/60 | No | Testis | SE | Negative | 7.0 | No | No | NED, 18 |
| 31/F/22 | No | Inguinal ligament | SE | Negative | 7.0 | No | No | NED, 14 |
| 32/M/21 | No | Diaphragm | SE | Negative | 24.0 | No | No | NED, 12 |
| 33/M/72 | No | Lung | SE | Negative | 6.0 | No | No | NED, 11 |
| 34/F/16 | No | Chest wall | SE | Negative | 8.5 | No | No | NED, 10 |
| 35/F/34 | Abdominal pain | Stomach | SE | Negative | 11.0 | No | No | NED, 9 |
| 36/M/24 | Constipation | Pelvic cavity | SE | Negative | NA | No | No | NED, 10 |
| 37/F/50 | No | Sigmoid mesocolon | SE | Negative | 25.0 | No | No | NED, 5 |
Age is measured in years. F, female; M, male; SE, surgical excision; NA, data not available; NED, no evidence of disease.
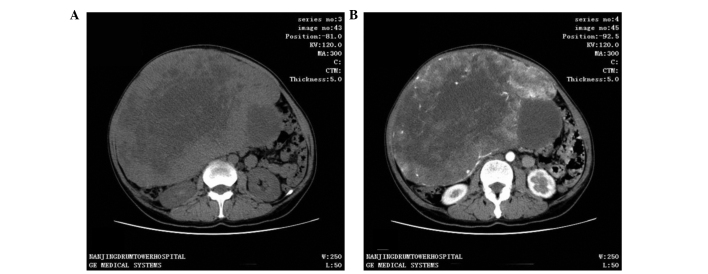
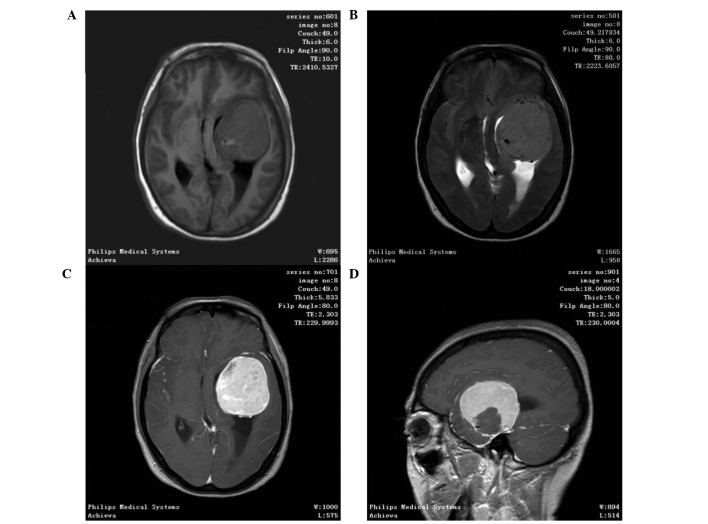
CT scan was the most frequent diagnostic imaging technique used. CT images captured following iodinated contrast administration from 30 of the 37 cases showed SFTs as well-defined, cystic or solid mass, and enhanced (Fig. 1). In total, 11 out of 37 patients underwent an MRI scan, in which SFTs were shown as lobulated, heterogeneous soft tissue with short T1-weighted (T1WI) and a flake long T2-weighted imaging (T2WI) signal. Contrast-enhanced MRI scans demonstrated heterogeneous contrast enhancement (Fig. 2). Color Doppler ultrasound was performed in 14 out of 37 patients and found the tumors to be hypoechoic, clear, irregular masses. All patients underwent successful surgical resection with no serious complications, and no postoperative mortality was recorded. No patient underwent other adjuvant therapy.
Figure 1.
A 50-year-old female presented with a large abdominal mass. Computed tomography scan showing (A) a well-fined cystic or solid mass measuring 24.5×17.4 cm and (B) an uneven enhancement of the tumor following iodinated contrast administration.
Figure 2.
A 48-year-old female presented with a headache that had lasted for 1 month. Magnetic resonance imaging scan showing a well-defined and round soft tissue mass measuring 5×5 cm. (A) T1-weighted image; (B), T2-weighted image; (C) arterial phase; (D) sagittal view.
Pathological findings
All patients were definitively diagnosed with SFT by postoperative pathological and immunohistochemical examination. Macroscopically, most tumors appeared as solid, well-encapsulated, smooth to firm soft tissue masses, with a gray-white to red-brown color on the cut surface. Microscopically, the tumors were shown to be comprised of spindle or short spindle cells and various quantities of vascular tissue. In certain regions, the cells were arranged in short, ill-defined fascicles, whereas in others they were arranged irregularly.
Immunohistochemical findings
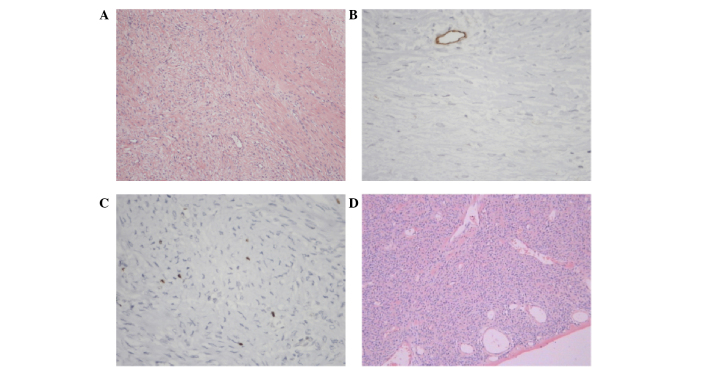
Immunohistochemistry showed the following: The positive rates of vimentin, CD34, CD99, bcl-2, actin, desmin, CK, S100, CD117, EMA and SMA were 13/13 (100.0%), 40/46 (87.0%), 25/31 (80.6%), 30/38 (79.0%), 6/21 (28.6%), 2/35 (5.7%), 0/18 (0%), 6/40 (15%), 2/18 (11.1%), 3/13 (23.1%) and 7/15 (46.7%), respectively. The immunohistochemical indexes were selected according to the location of primary lesion, as different tissues have different specific markers. The representative pathological and immunohistochemical images of a patient that presented with SFT in the diaphragm are shown in Fig. 3. The immunohistochemical results for this patient were as follows: CD34−, CD99−, neurofilament−, bc1-2+, desmin−, actin−, S100−, Ki67+ 1% and β-catenin+.
Figure 3.
Microscopic examination of a solitary fibrous tumor located in the diaphragm of a patient, showing proliferating spindle cells with a bundle or flow pattern, determined by hematoxylin and eosin staining. Clusters of spindle cells were readily identifiable, and no nuclear atypia, mitotic activity or necrosis was evident; magnification, (A) ×100 and (B) ×400. Immunohistochemical examination showing that the tissue was (C) β-catenin and (D) B-cell lymphoma-2 positive.
Follow-up information
A total of 25 patients received complete follow-up lasting 5–130 months (median follow-up period, 35.2 months), during which they underwent a color Doppler ultrasound or CT scan every 6–12 months. At the time of writing, all patients were alive and healthy. Only 1 patient (4%) presented with recurrent SFT 15 months after the first surgery, and had a disease-free survival following the second operation. The status of each patient at the last follow-up is summarized in Table I. The follow-up data from 22 patients of the total 47 (12 of the 37) were either lost or could not be obtained.
Discussion
SFT was first described as a rare spindle cell tumor that arises from the visceral pleura (4); however, over time, SFT was identified in various other locations outside of the thoracic cavity, such as the meninges (5,6), orbit (7), nasal cavity (8), salivary gland (9), parapharyngeal space (10) and paranasal sinuses (11). The present study reviewed numerous cases of SFT manifesting in locations outside the thoracic cavity, such as the thyroid, groin, bladder, sigmoid mesocolon and posterior superior iliac spine, locations that had not been previously reported. According to the literature (12), SFT is usually encountered in middle-aged people with an equal distribution between genders; however, it has also been reported in young patients (13). Consistent with these findings, the gender ratio in the present study was 0.95 (18 men and 19 women) and the mean age was 44.1 years. The symptoms varied depending on the lesions; the majority of patients (23/37) presented with physical symptoms. The diameter of SFTs is usually large (commonly >8cm) (14). In the present study, 50% patients had a tumor measuring >8 cm.
SFTs do not express any tumor markers, and diagnostic techniques include CT, MRI scans and color Doppler ultrasound, despite the fact that their results may not be specific (9). These imaging tests often provide the first clue to the identification of the tumors, depiction of local extent and invasion of adjacent structures, which is useful in guiding surgery. On CT scan, SFT appears as a well-defined, heterogeneous or homogeneous isodense mass, and shows moderate to marked enhancement following contrast administration (15,16). On color Doppler ultrasound, it has the appearance of a hypoecho, clear, and irregular mass (17). On MRI, it has been shown to exhibit intermediate signal intensity on T1WI images, and enhancement on T2WI images, which was in accordance with the findings of the present study (18,19). None of these methods, however, could achieve accurate diagnosis; the final diagnosis has been reported to rely on surgical resection and pathological examination (20,21).
In the present study, all patients underwent surgical resection. Macroscopically, the majority of SFTs appear as rounded, encapsulated masses of homogenous density, with a yellow/brown-to-white whorled appearance of the cut surface (22). Microscopically, SFTs are shown to be comprised of spindle or short spindle cells and varying quantities of vascular tissue (9). The micro and macroscopic results of the present study were in accordance with the findings of previous studies. Immunohistochemically, SFT shows immunoreactivity with vimentin and CD34, with the largest part of tumor also displaying positive results for bcl-2 and CD99; however, the specimens do not express EMA or S-100 proteins (23). The immunohistochemical analysis results of the current study validated the previous findings.
The primary treatment of SFT is surgical resection with negative margins (24). All patients from the present study underwent complete resection without adjuvant treatments, such as radiotherapy or chemotherapy. Tumors that cannot be completely excised or that show malignant histological features may respond to radiation and/or chemotherapy. Xue et al (25) described a case of non-recurrent malignant SFT of the nasal and paranasal areas, and found that the combination of cytoreductive surgery with intensity-modulated radiation and stereotactic body radiation therapies have a good result. Studies have also demonstrated that preoperative embolization may be employed prior to surgical resection for highly vascular tumors (26,27). Immunotherapy, for example with interferon, may also be effective (28).
A previous study showed that the prognosis of patients with SFT is favorable (29). Patients that underwent complete tumor resection showed 100% survival at a mean follow-up period of 1.9 years (29). In the present study, all 25 patients that received a complete follow-up were alive at the time of writing. The longest follow-up period among these patients was 130 months. The recurrence rate of SFT was 4%.
In conclusion, SFT is a rare systemic disease with no particular clinical manifestations. In the cases reviewed in the present study, CT and MRI scans and color Doppler ultrasound were important for the diagnosis of SFT; however, the final diagnosis relied on pathological and immunohistochemical examinations. Surgery was the primary treatment for SFT, and the prognosis following complete tumor resection was favorable.
References
- 1.Klemperer P, Coleman BR. Primary neoplasms of the pleura. A report of five cases. Am J Ind Med. 1992;22:1–31. doi: 10.1002/ajim.4700220103. [DOI] [PubMed] [Google Scholar]
- 2.Gold JS, Antonescu CR, Hajdu C, Ferrone CR, Hussain M, Lewis JJ, Brennan MF, Coit DG. Clinicopathologic correlates of solitary fibrous tumors. Cancer. 2002;94:1057–1068. doi: 10.1002/cncr.10328. [DOI] [PubMed] [Google Scholar]
- 3.vanHoudt WJ, Westerveld CM, Vrijenhoek JE, van Gorp J, van Coevorden F, Verhoef C, van Dalen T. Prognosis of solitary fibrous tumors: A multicenter study. Ann Surg Oncol. 2013;20:4090–4095. doi: 10.1245/s10434-013-3242-9. [DOI] [PubMed] [Google Scholar]
- 4.England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol. 1989;13:640–658. doi: 10.1097/00000478-198908000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa K, Tada T, Takahashi S, Sugiyama N, Inaguma S, Takahashi SS, Shirai T. Malignant solitary fibrous tumor of the meninges. Virchows Arch. 2004;444:459–464. doi: 10.1007/s00428-004-0991-7. [DOI] [PubMed] [Google Scholar]
- 6.Saceda-Gutiérrez JM, Isla-Guerrero AJ, Pérez-López C, Ortega-Martínez R, de la Riva A Gómez, Gandia-González ML, Gutiérrez-Molina M, Rey-Herranz JA. Solitary fibrous tumors of the meninges: Report of three cases and literature review. Neurocirugia (Astur) 2007;18:496–504. doi: 10.1016/S1130-1473(07)70258-0. (In Spanish) [DOI] [PubMed] [Google Scholar]
- 7.Adeleye AO, Ogun OA, Ogun GO. Orbital solitary fibrous tumor. Another rare case from Africa. Int Ophthalmol. 2010;30:315–318. doi: 10.1007/s10792-009-9320-0. [DOI] [PubMed] [Google Scholar]
- 8.Kessler A, Lapinsky J, Berenholz L, Sarfaty S, Segal S. Solitary fibrous tumor of the nasal cavity. Otolaryngol Head Neck Surg. 1999;121:826–828. doi: 10.1053/hn.1999.v121.a95230. [DOI] [PubMed] [Google Scholar]
- 9.O'Regan EM, Vanguri V, Allen CM, Eversole LR, Wright JM, Woo SB. Solitary fibrous tumor of the oral cavity: Clinicopathologic and immunohistochemical study of 21 cases. Head Neck Pathol. 2009;3:106–115. doi: 10.1007/s12105-009-0111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong AK, Lee HK, Kim SY, Cho KJ. Solitary fibrous tumor of the parapharyngeal space: MR imaging findings. AJNR Am J Neuroradiol. 2002;23:473–475. [PMC free article] [PubMed] [Google Scholar]
- 11.Kim TA, Brunberg JA, Pearson JP, Ross DA. Solitary fibrous tumor of the paranasal sinuses: CT and MR appearance. AJNR Am J Neuroradiol. 1996;17:1767–1772. [PMC free article] [PubMed] [Google Scholar]
- 12.Cho KJ, Ro JY, Choi J, Choi SH, Nam SY, Kim SY. Mesenchymal neoplasms of the major salivary glands: Clinicopathological features of 18 cases. Eur Arch Otorhinolaryngol. 2008;265(Suppl 1):S47–S56. doi: 10.1007/s00405-007-0488-5. [DOI] [PubMed] [Google Scholar]
- 13.Mathew GA, Ashish G, Tyagi AK, Chandrashekharan R, Paul RR. Solitary Fibrous Tumor of Nasal Cavity: A Case Report. Iran J Otorhinolaryngol. 2015;27:307–312. [PMC free article] [PubMed] [Google Scholar]
- 14.Huang SC, Li CF, Kao YC, Chuang IC, Tai HC, Tsai JW, Yu SC, Huang HY, Lan J, Yen SL, et al. The clinicopathological significance of NAB2-STAT6 gene fusions in 52 cases of intrathoracic solitary fibrous tumors. Cancer Med. 2016;5:159–168. doi: 10.1002/cam4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chis O, Albu S. Giant solitary fibrous tumor of the parotid gland. Case Rep Med. 2014;2014:950712. doi: 10.1155/2014/950712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li XM, Reng J, Zhou P, Cao Y, Cheng ZZ, Xiao Y, Xu GH. Solitary fibrous tumors in abdomen and pelvis: Imaging characteristics and radiologic-pathologic correlation. World J Gastroenterol. 2014;20:5066–5073. doi: 10.3748/wjg.v20.i17.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SB, Park YS, Kim JK, Kim MH, Oh YT, Kim KA, Cho KS. Solitary fibrous tumor of the genitourinary tract. AJR Am J Roentgenol. 2011;196:W132–W137. doi: 10.2214/AJR.09.3787. [DOI] [PubMed] [Google Scholar]
- 18.Bauer JL, Miklos AZ, Thompson LD. Parotid gland solitary fibrous tumor: A case report and clinicopathologic review of 22 cases from the literature. Head Neck Pathol. 2012;6:21–31. doi: 10.1007/s12105-011-0305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messa-Botero OA, Romero-Rojas AE, Olaya SI Chinchilla, Díaz-Pérez JA, Tapias-Vargas LF. Primary malignant solitary fibrous tumor/hemangiopericytoma of the parotid gland. Acta Otorrinolaringol Esp. 2011;62:242–245. doi: 10.1016/j.otorri.2010.02.007. (In Spanish) [DOI] [PubMed] [Google Scholar]
- 20.Hunt I, Ewanowich C, Reid A, Stewart K, Bédard EL, Valji A. Managing a solitary fibrous tumour of the diaphragm from above and below. ANZ J Surg. 2010;80:370–371. doi: 10.1111/j.1445-2197.2010.05282.x. [DOI] [PubMed] [Google Scholar]
- 21.Kita Y. Pleural solitary fibrous tumor from diaphragm, being suspected of liver invasion; report of a case. Kyobu Geka. 2012;65:338–340. (In Japanese) [PubMed] [Google Scholar]
- 22.Wang H, Chen P, Zhao W, Shi L, Gu X, Xu Q. Clinicopathological findings in a case series of abdominopelvic solitary fibrous tumors. Oncol Lett. 2014;7:1067–1072. doi: 10.3892/ol.2014.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong Q, Yuan S. Total resection of a solitary fibrous tumor of the sellar diaphragm: A case report. Oncol Lett. 2013;5:1783–1786. doi: 10.3892/ol.2013.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gengler C, Guillou L. Solitary fibrous tumour and haemangiopericytoma: Evolution of a concept. Histopathology. 2006;48:63–74. doi: 10.1111/j.1365-2559.2005.02290.x. [DOI] [PubMed] [Google Scholar]
- 25.Xue Y, Chai G, Xiao F, Wang N, Mu Y, Wang Y, Shi M. Post-operative radiotherapy for the treatment of malignant solitary fibrous tumor of the nasal and paranasal area. Jpn J Clin Oncol. 2014;44:926–931. doi: 10.1093/jjco/hyu100. [DOI] [PubMed] [Google Scholar]
- 26.Zerón-Medina J, Rodríguez-Covarrubias F, García-Mora A, Guerrero-Hernandez M, Chablé-Montero F, Albores-Saavedra J, Medina-Franco H. Solitary fibrous tumor of the pelvis treated with preoperative embolization and pelvic exenteration. Am Surg. 2011;77:112–113. [PubMed] [Google Scholar]
- 27.Botchu R, Khan AN, Adair W, Elabassy M. Solitary fibrous tumor made resectable after successful endovascular embolization. J Gastrointest Cancer. 2011;42:287–291. doi: 10.1007/s12029-010-9247-8. [DOI] [PubMed] [Google Scholar]
- 28.Cuello J, Brugés R. Malignant solitary fibrous tumor of the kidney: Report of the first case managed with interferon. Case Rep Oncol Med. 2013;2013:564980. doi: 10.1155/2013/564980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manglik N, Patil S, Reed MF. Solitary fibrous tumour of the parotid gland. Pathology. 2008;40:89–91. doi: 10.1080/00313020701716334. [DOI] [PubMed] [Google Scholar]