Abstract
Alien hand syndrome (AHS) is characterized by involuntary and autonomous activity of the affected limbs, and consists of the frontal, callosal and posterior AHS variants. The callosal subtype, resulting from damage to the corpus callosum, frequently features intermanual conflict. However, infarction of the corpus callosum is rare due to abundant blood supply. The present study reported a case of AHS (callosal subtype, in the right hand) caused by callosal infarction. Infarction of the left corpus callosum was confirmed with magnetic resonance imaging. In addition, magnetic resonance angiography and digital subtraction angiography examinations revealed multiple lesions in the feeding arteries. Subsequent to antiplatelet therapy for 2 weeks following admission, the patient gradually recovered. Furthermore, the current study reviewed 31 previously reported cases of AHS following callosal infarction in the literature.
Keywords: alien hand syndrome, infarction, corpus callosum
Introduction
Alien hand syndrome (AHS) is a rare clinical syndrome; using the Mayo Clinic Medical Records Linkage System, Graff-Radford et al (1) identified 150 patients with alien limbs within the Department of Neurology between January 1, 1996, and July 11, 2011. Numerous medical and surgical conditions can cause AHS, such as cerebral infarction, cerebral hemorrhage, corticobasal degeneration, epilepsia partialis continua, Alzheimer's disease, progressive supranuclear palsy and Creutzfeldt-Jakob disease (2–9). However, there are few reports about the pharmacologic or rehabilitation treatment of AHS. Experience with pharmacologic treatment of AHS has been limited to platelet aggregation inhibitors (10,11). In addition, the rehabilitation treatment of AHS is lacking in the literature (12–14). Pooyania et al (14) identified that being educated about the diagnosis of AHS and the use of compensatory strategies (visualization, distraction of affected limb and maintaining a slow/steady pace during activities) decreased the frequency of patient's AHS movements (14).
AHS is a movement disorder characterized by involuntary and autonomous movements. AHS can be divided into three variants, including the frontal, callosal and posterior AHS subtypes (5,15). The callosal subtype is characterized by intermanual conflict (antagonizing movements of the two hands), mostly due to the disconnection between the two cerebral hemispheres (16). The blood supply to the corpus callosum is ample; thus, infraction in this area is rare. The present study reports a case of callosal-subtype AHS presenting following corpus callosum infarction. In addition, the current case was compared with a collection of 31 previously reported cases of AHS that were caused by callosal infarction.
Case report
A 56-year-old woman presented at the Yantai Yuhuangding Hospital (Yantai, China) in November 2011 with involuntary and autonomous activity of the right hand that persisted for 1 month, without apparent cause. Intermanual conflict was the most troubling feature experienced. For instance, the patient's right hand took off her clothing while she attempted to dress with the left hand. The patient also reported weak right limb and dysarthria. She had a history of hypertension, coronary artery disease and type 2 diabetes mellitus, but no substance abuse history, including smoking and drinking. No similar disease or syndrome was reported for any family members. Written informed consent was obtained from the patient.
Physical examination failed to revealed anepia, anarthria, autotopagnosia or apraxia. No signs of meningeal irritation were observed. In addition, an ophthalmic examination failed to reveal any abnormalities, whereas a slightly superficial right nasolabial fold was detected. Muscle tension was normal, with level-5 muscle force in the right upper extremity and level-4 in the right lower extremity (17). Sensory, finger-to-nose and left heel-knee-shin tests were normal. However, the heel-knee-shin test on the right side was unsuccessful. Deep tendon reflex was normal, with no pathological signs. The mini-mental state examination (MMSE) score was 30 (18).
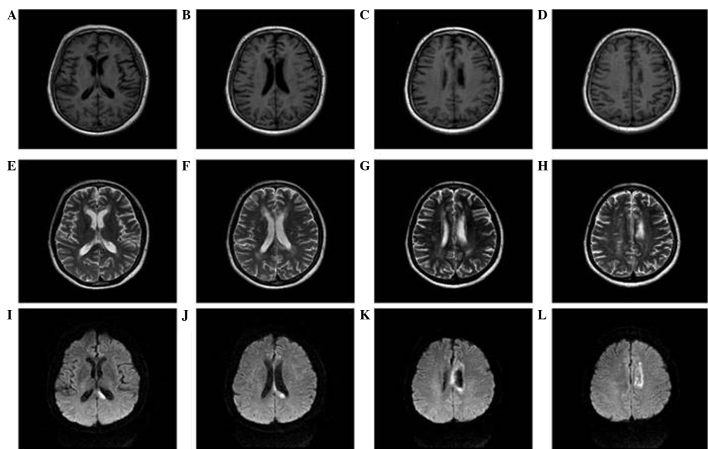
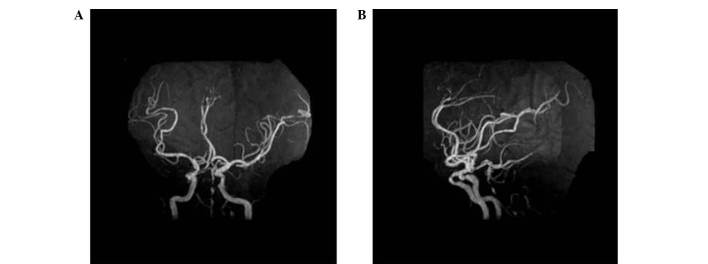
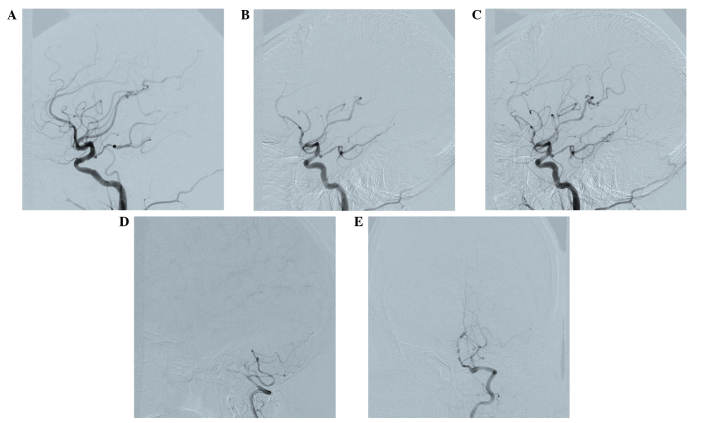
Doppler color imaging demonstrated atherosclerotic plaques in bilateral carotid arteries and increased resistance in bilateral vertebral arteries. Cranial magnetic resonance imaging (MRI) displayed long T1 and T2 signals, as well as high signals on diffusion weighted imaging (DWI), in the body and splenium of the left corpus callosum (Fig. 1). Brain magnetic resonance angiography (MRA) 3 days following admission revealed extensive atherosclerosis and intermittent visualization of the basilar artery (Fig. 2A). Cerebral digital subtraction angiography (DSA) of the right or left internal carotid artery showed the opening of the corresponding posterior communicating artery. Furthermore, bilateral posterior cerebral arteries and basilar artery apex were visualized. The left anterior cerebral artery and left pericallosal arteries appeared faint. The right vertebral artery was narrow, and the distal segment of the intracranial branch was occluded. In addition, the intracranial branch of the left vertebral artery and basilar artery was significantly narrowed (Fig. 3). A diagnosis of callosal AHS was established based on the aforementioned observations. The characteristic features of callosal AHS reported in the present patient included involuntary autonomous movement, and intermanual conflict. Infarction of the corpus callosum was clearly demonstrated in MRI scans, while MRA and DSA revealed multiple lesions in the feeding arteries.
Figure 1.
Multi-modal brain magnetic resonance imaging (MRI). (A and B) T1 hypo-intensity in the splenium of the left corpus callosum. (C and D) T1 hypo-intensity in the body of the left corpus callosum. (E and F) T2 hyper-intensity in the splenium of the left corpus callosum. (G and H) T2 hyper-intensity in body of the left corpus callosum. (I-L) Axial diffusion weighted imaging of MRI. (I and J) Diffusion weighted imaging showed high signal intensity in the splenium of the corpus callosum. (K and L) Diffusion weighted imaging demonstrated high signal intensity in the body of the corpus callosum. All these confirm the infarction of left corpus callosum.
Figure 2.
Brain MRA. (A) MRA revealed extensive stenosis in basilar artery. (B) Lateral view of MRA showed multiple severe stenosis of basilar artery. MRA, magnetic resonance angiography.
Figure 3.
Cerebral digital subtraction angiography. (A) Angiography of the right internal carotid artery showed opening of the posterior communicating artery in the right cerebral artery. (B) Angiography of the left internal carotid artery revealed opening of the posterior communicating artery in the left cerebral artery, while the bilateral posterior cerebral artery and basilar artery apex are also visualized. (C) Faint visualization of the left anterior cerebral artery and pericallosal artery are shown. (D) Fine right vertebral artery and occlusion of distal segment of the intracranial branch in the right vertebral artery are shown. (E) Fine and occlusive intracranial branch of the left vertebral and basilar arteries were visualized, with ~90% stenosis observed.
The patient was treated with aspirin enteric coated tablets (100 mg, qd; Bayer AG, Leverkusen, Germany) atorvastatin calcium (20 mg, qn; Pfizer), amlodipine besylate tablets (5 mg, qd; Pfizer, Inc., New York, NY, USA) and metformi (500 mg, bid; Bristol-Myers Squibb, New York, NY, USA) during the 2-week hospital stay, and continued to receive the same treatment until the last visit in March 2015. No stroke recurrence was observed.
Discussion
Frontal AHS is characterized by forced grasping of objects and impulsive reaching and groping movements toward nearby objects within the visual field. This type of AHS is typically caused by lesions located in the anterior corpus callosum, the supplementary motor area, the anterior cingulate gyrus and the medial prefrontal cortex of the dominant hemisphere. By contrast, callosal AHS typically results from a callosal lesion and is characterized primarily by intermanual conflict and apraxia of the non-dominant limb (5). Posterior AHS is characterized by the feeling of an alien hand and left hemianesthesia, which lesions primarily in the cortex or subcortical structures, such as the thalamus, parietal lobe and medial temporal lobe that are supplied by the posterior cerebral artery (10,12,13,15). The current study reported a case with characteristic features of callosal AHS, including involuntary autonomous movement, as well as intermanual conflict. Infarction of the corpus callosum was clearly demonstrated according to the imaging results.
In the present study, a search of the PubMed database (www.ncbi.nlm.nih.gov/pubmed) was performed (keyword, alien hand syndrome) in order to identify previous AHS cases presenting after callosal infraction. The search yielded 23 studies reporting 31 cases of AHS following callosal infarction between 1990 and 2014 (5,19–40). These callosal infarction cases were divided into simple corpus callosum infarction and complex corpus callosum infarction. Simple corpus callosum infarction was defined as infarction occurring in the corpus callosum alone; complex corpus callosum infarction was defined as corpus callosum with involvement of other brain sites.
The search yielded a total of 31 previous AHS cases, with 7 cases of simple corpus callosum infarction (22.6%) and 24 cases of complex corpus callosum infarction (77.4%). As shown in Table I, the corpus callosum was affected on the left side in 10 out of the 31 cases (5,19,25–30) and on the right side in 17 cases (20,21,25–27,31–40). Both sides of the corpus callosum were affected in 4 cases (20,22–24), all of which presented simple corpus callosum infarction (Table I). In complex corpus callosum infarction cases, the majority of lesion sites included the medial frontal cortex (16/24; 66.7%), the anterior cingulate gyrus (7/24; 29.2%) and the supplementary motor cortex (3/24; 12.5%).
Table I.
Summary of reported cases of alien hand syndrome presenting following simple and complex callosal infarction.
| A, Simple callosal infarction (n=7)a | ||||
|---|---|---|---|---|
| Studies | Nc | Symptoms | Ref. | |
| Unilateral (left) | ||||
| Verleger et al | IC | (19) | ||
| Unilateral (right) | ||||
| Suwanwela and Leelacheavasit | 2 | IC, PC | (20) | |
| Nicholas et al | GR, SR | (21) | ||
| Bilateral | ||||
| Muangpaisan et al | IC, agonistic dyspraxia, mirror movement | (22) | ||
| Lin et al | IC of left hand, GR of right hand | (23) | ||
| Yuan et al | IC, AH sign | (24) | ||
| Suwanwela and Leelacheavasit | 1 | IC, PC, mirror movement | (20) | |
| B, Complex callosal infarction (n=24)b | ||||
| Studies | Nc | Concurrent infarction | Symptoms | Ref. |
| Unilateral (left) | ||||
| Feinberg et al | Medial frontal lobe | IC, GR | (5) | |
| Goldberg and Bloom | 2, 4 | Medial surface of frontal lobe | IC and GR, or GR only | (25) |
| Giroud and Dumas | 7 | Centrum ovale | GR | (26) |
| Chan and Ross | 1 | Mesiofrontal cortex | IC | (27) |
| Biran et al | Mesial frontal cortex | IC, GR, PC | (28) | |
| Jang et al | Anterior portion of left cingulate gyrus | GR, compulsive manipulation | (29) | |
| Nowak et al | 1 | Paramedian periventricular white matter and paramedian cortex | IC, GR | (30) |
| Nowak et al (30) | 2 | Anterior of corpus callosum and overlying white matter | GR | (30) |
| Unilateral (right) | ||||
| Tanaka et al | Anterior third cingulate gyrus, lower or medial superior frontal gyrus | IC | (31) | |
| Goldberg and Bloom | 1 | Medial frontal gyrus | IC, GR, SR | (25) |
| Goldberg and Bloom | 3 | Medial frontal lobe | GR | (25) |
| Gottlieb et al | 2 | Multiple lacunae (both cerebral hemispheres) | IC | (32) |
| Doody and Jankovic | 6 | Internal capsule lacuna | LE, PE | (33) |
| Trojano et al | 1 | Frontomesial region extending to anterior cingulate gyrus | IC, GR, SR, AH sign | (34) |
| Giroud and Dumas | 8 | Internal frontal area | GR | (26) |
| Chan et al | Right anterior cingulate gyrus, supplementary motor area, medial prefrontal cortex | IC, GR, SR, leg symptoms | (35) | |
| Chan and Ross | 2 | Mesial prefrontal cortex, anterior cingulate gyrus, supplementary motor cortex | IC, GR | (27) |
| Chan and Ross | 3 | Anterior cingulate gyrus, prefrontal cortex, partial supplementary motor cortex | GR | (27) |
| Ay et al | Thalamus, hippocampus, inferior temporal lobe, occipital lobe | AH sign, PE | (36) | |
| Ong Hai and Odderson | Mesial frontal cortex, right anterior cingulate gyrus | IC, GR, PC, involuntary masturbation | (37) | |
| Bejot et al | Bilateral medial frontal lobes | GR, involuntary masturbation | (38) | |
| Espinosa et al | Parietal area | Mirror movement | (39) | |
| Park et al | 2 | Frontal lobe | IC | (40) |
Infarction at the corpus callosum only
concurrent infarction at other sites of the brain apart from the corpus callosum
order of the case if the article reported several cases. IC, intermanual conflict; GR, grasp reflex; AH, alien hand; LE, levitation; PE, personification; SR, self-restriction.
Major AHS symptoms included intermanual conflict (19/31; 61.3%), grasp reflect (19/31; 61.3%) and alien hand signs (3/24; 12.5%). Among the 7 patients with simple corpus callosum infarction, intermanual conflict occurred in 6 cases (85.7%), while grasp reflex was seen in 2 cases (28.5%). Among the 24 patients with complex corpus callosum infarction, intermanual conflict was reported in 13/24 (54.2%) patients, while grasp reflex was observed in 17 cases (70.8%). Within the 17 grasp reflex patients, 14 (82.3%) had infarction at the medial frontal cortex, cingulate gyrus or supplementary motor cortex. In addition, 2/24 (8.3%) complex corpus callosum infarction cases were reported to experience involuntary masturbation.
As shown in Table II, 16 (51.6%) (19,24,25,27,30–33, 35–38) out of the 31 included cases presented disappearance or significant alleviation of AHS symptoms during the follow-up (between several days and 15 months), while no improvement was observed in 4 cases (5,21,27,34) for at least 6 months. In the remaining 11 cases (20,22,23,25,26,28,29,39,40), the outcome was unknown.
Table II.
Outcomes of reported cases of AHS presenting following simple and complex callosal infarction (n=31).
| Authors | Na | Duration | AHS symptoms | Ref. |
|---|---|---|---|---|
| Feinberg et al | 1 | 1 year | Absence | (5) |
| Verleger et al | 1 | 15 months | Decrease | (19) |
| Suwanwela and Leelacheavasit | 2 | N/A | N/A | (20) |
| Nicholas et al | 1 | 8 months | Absence | (21) |
| Muangpaisan et al | 1 | N/A | N/A | (22) |
| Lin et al | 1 | N/A | N/A | (23) |
| Yuan et al | 1 | 2 weeks | Decrease | (24) |
| Goldberg and Bloom | 4 | 1 year, 7 months, N/A | Decrease | (25) |
| N/A | N/A | |||
| Giroud and Dumas | 2 | N/A | N/A | (26) |
| Chan and Ross | 3 | 6 and 3 months | Decrease | (27) |
| 6 months | Absence | |||
| Biran et al | 1 | N/A | N/A | (28) |
| Jang et al | 1 | N/A | N/A | (29) |
| Nowak et al | 2 | 28 and 37 weeks | Decrease | (30) |
| Tanaka et al | 1 | 7 months | Decrease | (31) |
| Gottlieb et al | 1 | Next few months | Decrease | (32) |
| Doody and Jankovic | 1 | Within days | Decrease | (33) |
| Trojano et al | 1 | 11 months | Absence | (34) |
| Chan et al | 1 | 6 months | Decrease | (35) |
| Ay et al | 1 | 2 weeks | Decrease | (36) |
| Ong Hai and Odderson | 1 | 7 weeks | Decrease | (37) |
| Bejot et al | 1 | 2 weeks | Decrease | (38) |
| Espinosa et al | 1 | N/A | N/A | (39) |
| Park et al | 1 | N/A | N/A | (40) |
Number of cases in each study. AHS, alien hand syndrome; N/A, information not available (inconclusive or missing).
In general, the literature search revealed that the lesion was complex in the majority (77.4%) of cases. Intermanual conflict was more common in simple corpus callosum infarction patients (85.7%), and grasp reflex was more frequently reported in complex corpus callosum infarction patients (70.8%). Involuntary masturbation seems to be unique to infarction of the right anterior corpus callosum (37,38). Table I also suggests that the right hemisphere is more frequently involved, since the right and left sides of the corpus callosum were affected in 17 and 10 patients, respectively.
The corpus callosum is a fiber plate that conveys information between the two cerebral hemispheres (41). Callosal AHS putatively arises from failure in connection between the motor area and the supplementary motor area in the two cerebral hemispheres (16,23). Damage to the corpus callosum may manifest as frontal or callosal AHS. Mixed AHS has been also reported following corpus callosum infarction (24), in which the symptoms include intermanual conflict (as in callosal AHS), feeling of an alien hand and hemianesthesia (as in posterior AHS that involves the thalamus, parietal lobe or medial temporal lobe) (10,12,13,15).
Table I indicates that infarction of the corpus callosum typically occurs with lesions to other sites, including the medial frontal cortex, anterior cingulate gyrus and supplementary motor cortex. Similar to the genu and body of the corpus callosum, these regions all receive blood supply from the anterior cerebral artery. By contrast, the splenium of the corpus callosum receives blood from the posterior cerebral artery (42). Infarction at the splenium of the corpus callosum is reportedly caused by embolism, whereas atherosclerotic cerebral infarction is the more common form of infarction in the genu and body of the corpus callosum (43). The corpus callosum receives blood supply from the anterior and the posterior cerebral arteries with extensive anastomosis, and thus is rarely affected by infarction (42). In the present case, MRA and cerebral angiography revealed extensive atherosclerosis throughout the intracranial vasculature, notably in the left anterior cerebral and the vertebral basilar arteries. These imaging findings suggested the presence of atherosclerotic lesions in the anterior and posterior circulation.
AHS tends to be transient in patients with partial loss of the corpus callosum genu and splenium; in addition, patients with unilateral callosal lesions may regain connectivity between the two hemispheres if the remaining corpus callosum is functional (44). Permanent AHS may develop when the infarction involves two thirds of the anterior corpus callosum (45). Quick recovery was noticed following antiplatelet therapy in the present case, suggesting that the remaining corpus callosum may have compensated for the lesioned site, and that connectivity between the bilateral cerebral hemispheres was re-established.
In conclusion, the present study reported a prototypic case of callosal AHS, which was apparently caused by compromised anterior and posterior circulation. A notable issue in this case is insufficient blood supply of the vertebra-basilar arteries and the left anterior cerebral artery. This finding highlights the need for comprehensive investigation of the cerebral vasculature in patients with AHS caused by infarction of the corpus callosum.
Acknowledgements
The authors would like to thank the patient for her participation in the present study. The present study was funded by the Yantai Science and Technology Development Plan (grant no. 2013ws220).
Glossary
Abbreviations
- AHS
alien hand syndrome
- MRA
magnetic resonance angiography
- MRI
magnetic resonance imaging
- MMSE
mini-mental state examination
References
- 1.Graff-Radford J, Rubin MN, Jones DT, Aksamit AJ, Ahlskog JE, Knopman DS, Petersen RC, Boeve BF, Josephs KA. The alien limb phenomenon. J Neurol. 2013;260:1880–1888. doi: 10.1007/s00415-013-6898-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeeves MA, Simpson DA, Geffen G. Functional consequences of the transcallosal removal of intraventricular tumors. J Neurol Neurosurg Pshychiatry. 1979;42:134–142. doi: 10.1136/jnnp.42.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Y, Jia JP. Corpus callosum hematoma secondary to cerebral venous malformation presenting as alien hand syndrome. Neurocase. 2012;19:377–381. doi: 10.1080/13554794.2012.690420. [DOI] [PubMed] [Google Scholar]
- 4.Gibb WRG, Luther PJ, Marsden CD. Corticobasal degeneration. Brain. 1989;112:1171–1192. doi: 10.1093/brain/112.5.1171. [DOI] [PubMed] [Google Scholar]
- 5.Feinberg TE, Schindler RJ, Flanagan NG, Haber LD. Two alien hand syndromes. Neurology. 1992;42:19–24. doi: 10.1212/WNL.42.1.19. [DOI] [PubMed] [Google Scholar]
- 6.Kim HY, Kim JY, Kim GU, Han HJ, Shin DI. Alien hand syndrome after epilepsia partialis continua: FDG PET and MRI studies. Epilepsy Behav. 2012;23:71–73. doi: 10.1016/j.yebeh.2011.08.043. [DOI] [PubMed] [Google Scholar]
- 7.Ball JA, Lantos PL, Jackson M, Marsden CD, Scadding JW, Rossor MN. Alien hand sign in association with Alzheimer's histopathology. J Neurol Neurosurg Psychiatry. 1993;56:1020–1023. doi: 10.1136/jnnp.56.9.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barclay CL, Bergeron C, Lang AE. Arm levitation in progressive supranuclear palsy. Neurology. 1999;52:879–882. doi: 10.1212/WNL.52.4.879. [DOI] [PubMed] [Google Scholar]
- 9.Rubin M, Graff-Radford J, Boeve B, Josephs KA, Aksamit AJ. The alien limb phenomenon and Creutzfeldt-Jakob disease. Parkinsonism Relat Disord. 2012;18:842–846. doi: 10.1016/j.parkreldis.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Marey-Lopez J, Rubio-Nazabal E, Alonso-Magdalena L, Lopez-Facal S. Posterior alien hand syndrome after a right thalamic infarct. J Neurol Neurosurg Psychiatry. 2002;73:447–449. doi: 10.1136/jnnp.73.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolado AM, Castrillo C, Urra DG, de Seijas E Varela. Alien hand sign or alien hand syndrome? J Neurol Neurosurg Psychiatry. 1995;59:100–101. doi: 10.1136/jnnp.59.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pack BC, Stewart KJ, Diamond PT, Gale SD. Posterior-variant alien hand syndrome: Clinical features and response to rehabilitation. Disabil Rehabil. 2002;24:817–818. doi: 10.1080/09638280210142202. [DOI] [PubMed] [Google Scholar]
- 13.Pappalardo A, Ciancio MR, Reggio E, Patti F. Posterior alien hand syndrome: Case report and rehabilitative treatment. Neurorehabil Neural Repair. 2004;18:176–181. doi: 10.1177/0888439004269031. [DOI] [PubMed] [Google Scholar]
- 14.Pooyania S, Mohr S, Gray S. Alien hand syndrome: A case report and description to rehabilitation. Disabil Rehabil. 2011;33:1715–1718. doi: 10.3109/09638288.2010.540290. [DOI] [PubMed] [Google Scholar]
- 15.Kato H, Iijima M, Hiroi A, Kubo M, Uchigata M. A case of alien hand syndrome after right posterior cerebral artery territory infarction. Rinsho Shinkeigaku. 2003;43:487–490. [PubMed] [Google Scholar]
- 16.Geschwind DH, Iacoboni M, Mega MS, Zaidel DW, Cloughesy T, Zaidel E. Alien hand syndrome: Interhemispheric motor disconnection due to a lesion in the midbody of the corpus callosum. Neurology. 1995;45:802–808. doi: 10.1212/WNL.45.4.802. [DOI] [PubMed] [Google Scholar]
- 17.Shi YQ, Zhou XD. Pratical Neurology. 3rd. Shanghai Scientific and Technical Publishers; Shanghai: 2004. p. 49. [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. Mini mental state. A practical method for grading the cognitive state of patients for clinicians. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Verleger R, Binkofski F, Friedrich M, Sedlmeier P, Kömpf D. Anarchic-hand syndrome: ERP reflections of lost control over the right hemisphere. Brain Cogn. 2011;77:138–150. doi: 10.1016/j.bandc.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Suwanwela NC, Leelacheavasit N. Isolated corpus callosal infarction secondary to pericallosal artery disease presenting as alien hand syndrome. J Neurol Neurosurg Psychiatry. 2002;72:533–536. doi: 10.1136/jnnp.72.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholas JJ, Wichner MH, Gorelick PB, Ramsey MM. Naturalization of the alien hand: Case report. Arch Phys Med Rehabil. 1998;79:113–114. doi: 10.1016/S0003-9993(98)90218-0. [DOI] [PubMed] [Google Scholar]
- 22.Muangpaisan W, Srisajjakul S, Chiewvit P. The alien hand syndrome: Report of a case and review of the literature. J Med Assoc Thai. 2005;88:1447–1452. [PubMed] [Google Scholar]
- 23.Lin JH, Kwan SY, Wu D. Mixed alien hand syndrome coexisting with left-sided extinction secondary to a left corpus callosal lesion: A case report. Mov Disord. 2007;22:248–251. doi: 10.1002/mds.21241. [DOI] [PubMed] [Google Scholar]
- 24.Yuan JL, Wang SK, Guo XJ, Hu WL. Acute infarct of the corpus callosum presenting as alien hand syndrome: Evidence of diffusion weighted imaging and magnetic resonance angiography. BMC Neurol. 2011;11:142. doi: 10.1186/1471-2377-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldberg G, Bloom KK. The alien hand sign. Localization, lateralization and recovery. Am J Phys Med Rehabil. 1990;69:228–238. doi: 10.1097/00002060-199010000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Giroud M, Dumas R. Clinical and topographical range of callosal infarction: A clinical and radiological correlation study. J Neurol Neurosurg Psychiatry. 1995;59:238–242. doi: 10.1136/jnnp.59.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan JL, Ross ED. Alien hand syndrome: Influence of neglect on the clinical presentation of frontal and callosal variants. Cortex. 1997;33:287–299. doi: 10.1016/S0010-9452(08)70005-4. [DOI] [PubMed] [Google Scholar]
- 28.Biran I, Giovannetti T, Buxbaum L, Chatterjee A. The alien hand syndrome: What makes the alien hand alien? Cogn Neuropsychol. 2006;23:563–582. doi: 10.1080/02643290500180282. [DOI] [PubMed] [Google Scholar]
- 29.Jang SH, Lee J, Yeo SS, Chang MC. Callosal disconnection syndrome after corpus callosum infarct: A diffusion tensor tractography study. J Stroke Cerebrovasc Dis. 2013;22:e240–e244. doi: 10.1016/j.jstrokecerebrovasdis.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Nowak DA, Bösl K, Lüdemann-Podubecka J, Gdynia HJ, Ponfick M. Recovery and outcome of frontal alien hand syndrome after anterior cerebral artery stroke. J Neurol Sci. 2014;338:203–206. doi: 10.1016/j.jns.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka Y, Iwasa H, Yoshida M. Diagonistic dyspraxia: Case report and movement-related potentials. Neurology. 1990;40:657–661. doi: 10.1212/WNL.40.4.657. [DOI] [PubMed] [Google Scholar]
- 32.Gottlieb D, Robb K, Day B. Mirror movements in the alien hand syndrome. Case report. Am J Phys Med Rehabil. 1992;71:297–300. doi: 10.1097/00002060-199210000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Doody RS, Jankovic J. The alien hand and related signs. J Neurol Neurosurg Psychiatry. 1992;55:806–810. doi: 10.1136/jnnp.55.9.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trojano L, Crisci C, Lanzillo B, Elefante R, Caruso G. How many alien hand syndromes? Follow-up of a case. Neurology. 1993;43:2710–2712. doi: 10.1212/WNL.43.12.2710. [DOI] [PubMed] [Google Scholar]
- 35.Chan JL, Chen RS, Ng KK. Leg manifestation in alien hand syndrome. J Formos Med Assoc. 1996;95:342–346. [PubMed] [Google Scholar]
- 36.Ay H, Buonanno FS, Price BH, Le DA, Koroshetz WJ. Sensory alien hand syndrome: Case report and review of the literature. J Neurol Neurosurg Psychiatry. 1998;65:366–369. doi: 10.1136/jnnp.65.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong Hai BG, Odderson IR. Involuntary masturbation as a manifestation of stroke-related alien hand syndrome. Am J Phys Med Rehabil. 2000;79:395–398. doi: 10.1097/00002060-200007000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Bejot Y, Caillier M, Osseby GV, Didi R, Ben Salem D, Moreau T, Giroud M. Involuntary masturbation and hemiballismus after bilateral anterior cerebral artery infarction. Clin Neurol Neurosurg. 2008;110:190–193. doi: 10.1016/j.clineuro.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Espinosa PS, Smith CD, Berger JR. Alien hand syndrome. Neurology. 2006;67:e21. doi: 10.1212/01.wnl.0000249303.88754.48. [DOI] [PubMed] [Google Scholar]
- 40.Park YW, Kim CH, Kim MO, Jeong HJ, Jung HY. Alien hand syndrome in stroke - case report & neurophysiologic study. Ann Rehabil Med. 2012;36:556–560. doi: 10.5535/arm.2012.36.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gazzaniga MS. Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition? Brain. 2000;123:1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- 42.Kahilogullari G, Comert A, Arslan M, Esmer AF, Tuccar E, Elhan A, Tubbs RS, Ugur HC. Callosal branches of the anterior cerebral artery: An anatomical report. Clin Anat. 2008;21:383–388. doi: 10.1002/ca.20647. [DOI] [PubMed] [Google Scholar]
- 43.Kasow DL, Destian S, Braun C, Quintas JC, Kagtsu NJ, Johnson CE. Corpus callosum infarcts with atypical clinical and radiologic presentations. AJNR Am J Neuroradiol. 2000;21:1876–1880. [PMC free article] [PubMed] [Google Scholar]
- 44.Barbeau E, Joubert S, Poncet M. A single case-study of diagonistic dyspraxia. Brain Cogn. 2004;54:215–217. doi: 10.1016/j.bandc.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Papagno C, Marsile C. Transient left-sided alien hand with callosal and unilateral fronto-mesial damage: A case study. Neuropsychologia. 1995;33:1703–1709. doi: 10.1016/0028-3932(95)00044-5. [DOI] [PubMed] [Google Scholar]