Abstract
More effective drugs may reduce the requirement for palliative external beam radiotherapy for bony target volumes; however, living with metastases for prolonged periods of time may result in more frequent episodes of bone pain or serious skeletal-related events. The purpose of the present study was to evaluate how recent advances in systemic therapy impact radiotherapy utilization. A retrospective analysis of a comprehensive regional database was performed. All oncology care in this region was provided by only one center, assuring complete data. Patients that had succumbed between June 1, 2004 and June 1, 2015 were included. For all 236 patients, the median age at diagnosis of bone metastases was 75 years and median overall survival was 20 months. More intense systemic therapy was associated with a significantly longer survival time. Only 69 patients (29%) did not receive palliative radiotherapy for bony target volumes, whilst 1 course was given to 101 patients (43%), 2 courses to 34 patients (14%) and >2 courses to 32 patients (14%). Radiotherapy was used more frequently in younger patients, those with spinal cord compressions or pathological fractures, and those treated with intense and long-standing systemic therapy. Radiotherapy utilization increased with survival time. For 100 poor-prognosis patients that succumbed within 12 months, 57 courses of palliative radiotherapy were administered, whilst 100 patients that survived for 12–24 months were administered 114 courses (24–36 months, 148 courses). In conclusion, the use of palliative radiotherapy did not decrease when more effective systemic therapy was administered. However, provided that only 5% of patients received radionuclide treatment, additional studies in other populations are required.
Keywords: prostate cancer, bone metastases, chemotherapy, radiotherapy, systemic treatment
Introduction
Metastatic prostate cancer commonly involves the skeleton, resulting in skeletal-related events (SRE), including pathological fractures and metastatic spinal cord compression (MSCC) (1). In addition to systemic treatment, a number of patients also require orthopedic surgery and, in particular, palliative radiotherapy. Systemic treatment options have expanded during the last decade, resulting in improved overall survival rates (2), but it is not entirely clear how these advances impact radiotherapy utilization. More effective drugs may reduce the requirement for radiotherapy; however, living with metastases for prolonged periods of time may result in more frequent episodes of bone pain or other more serious SREs. In a recent meta-analysis, the median survival time of patients with castration-resistant prostate cancer and bone metastases was 21.3 months (3). In a 15-year study from the USA, more than half of patients with bone metastases from prostate cancer had evidence of SREs, either at diagnosis of bone metastases or subsequently (4).
Generally, large databases or cancer registries contain useful data regarding radiotherapy utilization rates. However, there is often a lack of detailed information on systemic therapy and patient-associated baseline data, including comorbidity, blood tests or extent of metastatic disease. Consequently, the comprehensive present study was performed in a patient population treated in a well-defined geographical region with a publicly-funded healthcare system, which provides equal access to treatment, irrespective of income, place of living and other potential socioeconomic barriers. Current radiotherapy utilization rates are important for healthcare authorities and various stakeholders participating in the development of future healthcare services (5,6). An equitable access to specialized healthcare services based on requirements and not on each individual's economy has been a cornerstone in the Norwegian healthcare system. In the present study, the aim of which was to evaluate how recent advances in systemic therapy impact radiotherapy utilization, this aspect is elucidated from the radiotherapy in metastatic prostate cancer perspective.
Patients and methods
Patients and treatment
The present retrospective study included 236 consecutive male patients with bone metastases from prostate cancer, who received oncology care at the Nordland Hospital (Bodø, Norway), which is an academic teaching hospital. These patients succumbed to prostate cancer between June 1, 2004 and June 1, 2015. They were identified from the electronic patient record systems of the hospital and its radiotherapy unit (DIPS®, DIPS ASA, Bodø, Norway; ARIA®, Varian Medical Systems, Inc., Palo Alto, CA, USA). In order to ensure complete follow-up, patients that were alive on June 1, 2015 were excluded from the study.
The National Healthcare System in Norway is the responsibility of the state through state ownership of four regional health authority trusts. Within these, psychiatric and somatic hospitals are organized as health trusts. One of these is the Nordland Hospital Trust (Bodø, Norway), which provides oncology services to the complete population of the Nordland county, a geographically large, but sparsely populated, area (38,460 km2; 241,682 inhabitants). Municipalities are responsible for primary healthcare, and there are no private practices providing cancer treatment in the county. There are three centers that have radiation treatment facilities in the northern and central region of Norway (Bodø, Tromsø and Trondheim), one of which is The Nordland Hospital, which are separated by large distances; therefore, dilution effects, where a patient receives radiotherapy at other centre, does not apply in the collection of reliable data if the study population lives within close proximity to one of the centers. One of these centers is The Nordland Hospital, which forms the basis of the present and previous analyses (7).
Since the present study was a retrospective quality of care analysis, no approval from the Regional Committee for Medical and Health Research Ethics was required. Similarly no approval from the Norwegian Social Science Database had to be obtained.
Blood and imaging tests
Serum prostate-specific antigen (PSA), radioisotope bone scan and computed tomography (CT) of the chest, abdomen and pelvis were part of routine blood chemistry and imaging assessment in patients with metastatic prostate cancer, which were performed every three months. To confirm suspicious findings, ultrasound and/or magnetic resonance imaging were performed. Positron emission tomography was not available.
Statistical analysis
All analyses were performed with SPSS version 22 (SPSS IBM, Armonk, NY, USA). Actuarial survival data from imaging diagnosis of bone metastases was calculated using the Kaplan-Meier method, and compared between different groups with the log rank test. The date on which the patients succumbed was recorded. Associations between different variables of interest were assessed using χ2 or Fisher's exact probability tests (two-tailed). P≤0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
The median age at diagnosis of bone metastases was 75 years (range, 56–94 years). A total of 81 patients (34%) had bone metastases at the time of diagnosis with prostate cancer. The majority (n=155; 66%) had metachronous metastatic disease following a median of 67 months from initial cancer diagnosis. In total, 49 patients that had metastatic disease were diagnosed with bone metastases prior to the development of castration-resistant prostate cancer (CRPC), and 101 patients already had CRPC (unknown data in the remaining 5 patients). In patients with CRPC, the median time interval between the start of endocrine treatment and diagnosis of bone metastases was 38 months. Only 49 patients (21%) had other distant metastases, including to the liver or non-pelvic lymph nodes, when they were diagnosed with bone metastases. The median PSA level was 78.5 µg/l (range, 0.9–10,302.0 µg/l; normal range, <4 µg/l). Additional information is shown in Table I.
Table I.
Patient characteristics at time of diagnosis with bone metastases (n=236).
| Parameter | n (%) |
|---|---|
| Marital status | |
| Married/partner | 176 (75) |
| Single | 51 (22) |
| Unknown | 9 (4) |
| Residence | |
| Bodøa | 112 (48) |
| Surrounding communitiesb | 124 (53) |
| Charlson comorbidity index | |
| 0 | 109 (46) |
| 1 | 68 (29) |
| 2 | 31 (13) |
| >2 | 15 (6) |
| Unknown | 13 (6) |
| Gleason score | |
| ≥8 | 100 (42) |
| <8 | 83 (35) |
| Unknown | 53 (23) |
| NCCN risk category at first cancer diagnosis | |
| M1 | 81 (34) |
| N1 | 13 (6) |
| High | 97 (41) |
| Intermediate | 24 (10) |
| Unknown | 21 (9) |
| Initial treatment strategy | |
| Surgical treatment | 14 (6) |
| Radiotherapy ± endocrine treatment | 14 (6) |
| LHRH agonist | 116 (49) |
| Antiandrogen | 23 (10) |
| Orchiectomy | 10 (4) |
| Watchful waiting | 59 (25) |
| Bone metastases (isotope bone scan) | |
| 1 | 18 (8) |
| 2–4 | 52 (22) |
| 5–10 | 50 (21) |
| >10 or super scan | 99 (42) |
| Unknown | 17 (7) |
~50,000 inhabitants
~70,000 inhabitants. NCCN, National Comprehensive Cancer Network; LHRH, luteinizing hormone-releasing hormone.
Systemic treatment
The treatment regimes used to treat the present patients with bone metastases evolved in line with the approval of novel drugs in Norway. In general, adherence to national guidelines is extremely high throughout the country (8). The patients did not participate in clinical trials or early access programs. Early during the study period (2004–2011), a typical patient received endocrine therapy, including total androgen blockade, followed by anti-androgen withdrawal. Following the development of CRPC, patients were treated with prednisolone and taxotere, which may have been followed by mitoxantrone. Later in the study period (2012–2015), cabazitaxel, abiraterone and enzalutamide became available. All patients were continued on luteinizing hormone-releasing hormone agonists, unless orchiectomy had been performed. Individualized decisions were made regarding the sequence of treatments. Additional information is shown in Table II.
Table II.
Systemic therapy following diagnosis of bone metastases (n=236).
| Treatment | n (%) |
|---|---|
| Chemotherapy typea | |
| None | 149 (63) |
| One line | 46 (20) |
| Two lines | 27 (11) |
| Three lines | 14 (6) |
| Chemotherapy druga | |
| Taxotere | 79 (34) |
| Mitoxantrone | 9 (4) |
| Cabazitaxel | 5 (2) |
| Abiraterone | 28 (12) |
| Enzalutamide | 10 (4) |
| None | 105 (44) |
| Bisphosphonates/denosumab treatment | |
| None | 111 (47) |
| Monthly zoledronic acid | 98 (42) |
| Monthly denosumab | 23 (10) |
| Other bisphosphonate | 4 (2) |
| Overall systemic therapy | |
| None | 94 (40) |
| Bone-targeted | 55 (23) |
| Chemotherapya | 17 (7) |
| Both | 70 (30) |
| Radionuclide therapy | |
| None | 225 (95) |
| Radium-223 | 6 (3) |
| Other | 5 (2) |
Includes cytotoxic chemotherapy, abiraterone and enzalutamide.
Survival and other endpoints
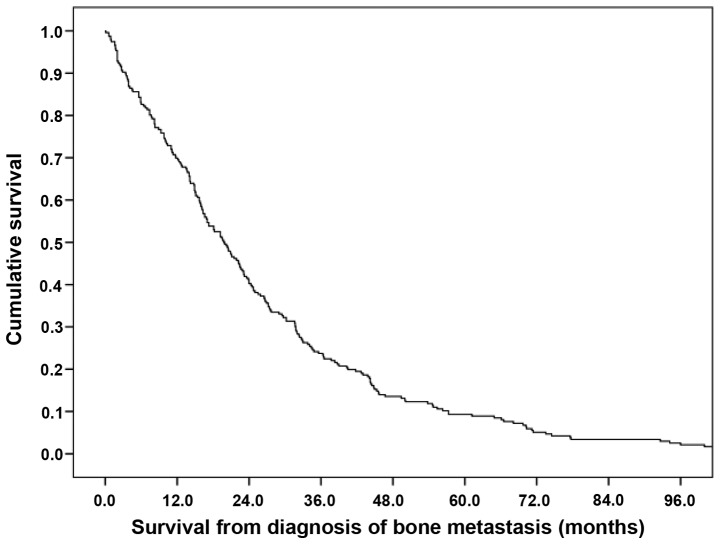
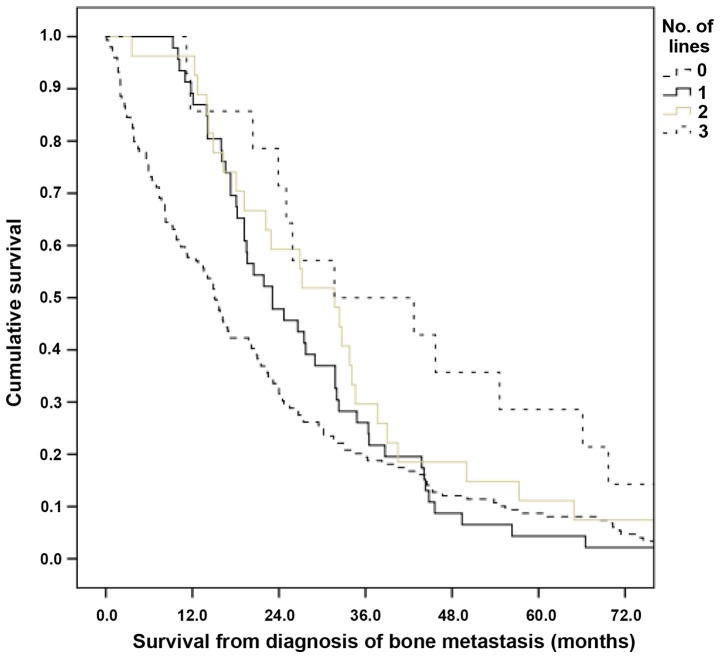
Median overall survival was 20 months (Fig. 1). More intense systemic therapy was associated with longer survival time over all strata (Fig. 2; P=0.01). Median values were 15.1, 23.1, 31.7 and 31.7 months for patients not treated and treated with 1, 2 or 3 lines chemotherapy, respectively. In order to reduce selection bias, a landmark analysis was performed, which included only patients alive 3 months following diagnosis of bone metastases. This confirmed the initial results; median survival was 17.0, 23.1, 31.7 and 31.7 months for patients not treated and treated with 1, 2 or 3 lines, respectively.
Figure 1.
Overall survival time of patients with bone metastasis from prostate cancer (n=236).
A total of 12 patients (5%) had pathological fractures or MSCC as the first sign of bone metastases. Overall, 44 patients (19%) developed pathological fractures [MSCC, 35 patients (15%)] during follow-up. In the majority of cases, fractures developed prior to the initiation of bisphosphonates or denosumab treatment (n=28). The median time between diagnosis of bone metastases and treatment with bone-targeting drugs was 6 months. A minority of patients (32%) that succumbed within 12 months were prescribed bone-targeting drugs. This figure increased to 57% in patients surviving 12–24 months, 69% in patients surviving 24–36 months, and 63% in those surviving >36 months.
Palliative external beam radiotherapy to skeletal target volumes
Three common fractionation regimes were prescribed: 8 Gy single fraction; 5 fractions of 4 Gy; and 10 fractions of 3 Gy. Stereotactic radiotherapy was not available. In total, 69 patients (29%) did not receive radiotherapy. One course of radiotherapy was administered to 101 patients (43%), two courses to 34 patients (14%), three courses to 20 patients (9%), and more than three courses to 12 patients (5%). One target volume was irradiated in 56 patients (24%), two in 50 patients (21%), three in 24 patients (10%), four in 13 patients (6%), and more than four in 24 patients (10%).
Predictors of radiotherapy utilization (Table III)
Table III.
Radiotherapy utilization, including external beam and skeletal target (n=236).
| Courses of radiotherapy, n (%) | ||||||
|---|---|---|---|---|---|---|
| Parameter | 0 | 1 | 3 | ≥3 | P-value | Median target volumesa |
| Age, years | ||||||
| <75 | 21 (20) | 42 (40) | 14 (13) | 28 (27) | 2.0 | |
| ≥75 | 48 (37) | 59 (45) | 20 (15) | 4 (3) | <0.0001 | 1.0 |
| Initial symptom | ||||||
| MSCC/PF | 0 (0) | 5 (42) | 3 (25) | 4 (33) | 2.0 | |
| Other | 69 (31) | 96 (43) | 31 (14) | 28 (13) | 0.0100 | 1.0 |
| MSCC/PFb | ||||||
| Not present | 67 (40) | 65 (39) | 22 (13) | 14 (8) | 1.0 | |
| Present | 2 (3) | 36 (53) | 12 (18) | 18 (26) | <0.0001 | 2.5 |
| Chemotherapyc | ||||||
| No chemotherapy | 57 (38) | 64 (43) | 22 (15) | 6 (4) | 1.0 | |
| One line only | 6 (13) | 21 (46) | 8 (17) | 11 (24) | 2.0 | |
| Two lines | 5 (19) | 10 (37) | 4 (15) | 8 (30) | 2.0 | |
| Three lines | 1 (7) | 6 (43) | 0 (0) | 7 (50) | 0.0001 | 2.5 |
| Overall systemic therapyd | ||||||
| None | 51 (54) | 34 (36) | 10 (11) | 0 (0) | 0 | |
| Bone-targeting | 6 (11) | 31 (56) | 12 (22) | 6 (11) | 2.0 | |
| Chemotherapy | 8 (50) | 5 (31) | 1 (6) | 2 (13) | 0.5 | |
| Both | 4 (6) | 31 (44) | 11 (16) | 24 (34) | 0.0001 | 2.0 |
| Survival, months | ||||||
| <12 | 30 (42) | 32 (45) | 8 (11) | 1 (1) | 1.0 | |
| 12–24 | 20 (28) | 33 (46) | 10 (14) | 9 (13) | 1.0 | |
| 24–36 | 12 (31) | 12 (31) | 6 (15) | 9 (23) | 2.0 | |
| 36–48 | 4 (17) | 10 (42) | 4 (17) | 6 (25) | 2.0 | |
| >48 | 3 (10) | 14 (47) | 6 (20) | 7 (23) | 0.0100 | 2.0 |
All courses combined.
Between diagnosis of bone metastases and death.
Includes cytotoxic chemotherapy, abiraterone and enzalutamide.
Includes cytotoxic chemotherapy, abiraterone, enzalutamide, bisphosphonates and denosumab. MSCC/PF, metastatic spinal cord compression/pathological fracture.
All aforementioned baseline characteristics and systemic therapy regimens were analyzed. No association was identified between radiotherapy utilization and the number of bone metastases at diagnosis of metastatic disease, synchronous vs. metachronous metastases, distance to radiotherapy center and the majority of other parameters. Radiotherapy was utilized more frequently in patients <75 years of age, patients with MSCC or pathological fracture as a first sign of bone metastases, and patients that developed MSCC or pathological fractures during the disease trajectory. Significant associations were also observed with regard to the number of lines of systemic treatment, and whether or not such treatment included various types of drugs. In general, patients with intense and long-standing systemic therapy also required more palliative radiotherapy (Table III).
Radiotherapy utilization increased with increasing patient survival time. Based on the results in Table III, utilization rates were calculated per 100 patients. For 100 poor-prognosis patients, who succumbed within 12 months, 57 appointments (or courses) for a consultation with a radiation oncologist, treatment planning and palliative radiotherapy were required. For 100 patients surviving 12–24 months, the corresponding figure was 114 appointments (24–36 months, 148 appointments; 36–48 months, 179 appointments; >48 months, 170 appointments).
Discussion
Recently, several novel systemic treatment options for patients with metastatic and/or CRPC have become available, the first being docetaxel (9). A retrospective study of CRPC patients treated with palliative radiotherapy for bony target volumes in BC, Canada, compared patients in the pre-docetaxel era (radiotherapy between 1998 and 2001) to those in the docetaxel era (radiotherapy between 2006 and 2009) (10). In that study, time of the first radiotherapy treatment to bone was used to select patients at a similar point in their disease state (i.e., onset of bone pain). The primary objective was to determine the median survival in the two eras; of the 919 patients in the pre-docetaxel era and the 957 in the docetaxel era, 7 and 37% received docetaxel, respectively, compared with 34% in the present study. The median survival time from the first palliative radiotherapy was 7.5 vs. 10.3 months (P<0.0001). Therefore, that study demonstrated that docetaxel improves survival time at a population level. By contrast, in a randomized trial (9), the effect of docetaxel treatment was moderate. Approximately the same magnitude of improvement in patient survival time was observed with cabazitaxel (11), abiraterone (12,13) and enzalutamide (14,15) treatment, although efficacy varied between post- and pre-chemotherapy settings. More effective drugs may reduce the requirement for palliative radiotherapy; however, living with metastases for prolonged periods of time may result in more frequent episodes of bone pain or other SREs treated with radiotherapy. The impact of altering treatment paradigms on radiotherapy utilization should be monitored regularly in order to adjust the necessary resources.
Radiotherapy utilization was the main endpoint of the present study. The secondary results from the present study were consistent with those of the landmark randomized trials (9,11,12,14); there was a prolongation of survival time with more available lines of therapy. However, it is important to note that the present patients differed from those included in the previous trials, such as differences between disease stage at bone metastases diagnosis. The present study included patients with primary metastatic disease (hormone sensitive) and secondary metastatic disease (prior to or following the development of CRPC), irrespective of performance status and prognosis. The role of performance status was not analyzed, since this variable alters unpredictably during the disease trajectory, such as following successful palliative radiotherapy. In addition, it is important to emphasize the limited sample size and statistical power of the present study. A larger study based on cancer registry data would have been possible. However, such registries collect fewer data concerning baseline characteristics and details of systemic therapy. Therefore, important insights are derived from smaller, but nevertheless population-based, studies.
Using the Surveillance, Epidemiology, and End Results-Medicare linked database, Murphy et al (16) analyzed patients with stage IV breast, prostate, lung or colorectal cancer diagnosed between 2000 and 2007, and observed these patients until 2009. A total of 41% of the study population received palliative radiotherapy, including 53% of patients with lung cancer, followed by those with breast (42%), prostate (40%), and colorectal cancers (12%). The present study observed a higher utilization rate of 71%. However, the health care system in the present study is different. The study by Murphy et al revealed that older patients and those with higher Charlson comorbidity scores were significantly less likely to receive palliative radiotherapy (16). Regarding age, comparable results were obtained to the present study, but comorbidity was not significant. As one would expect, patients with MSCC and/or pathological fractures were more likely to receive radiotherapy (97 vs. 60%).
The present study identified that patients with intense and long-standing systemic therapy also required more palliative radiotherapy. Radiotherapy utilization increased with increasing survival time. For 100 poor-prognosis patients, who died within 12 months, 57 appointments for consultation with a radiation oncologist, treatment planning and palliative radiotherapy were registered. For 100 patients surviving 12–24 months, the corresponding figure was 114, which is twice as high; however, with a longer survival time, the relative increase diminished (24–36 months, 148; 36–48 months 179; >48 months, 170). A possible explanation is the increasing use of bone-targeting drugs in patients with improved survival. Such drugs were prescribed in 32% of patients that succumbed within 12 months. This figure increased to 57% in patients surviving 12–24 months and 69% in patients surviving 24–36 months. It is well known that bone-targeting drugs significantly reduce the incidence of SREs (17). This effect has also been demonstrated for another novel systemic treatment options, including radionuclide treatment with the α-emitter radium-223 (18–20). In addition, survival improved significantly with radium-223 treatment compared with placebo (18). However, only 5% of the present patients received radionuclides; therefore, it is necessary to perform additional studies in patients treated with radium-223 and to update radiotherapy utilization rates as novel treatment options become available and others alter. For example, taxotere is currently used at diagnosis of metastatic disease rather than following the development of CRPC (21).
In conclusion, the present study demonstrated that the use of palliative radiotherapy did not decrease when more effective systemic therapy was administered. Palliative radiotherapy remains an important part of the multimodal management of patients with skeletal metastases from prostate cancer.
Figure 2.
Overall survival time of patients with bone metastasis from prostate cancer stratified by systemic therapy (P=0.01; pooled over all strata). Median values were 15.1, 23.1, 31.7 and 31.7 months for patients not treated and treated with 1, 2 or 3 lines of chemotherapy, respectively.
References
- 1.Gartrell BA, Coleman R, Efstathiou E, Fizazi K, Logothetis CJ, Smith MR, Sonpavde G, Sartor O, Saad F. Metastatic prostate cancer and the bone: Significance and therapeutic options. Eur Urol. 2015;68:850–858. doi: 10.1016/j.eururo.2015.06.039. [DOI] [PubMed] [Google Scholar]
- 2.Crawford ED, Higano CS, Shore ND, Hussain M, Petrylak DP. Treating patients with metastatic castration resistant prostate cancer: A comprehensive review of available therapies. J Urol. 2015;194:1537–1547. doi: 10.1016/j.juro.2015.06.106. [DOI] [PubMed] [Google Scholar]
- 3.Halabi S, Kelly WK, Ma H, Zhou H, Solomon NC, Fizazi K, Tangen CM, Rosenthal M, Petrylak DP, Hussain M, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;34:1652–1659. doi: 10.1200/JCO.2015.65.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oster G, Lamerato L, Glass AG, Richert-Boe KE, Lopez A, Chung K, Richhariya A, Dodge T, Wolff GG, Balakumaran A, Edelsberg J. Natural history of skeletal-related events in patients with breast, lung, or prostate cancer and metastases to bone: a 15-year study in two large US health systems. Support Care Cancer. 2013;21:3279–3286. doi: 10.1007/s00520-013-1887-3. [DOI] [PubMed] [Google Scholar]
- 5.Borras JM, Lievens Y, Dunscombe P, Coffey M, Malicki J, Corral J, Gasparotto C, Defourny N, Barton M, Verhoeven R, et al. The optimal utilization proportion of external beam radiotherapy in European countries: An ESTRO-HERO analysis. Radiother Oncol. 2015;116:38–44. doi: 10.1016/j.radonc.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Borras JM, Barton M, Grau C, Corral J, Verhoeven R, Lemmens V, van Eycken L, Henau K, Primic-Zakelj M, Strojan P, et al. The impact of cancer incidence and stage on optimal utilization of radiotherapy: Methodology of a population based analysis by the ESTRO-HERO project. Radiother Oncol. 2015;116:45–50. doi: 10.1016/j.radonc.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Nieder C, Pawinski A, Haukland E, Dokmo R, Phillipi I, Dalhaug A. Estimating need for palliative external beam radiotherapy in adult cancer patients. Int J Radiat Oncol Biol Phys. 2010;76:207–211. doi: 10.1016/j.ijrobp.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 8.Norwegian Directorate of Health, Oslo, corp-author. https://helsedirektoratet.no/Documents/Utgåtte%20publikasjoner/IS-1777%20(1)%20Prostatakreft%2017.12.09-16-10.12.pdf. National care program with guidelines for the examinations, treatments and follow up of patients with prostate cancer. 2015 Accessed on June 26, 2016. (In Norwegian)
- 9.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 10.Zielinski RR, Azad AA, Chi KN, Tyldesely S. Population-based impact on overall survival after the introduction of docetaxel as standard therapy for metastatic castration resistant prostate cancer. Can Urol Assoc J. 2014;8:E520–E523. doi: 10.5489/cuaj.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.deBono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 12.deBono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Jr, Saad F, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, Miller K, Logothetis CJ, Shore ND, Small EJ, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–160. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 14.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 15.Loriot Y, Miller K, Sternberg CN, Fizazi K, De Bono JS, Chowdhury S, Higano CS, Noonberg S, Holmstrom S, Mansbach H, et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): Results from a randomised, phase 3 trial. Lancet Oncol. 2015;16:509–521. doi: 10.1016/S1470-2045(15)70113-0. [DOI] [PubMed] [Google Scholar]
- 16.Murphy JD, Nelson LM, Chang DT, Mell LK, Le QT. Patterns of care in palliative radiotherapy: A population-based study. J Oncol Pract. 2013;9:e220–e227. doi: 10.1200/JOP.2012.000835. [DOI] [PubMed] [Google Scholar]
- 17.Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 19.Jong JM, Oprea-Lager DE, Hooft L, de Klerk JM, Bloemendal HJ, Verheul HM, Hoekstra OS, van den Eertwegh AJ. Radiopharmaceuticals for palliation of bone pain in patients with castration-resistant prostate cancer metastatic to bone: A systematic review. Eur Urol. 2015;pii:S0302–2838. doi: 10.1016/j.eururo.2015.09.005. 00862-00863. [DOI] [PubMed] [Google Scholar]
- 20.Norum J, Traasdahl ER, Totth A, Nieder C, Olsen JA. Health economics and radium-223 (Xofigo®) in the treatment of metastatic castration resistant prostate cancer (mCRPC): A case history and a systematic review of the literature. Glob J Health Sci. 2015;8:1–9. doi: 10.5539/gjhs.v8n4p1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]