Abstract
Despite the fact that testicular germ cell tumors (TGCTs) are one of the most chemosensitive solid tumors, a small proportion of patients fail to be cured following cisplatin-based first line chemotherapy. Upregulation of carbonic anhydrase IX (CA IX) in various solid tumors is associated with poor outcome. The current prospective study investigated the prognostic value of serum CA IX level in TGCTs. In total, 83 patients (16 non-metastatic following orchiectomy with no evidence of disease, 57 metastatic chemotherapy-naïve and 10 metastatic relapsed chemotherapy-pretreated) starting adjuvant and/or new line of chemotherapy and 35 healthy controls were enrolled in the study. Serum CA IX values were determined using an enzyme-linked immunosorbent assay, and intratumoral CA IX was analyzed by immunohistochemistry. Metastatic chemotherapy-naïve patients had significantly higher mean CA IX serum levels than healthy controls (490.6 vs. 249.6 pg/ml, P=0.005), while there was no difference in serum CA IX levels in non-metastatic or relapsed TGCT patients compared with healthy controls. There was no significant difference in the mean serum CA IX levels between different groups of patients and between the first and second cycle of chemotherapy, nor association with patients/tumor characteristics. Serum CA IX was not prognostic for progression-free survival [hazard ratio (HR)=0.81, P=0.730] or overall survival (HR=0.64, P=0.480). However, there was a significant association between intratumoral CA IX expression and serum CA IX concentration (rho=0.51, P=0.040). These results suggest that serum CA IX level correlates with tumor CA IX expression in TGCT patients, but fails to exhibit either a prognostic value or an association with patients/tumor characteristics.
Keywords: testicular germ cell tumors, carbonic anhydrase IX, hypoxia, prognostic value
Introduction
Testicular germ cell tumors (TGCTs) represent the most common type of cancer in young men (1). The highest incidence of the disease is between 15 and 35 years of age (2). Due to high sensitivity to chemotherapy, the majority of TGCT patients with metastatic disease may expect to be cured with first line cisplatin-based chemotherapy. TGCTs are therefore considered as a model of curable malignancy. However, there are ~20% of patients who fail to be cured following the first line treatment (3,4). Thus, delineation of the molecular basis of insufficient chemotherapy effect in relapsed patients may enable the identification of novel biomarkers and prognostic factors, which may be used as an effective tool for better stratification of patients prior to treatment.
It is well established that hypoxia and an hypoxic microenvironment are key factors in cancer pathogenesis (4). Hypoxic microenvironments are frequently associated with increased disease aggressiveness, tumor dissemination and poor prognosis (5–9). The main adaptive response of tumor cells to an hypoxic microenvironment is mediated by hypoxia inducible factor-1 (HIF-1) transcription factor, which is stabilized and activated under hypoxic conditions (10). HIF-1 binds to hypoxia-responsive elements in its target genes, including those encoding erythropoietin, vascular endothelial growth factor and matrix metalloproteinases, and is therefore implicated in several important processes involved in tumor biology, including cell proliferation, angiogenesis, cell metabolism, apoptosis and migration (5,11). One of the HIF-1 transactivated genes codes for carbonic anhydrase IX (CA IX), a zinc metalloenzyme catalyzing the reversible hydration of carbon dioxide to carbonic acid and participating in pH regulation as well as in cell adhesion-migration-invasion (12). Thus, CA IX plays a role in maintaining the normal intracellular pH in tumor tissue under hypoxic conditions. Furthermore, increased CA IX expression is associated with treatment resistance and cancer stem cell properties (13–15). As cancer stem cells in TGCTs resemble embryonic stem cells (16,17), the present authors supposed that CA IX could be involved in the pathogenesis of TGCTs. Several studies demonstrated the prognostic value of tissue CA IX in different types of cancer, including cervical (6,18), ovarian (19), hepatocellular (20), lung and renal cancer (21,22).
Peripheral blood is easily accessible and enables repeated examination in the course of the disease. In several studies evaluating the clinical utility of soluble CA IX levels in cancer patients, high serum or plasma CA IX levels were associated with an inferior outcome in cervical and vulvar cancer, oral squamous cell carcinoma, non-small cell lung cancer and metastatic breast cancer (23–27). The aim of the present study was to evaluate CA IX levels in serum and tumor samples from a cohort of chemotherapy-naive, chemotherapy-pretreated and relapsed TGCTs patients in order to investigate the potential role of CA IX as a non-invasive prognostic biomarker in TGCT.
Patients and methods
Study patients
The current prospective translational study (protocol IZLO1, chair Dr M. Mego) was approved by the Institutional Review Board (IRB) of the National Cancer Institute (Bratislava, Slovakia), and was conducted between March 2011 and April 2013. All consecutive patients with TGCTs treated with ≥1 cycle of cisplatin-based chemotherapy in the National Cancer Institute or St. Elizabeth Cancer Institute (Bratislava, Slovakia) were enrolled in the study. Serum samples from 35 healthy individuals were used as a control group. Data regarding age, tumor histological subtype, clinical stage, type and number of sites of metastasis and type of chemotherapy regimen were recorded for all patients and compared with CA IX expression. TGCTs patients and controls were recruited and provided informed consent according to the IRB approved protocol.
Serum samples collection
Peripheral blood samples were collected from all participants in the present translational study. Samples were collected into Vacutainer® Rapid Serum Tubes (BD Biosciences, Franklin Lakes, NJ, USA) containing silica clot activator polymer gel at baseline in the morning on days 1 or 0 of the first cycle of chemotherapy (n=73) and prior to the second cycle of chemotherapy (n=37), or before starting a new line of salvage chemotherapy in patients with relapsed disease (n=10). Patients' blood samples (1 ml) were centrifuged at 2,800 × g for 10 min to separate the serum from the blood cells. Serum aliquots were stored at −80°C until further analysis.
CA IX enzyme-linked immunosorbent assay (ELISA)
ELISA for the quantitative determination of serum CA IX concentration was performed at room temperature. The microplate wells were coated overnight with 100 µl/well of the anti-CA IX ectodomain (ECD) monoclonal antibody (MAb) that had been purified in a previous study (28). The MAb was diluted in phosphate-buffered saline (PBS) to a concentration of 10 µg/ml. Non-specific binding was blocked with PBS containing 1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA) and 0.05% Tween 20 for 2 h, and washed twice with PBS+0.05% Tween 20 (PBST). Serum samples diluted in PBST were added together with the peroxidase-conjugated MAb (diluted to 1:10,000 in PBST), which was created in-house in our previous study (28). Each component was added in a volume of 100 µl/well, and incubated for 3 h. Tested samples were washed three times with PBST. The bound MAb used for detection was visualized by addition of 10 mg o-phenylenediamine substrate (Sigma-Aldrich) with 10 µl H2O2 in citrate buffer (pH 5) for 5–15 min in the dark. Absorbance was measured at 492 nm. Data were processed, and the CA IX ECD was quantitated based on a calibration curve obtained using our in-house generated recombinant CA IX ECD (amino acids 38–406)-polyhistidine-tag fusion protein, which was used as a standard.
Diagnosis and tumor samples
The study included available tumor specimens resected from 17 patients prior to the administration of cisplatin-based chemotherapy, namely from 13 (76.5%) patients with primary testicular tumors and 4 (23.5%) patients with retroperitoneal germ cell tumors. All specimens were classified according to the most recent World Health Organization classification from 2004 (29).
Tumor pathology
A pathology review was performed at the Department of Pathology, Faculty of Medicine, Comenius University (Bratislava, Slovakia), by two pathologists (Z.C. and P.B.) associated with the study. All specimens were assessed by light microscopy following hematoxylin and eosin (HE) staining, and with the use of immunohistochemical markers typical for germ cell tumors.
Tissue microarray construction
From each histological subtype, 1–2 representative tumor areas were identified on HE-stained sections, according to tumor histology. If normal testicular tissue samples were present, they were also marked. Sections were matched to the donor blocks (corresponding wax blocks). Tumor cores (3-mm diameter) were removed from the donor blocks using the multipurpose sampling tool Harris Uni-Core (Sigma-Aldrich) and inserted into the recipient master block. The recipient block was cut into 5-µm sections, and sections were transferred to FLEX IHC Microscope slides (cat. no. K8020; Dako, Glostrup, Denmark).
Immunohistochemistry (IHC)
Deparaffinized slides were rehydrated and immersed in PBS (10 mM, pH 7.2). Tissue epitopes were demasked using the automated water bath heating process in PT Link (Dako). Briefly, the slides were incubated in Tris-ethylenediaminetetraacetic acid (EDTA) retrieval solution (10 mM Tris, 1 mM, pH 9.0) at 98°C for 20 min. CA IX expression was detected by IHC using the in-house generated monoclonal antibody M75 against the N-terminal domain of human CA IX, as described previously (30,31). Slides were incubated for 60 min at room temperature with the aforementioned primary antibody diluted to 1:100 in REAL Antibody Diluent (Dako) and immunostained using an anti-mouse/anti-rabbit immunoperoxidase polymer (EnVision FLEX/HRP; Dako) for 30 min at room temperature, according to the manufacturer's protocol. The reaction was visualized with a 3,3-diaminobenzidine substrate-chromogen solution (Dako Cytomation; Dako) for 5 min, and slides were counter-stained with hematoxylin. Clear cell renal cell carcinoma tissue was used as a positive control. As a negative control, the same tumor tissue was used, but omitting the primary antibody from the staining protocol.
IHC scoring
Two observers (Z.C. and P.B.), who were blinded to the patients clinicopathological data, independently assessed the tumor cores. In cases of disagreement, the result was reached by consensus. CA IX expression was stratified as negative or positive (any staining).
Statistical analyses
Patients' data were tabulated. The patients' characteristics were summarized using the mean ± standard error of the mean or median (range) for continuous variables, and frequency (percentage) for categorical variables. Statistical analysis was performed using non-parametric tests, as the distribution of CA IX expression was significantly different from a normal distribution (Shapiro-Wilk test). The Mann-Whitney U test was used for analysis of the association between serum CA IX expression and clinicopathological variables in 2 groups of patients, while the Kruskal-Wallis test was used to compare >2 groups. The Wilcoxon test was used to compare the serum CA IX level prior to the first and second cycles of chemotherapy.
The median follow-up period was calculated as a median observation time among all patients and among those who were still alive at the time of their last follow-up. Progression-free survival (PFS) was calculated from the date of treatment initiation with cisplatin-based chemotherapy to the date of progression, mortality or last follow-up. Overall survival (OS) was calculated from the date of treatment initiation with cisplatin-based chemotherapy to the date of mortality or last follow-up. Survival rates were estimated using the Kaplan-Meier product limit method, and were compared with the log-rank test to determine their significance. CA IX expression data were dichotomized into high and low groups based on the CA IX expression median value of all samples. The Spearman correlation coefficient was used to examine a potential correlation between CA IX serum concentration and lactate dehydrogenase (LDH), human chorionic gonadotropin (HCG) and alpha-fetoprotein (AFP) levels as well as CA IX expression in tumor specimens. All statistical tests were two-sided, and P<0.05 was considered to indicate a statistical significant difference. Statistical analyses were performed using NCSS 2007 software (NCSS, LLC, Kaysville, UT, USA).
Results
Patients' characteristics
From March 2011 to April 2013, 83 patients, including 16 non-metastatic patients subjected to orchiectomy with no evidence of disease (group 1), 57 metastatic chemotherapy-naïve patients (group 2) and 10 metastatic relapsed chemotherapy-pretreated patients (group 3), who were starting adjuvant and/or a new line of chemotherapy were registered to participate in the present study at the National Cancer Institute and St. Elizabeth Cancer Institute of Slovakia. The characteristics of the patients are shown in Table I.
Table I.
Patients' characteristics (n=83).
| Chemotherapy-naïve TGCTs | Chemotherapy-pretreated relapsed TGCTs | |||
|---|---|---|---|---|
| Characteristics | N=73 | % | N=10 | % |
| Age, years | ||||
| Median (range) | 34 (19–67) | 34 (24–50) | ||
| Primary tumor | ||||
| Gonadal | 70 | 95.9 | 9 | 90.0 |
| Retroperitoneal | 3 | 4.1 | 1 | 10.0 |
| Histologya | ||||
| Seminoma | 12 | 16.4 | 3 | 30.0 |
| Non-seminoma | 60 | 82.2 | 7 | 70.0 |
| Stage of TGCTs | ||||
| I.A-I.B | 16 | 21.9 | 0c | 0.0 |
| I.S | 6 | 8.2 | 0 | 0.0 |
| II.A-III.A | 27 | 37.0 | 2 | 20.0 |
| III.B | 13 | 17.8 | 2 | 20.0 |
| III.C | 11 | 15.1 | 6 | 60.0 |
| Sites of metastasesb | ||||
| Retroperitoneum | 48 | 84.2 | 10 | 100.0 |
| Mediastinum | 9 | 15.8 | 0 | 0.0 |
| Lung | 18 | 31.6 | 7 | 70.0 |
| Liver | 4 | 7.0 | 1 | 10.0 |
| Brain | 2 | 3.5 | 0 | 0.0 |
| Other | 5 | 8.8 | 2 | 20.0 |
| Visceral non-pulmonary | 7 | 12.3 | 1 | 10.0 |
| IGCCCG risk groupb | ||||
| Good prognosis | 36 | 63.2 | NA | NA |
| Intermediate prognosis | 11 | 19.3 | NA | NA |
| Poor prognosis | 10 | 17.5 | NA | NA |
| Mean (range) | ||||
| AFP, mIU/ml | 869.9 (0.9–13,936.0)b | 10,376.2 (1.6–89,954.0) | ||
| HCG, IU/ml | 81,602.5 (0.0–1,840,510.0)b | 53,742.2 (0.1–480,259.0) | ||
| LDH, mkat/l | 11.8 (1.8–76.0)b | 11.3 (2.3–33.2) | ||
In 1 patient, chemotherapy started without histological confirmation due to very advanced disease.
Only patients with metastatic disease (n=57).
Initial stage of disease. HCG, human chorionic gonadotropin; AFP, alpha-fetoprotein; LDH, lactate dehydrogenase; IGCCCG, International Germ Cell Consensus Classification Group; NA, not applicable; TGCT, testicular germ cell tumor.
The majority of patients had non-seminoma histology and primary testicular cancer. The majority of chemotherapy-naïve patients (61; 83.6%) were treated with the BEP (bleomycin, etoposide, cisplatin) regimen; 8 patients (11.0%) received EP (etoposide, cisplatin) chemotherapy, while 2 patients (2.7%) received VIP (ifosfamide, etoposide, cisplatin) chemotherapy and 2 patients (2.7%) were treated with dose-dense chemotherapy, as described previously (32,33). Patients with relapsed disease were pretreated with ≥2 lines of cisplatin-based chemotherapy, with a median of 3 lines of treatment. Of the 10 patients, 3 (30.0%) were platinum resistant, while 7 (70.0%) had platinum-sensitive disease.
Association between serum CA IX level and patients/tumor characteristics
The mean serum level of CA IX in TGCT patients was significantly higher compared with healthy controls (405.2±90.1 vs. 249.6±100.0 pg/ml; P=0.007). Metastatic chemotherapy-naïve patients had significantly higher mean serum levels of CA IX compared with serum samples of an independent group of healthy individuals (490.6±111.8 vs. 249.6±100.0 pg/ml; P=0.005), whereas there was no significant difference in the mean serum CA IX levels between metastatic relapsed chemotherapy-pretreated patients and healthy controls (216.0±170.2 vs. 249.6±100.0 pg/ml; P=0.370). Similarly, the mean serum levels of CA IX in non-metastatic upon orchiectomy with no evidence of disease patients were not significantly different compared with those in healthy donors (218.9±128.8 vs. 249.6±100.0 pg/ml; P=0.080).
There was no significant difference in the mean CA IX serum levels between non-metastatic, metastatic chemotherapy-naïve and chemotherapy-pretreated TGCT patients (Fig. 1). For 37 chemotherapy-naïve patients, the serum was available for the CA IX measurement on days 1 and 2. There was no significant difference in the median CA IX level in serum prior to chemotherapy (157.8 pg/ml) and prior to the second cycle of chemotherapy (52.1 pg/ml, P=0.360) (Fig. 2).
Figure 1.

Serum CA IX in different groups of TGCT patients (group 1, adjuvant chemotherapy-naïve patients, n=16; group 2, metastatic chemotherapy-naïve patients, n=57; group 3, relapsed chemotherapy-pretreated patients, n=10). The median level of CA IX in serum in groups 1, 2 and 3 was 93.7, 186.7 and 141.7 pg/ml, respectively (P=0.630). CA IX, carbonic anhydrase IX; TGCT, testicular germ cell tumor.
Figure 2.
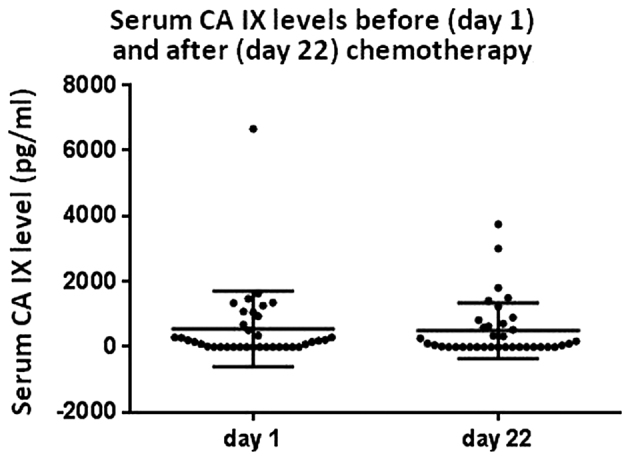
Serum CA IX level before (day 1) starting chemotherapy and before (day 22) the second cycle of chemotherapy. Serum CA IX levels displayed no significant changes during therapy (median serum level before and after chemotherapy, 157.8 and 52.1 pg/ml, respectively, P=0.360). CA IX, carbonic anhydrase IX.
The associations between the histological subtypes of the primary germ cell tumors and the mean CA IX levels detected by ELISA in the patients' serum samples are summarized in Table II. No significant correlation was observed between the mean serum CA IX levels and clinicopathological variables (Table III). The Spearman's correlation coefficient (rho) between the mean serum CA IX concentration and LDH, HCG and AFP levels was −0.11 (P=0.440), −0.20 (P=0.180) and 0.12 (P=0.430), respectively.
Table II.
CA IX concentration in serum of metastatic chemotherapy-naïve patients with different histological subtypes of primary germ cell tumors (n=55).
| Serum CA IX level (pg/ml) | |||||
|---|---|---|---|---|---|
| Histological subtypea | N | Mean | SEM | Median | P-value |
| Seminoma | 25 | 417.5 | 196.3 | 207.8 | 0.830 |
| Embryonal carcinoma | 26 | 644.8 | 189.7 | 198.7 | 0.570 |
| Yolk sac tumor | 25 | 372.1 | 195.7 | 186.7 | 0.570 |
| Choriocarcinoma | 12 | 261.7 | 281.8 | 152.3 | 0.410 |
| Teratoma | 20 | 304.1 | 217.9 | 77.9 | 0.510 |
In 1 patient, chemotherapy started without histological confirmation due to very advanced disease. The percentages of certain histological types of non-seminoma germ cell tumors were as follows: Pure embryonal carcinoma occurred in 14.5% (8 patients), pure choriocarcinoma in 7.3% (4 patients), pure form of yolk sac tumor in 5.5% (3 patients) and immature teratoma in 1.8% (1 patient). Mixed germ cell tumors were the most commonly presented histological subtype of non-seminomas. CA IX, carbonic anhydrase IX; SEM, standard error of the mean.
Table III.
Association between serum CA IX level and patients/tumor characteristics in metastatic chemotherapy-naïve testicular germ cell tumor patients (n=57).
| Serum CA IX level (pg/ml) | |||||
|---|---|---|---|---|---|
| Variable | N | Mean | SEM | Median | P-value |
| All patients | 57 | 490.6 | 127.9 | 186.7 | NA |
| Primary tumora | 0.750 | ||||
| Seminoma | 12 | 315.0 | 282.6 | 142.2 | |
| Non-seminoma | 43 | 510.4 | 149.3 | 157.8 | |
| IGCCCG risk group | 1.000 | ||||
| Good prognosis | 36 | 590.4 | 162.2 | 169.4 | |
| Intermediate prognosis | 11 | 382.6 | 231.7 | 231.7 | |
| Poor prognosis | 10 | 250.1 | 78.9 | 78.9 | |
| Number of metastatic sites | 0.200 | ||||
| 0–1 | 31 | 409.5 | 174.3 | 86.7 | |
| >2 | 26 | 587.3 | 190.3 | 219.8 | |
| Retroperitoneal lymph nodes metastases | 0.420 | ||||
| Present | 9 | 266.1 | 323.1 | 221.3 | |
| Absent | 48 | 532.7 | 139.9 | 209.3 | |
| Mediastinal lymph nodes metastases | 0.770 | ||||
| Present | 48 | 534.6 | 139.9 | 172.2 | |
| Absent | 9 | 255.9 | 323.0 | 231.7 | |
| Lung metastases | 0.670 | ||||
| Present | 39 | 419.1 | 155.1 | 210.8 | |
| Absent | 18 | 645.7 | 228.3 | 149.4 | |
| Non-pulmonary visceral metastases | 0.310 | ||||
| Present | 50 | 494.8 | 137.8 | 149.4 | |
| Absent | 7 | 460.7 | 368.3 | 286.9 | |
| S-stage | 0.460 | ||||
| 0 | 11 | 378.1 | 290.7 | 285.7 | |
| 1 | 22 | 773.6 | 205.6 | 197.3 | |
| 2 | 16 | 290.0 | 241.1 | 221.3 | |
| 3 | 8 | 268.3 | 341.0 | 0.0 | |
In 1 patient, chemotherapy started without histological confirmation due to very advanced disease, and in 1 patient, histology data were not available. NA, not applicable; SEM, standard error of the mean; CA IX, carbonic anhydrase IX; IGCCCG, International Germ Cell Consensus Classification Group.
Correlation between CA IX in serum samples and tumor specimens
Intratumoral CA IX expression was evaluated by IHC in 17 tumor tissue specimens collected on tissue microarray. CA IX protein was detected in 8 specimens (47.1%) and exhibited mostly a focal expression pattern. CA IX staining was present in cancer cells, but in certain specimens, it was visible in the stroma (Fig. 3). Using Spearman correlation analysis, a significant association was identified between CA IX expression in tumor specimens and CA IX values in the corresponding serum samples from 17 patients (Spearman's rho=0.51, P=0.040).
Figure 3.
Immunohistochemical staining of carbonic anhydrase IX (CA IX) in testicular germ cell tumor tissue specimens using the M75 monoclonal antibody targeting the N-terminal extracellular proteoglycan domain of both native and denatured CA IX. Focal staining pattern includes (A) seminoma cells and (B) stroma. Immunoperoxidase and 3,3-diaminobenzidine staining. Magnification, ×200.
Prognostic value of serum CA IX
In the median follow-up time of 31.5 months (range, 0.3–46.8 months), 11 patients (13.3%) experienced disease progression (4 in the chemotherapy-naïve group and 7 in the chemotherapy-pretreated group) and 10 patients (12.0%) succumbed to the disease (4 in the chemotherapy-naïve group and 6 in the chemotherapy-pretreated group).
No significant differences in PFS were observed based on the CA IX levels in serum of the TGCT patients enrolled in the present study [hazard ratio (HR)=0.81, 95% confidence interval (CI)=0.25–2.65, P=0.730). Similarly, there was no difference in OS according to the serum CA IX level (HR=0.64, 95% CI=0.18–2.20, P=0.48) (Figs. 4 and 5). Of 37 patients who exhibited CA IX overexpression prior to the second cycle of chemotherapy, 3 patients experienced disease progression and succumbed to the disease. All these patients had ‘high’ CA IX level in serum; however this difference did not reach statistical significance (P=0.070).
Figure 4.
Kaplan-Meier estimates of PFS according to CA IX expression in serum. Patients with ‘low’ CA IX had similar PFS to patients with ‘high’ CA IX (hazard ratio=0.81, P=0.730). CA IX, carbonic anhydrase IX; PFS, progression-free survival.
Figure 5.
Kaplan-Meier estimates of OS according to CA IX expression in serum. Patients with ‘low’ CA IX had similar OS to patients with ‘high’ CA IX (hazard ratio=0.64, P=0.480). CA IX, carbonic anhydrase IX; OS, overall survival.
Discussion
TGCTs belong to the most chemosensitive group of tumors, and represent a model for the cure of cancer (34). However, a small number of patients fail to achieve complete remission with initial cisplatin-based chemotherapy (35). Biomarkers and pathways involved in the treatment failure in TGCT patients remain poorly defined. Emerging data suggest that microenvironmental factors such as hypoxia may play a role in chemoresistance and in the acquisition of an aggressive phenotype by TGCT cells (36,37). In the present prospective translational study, serum CA IX, which was used as a marker of tumor tissue hypoxia, was significantly increased in TGCT patients compared with healthy controls. However, serum CA IX failed to display a prognostic value in TGCTs. This observation was consistent for chemotherapy-naïve non-metastatic and metastatic patients, as well as for relapsed chemotherapy-pretreated TGCT patients. In addition, there was no significant difference in the mean serum CA IX level between different groups of patients and between CA IX levels prior to the first and second cycles of chemotherapy. By contrast, there was a significant association between CA IX expression in tumor samples and CA IX levels in serum.
The absence of a significant correlation between CA IX levels in the serum of the analyzed TGCT patients and their treatment outcome is not an unusual finding. Recently published data suggest that the potential clinical value of the CA IX ECD shed from the surface of tumor cells to the serum of patients is inconsistent. While the studies by Műller et al (25), Ostheimer et al (38) and Kock et al (24) support the prognostic role of CA IX in metastatic breast cancer, non-small cell lung cancer and vulvar cancer, respectively, the study by Hyrsl et al (39) fails to identify any significant correlation between CA IX EDC values and clinicopathological features. Based on the present findings, it may be supposed that the CA IX EDC level cannot reliably reflect the expression of CA IX or the activation of the HIF-1 signaling pathway in TGCT.
For survival analysis, the CA IX level was dichotomized based on the median values of all analyzed samples. Although it cannot be excluded that using different cut-off values may be able to discriminate good vs. poor prognosis patients, there was no correlation between serum CA IX level and disease stage, International Germ Cell Consensus Classification Group prognostic group, disease burden or any other known prognostic factors, thus supporting the limited prognostic value of serum CA IX in TGCTs. To increase the statistical power, chemotherapy-naïve and chemotherapy-pretreated patients were combined. However, when separate analyses of these subgroups were performed, the study results remained the same. It is also possible that, due to the good prognosis of TGCTs, all pathophysiological aspects associated with the activation of CA IX and HIF pathways do not affect the chemosensitivity of TGCTs. This would be consistent with the findings of Vranic et al, who observed low frequency of HIF-1α overexpression in TGCTs (40).
A significant association between CA IX expression in tumor samples and CA IX values in serum was observed. However, there were several cases of CA IX positivity in serum samples and CA IX negativity in the corresponding tumors. Since the hypoxia-related expression of CA IX in tumors is generally very heterogeneous and often focal, it is probable that the available region encompassed in the tissue microarray subjected to IHC staining was actually devoid of CA IX, but this does not exclude the presence of CA IX in another tumor region. Thus, the correlation observed in the present study may actually be an underestimation of the real association. The limited sample size (n=17) and time period between orchiectomy and administration of chemotherapy could represent limitations that may affect the study results. The association between CA IX expression in tumor samples and the CA IX values in serum could be explained by intratumoral hypoxia, which leads to the release of the soluble form of CA IX into the bloodstream. Studies on the association of intratumoral CA IX expression and detection of its soluble isoform are limited. A study by Zhou et al demonstrated an association between CA IX serum level and tumor size, but not between intratumoral CA IX expression and tumor size in renal cell cancer (41). In contrast to these results, a study on urinary CA IX in renal cell cancer patients identified a coherence of soluble CA IX and intratumoral CA IX expression (39).
In a previous study, CA IX expression was identified in the flat surface epithelium (modified mesothelium) of all male and female genital organs (42). These findings indicated that, during human development, all CA IX-expressing cells have a mesodermal origin and thus, one would have expected higher CA IX expression in teratoma and teratocarcinoma compared with other TGCT histological subtypes. The presents results revealed no correlation between primary germ cell tumors histological subtypes and CA IX levels detected in serum, despite the fact that an independent analysis of a much larger collection of TGCT tissue specimens revealed a significant correlation between CA IX expression in tumors and patients' prognosis (data not shown). The reasons for the lack of tissue-serum CA IX correlation remain unknown, but they may be linked to the fact that the activation of the HIF-1 signaling pathway is relatively rare in TGCTs, and CA IX expression may also be driven by factors other than hypoxia (40). In addition, CA IX is often visible in the stroma of TGCTs; thus, it is conceivable that the ECD of CA IX could remain deposited in the extracellular matrix surrounding the stromal cells and act locally rather then being released into the circulation.
In the present study, there were no differences in serum CA IX levels between non-metastatic, metastatic chemotherapy-naïve and chemotherapy-pretreated TGCT patients, but significant differences were observed between CA IX serum levels in TGCT patients and healthy controls. Unexpectedly, there was no difference when comparing only relapsed chemotherapy-pretreated patients, suggesting a different biology and role of hypoxia in relapsed TGCTs compared with chemotherapy-naïve patients. Analysis of serum CA IX levels revealed no significant changes during therapy (prior to chemotherapy and prior to the initiation of the second cycle of chemotherapy). However, in the cohort of 37 patients prior to receiving the second line of chemotherapy, 3 patients experienced disease progression and succumbed to the disease. All these patients had CA IX levels above the median values. However, the limited sample size precludes the drawing of any definitive conclusions from these data.
In summary, the present study is the first aimed to assess the prognostic role of serum CA IX level in TGCTs. The present data suggest that there is an increased level of serum CA IX in metastatic chemotherapy-naïve TGCTs compared with healthy controls, and that a weak correlation exists between serum CA IX levels and tissue CA IX expression. These data suggest neither a prognostic value for serum CA IX levels nor an association between serum CA IX levels and patients/tumor characteristics. Despite the limited clinical utility of serum CA IX, the biological and clinical value of CA IX expression in TCGT tissues cannot be excluded, and therefore, further research into this area is warranted.
Acknowledgements
The authors would like to acknowledge Mrs. Daniela Jantekova from the Population Registry of Slovak Republic (Bratislava, Slovakia) for helping to update the patient database; Dr Maria Reckova from the 2nd Department of Oncology, Faculty of Medicine, Comenius University for discussion and critical input; Mrs. Zlatica Pekova from the Department of Oncology, National Cancer Institute for administration support; and Mrs. Emilia Klincova from the Department of Pathology, Faculty of Medicine, Comenius University for the excellent technical assistance. The present study was supported by the Slovak Research and Development Agency (Bratislava, Slovakia; contract nos. APVV-0016-11 and APVV-15-0086), the European Regional Development Fund (Brussels, Belgium), the State Budget of the Slovak Republic (Bratislava, Slovakia; project no. ITMS 26240220071) and the Slovak Scientific Grant Agency (Bratislava, Slovakia; grant no. VEGA-2/0108/16).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Rijlaarsdam MA, Looijenga LHJ. An oncofetal and developmental perspective on testicular germ cell cancer. Semin Cancer Biol. 2014;29:59–74. doi: 10.1016/j.semcancer.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Feldman DR, Bosl GJ, Sheinfeld J, Motzer RJ. Medical treatment of advanced testicular cancer. JAMA. 2008;299:672–684. doi: 10.1001/jama.299.6.672. [DOI] [PubMed] [Google Scholar]
- 4.Einhorn LH. Clinical trials in testicular cancer. Cancer. 1993;71:3182–3184. doi: 10.1002/1097-0142(19930515)71:10<3182::AID-CNCR2820711046>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 5.Wykoff CC, Beasly NJP, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]
- 6.Loncaster JA, Harris AL, Davidson SE, Logue JP, Hunter RD, Wycoff CC, Pastorek J, Ratcliffe PJ, Stratford IJ, West CM. Carbonic anhydrase IX (CA IX) expression, a potential new intrinsic marker of hypoxia: Correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001;61:6394–6397. [PubMed] [Google Scholar]
- 7.Vordermark D, Kaffer A, Riedl S, Katzer A, Flentje M. Characterisation of carbonic anhydrase IX (CAIX) as an andogenous marker of chronic hypoxia in live human tumor cells. Int J Radiant Oncol Biol Phys. 2005;61:1197–1207. doi: 10.1016/j.ijrobp.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Kim SJ, Shin HJ, Jung KY, Baek SK, Shin BK, Choi J, Kim BS, Shin SW, Kim YH, Kim JS, et al. Prognostic value of carbonic anhydrase IX and Ki-67 expression in squamous cell carcinoma of the tongue. Jpn J Clin Oncol. 2007;37:812–819. doi: 10.1093/jjco/hym121. [DOI] [PubMed] [Google Scholar]
- 9.Harris AL. Hypoxia-a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 10.Ratcliffe PJ. Oxygen sensing and hypoxia signalling pathways in animals: The implications of physiology for cancer. J Physiol. 2013;591:2027–2042. doi: 10.1113/jphysiol.2013.251470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon EJ, Brizel DM, Chi JT, Dewhirst MW. The potential role of intrinsic hypoxia markers as prognostic variables in cancer. Antioxid Redox Signal. 2007;9:1237–1294. doi: 10.1089/ars.2007.1623. [DOI] [PubMed] [Google Scholar]
- 12.Pastorek J, Pastorekova S. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: From biology to clinical use. Semin Cancer Biol. 2015;31:52–64. doi: 10.1016/j.semcancer.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 13.McCord AM, Jamal M, Shankavarum UT, Lang FF, Camphausen K, Tofilon PJ. Physiologic oxygen concentration enhances the stem-like properties of CD133+ human glioblastoma cells in vitro. Mol Cancer Res. 2009;7:489–497. doi: 10.1158/1541-7786.MCR-08-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lock FE, McDonald PS, Lou Y, Serrano I, Chafe SC, Ostlund C, Aparicio S, Winum JY, Supuran CT, Dedhar S. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene. 2013;32:5210–5219. doi: 10.1038/onc.2012.550. [DOI] [PubMed] [Google Scholar]
- 15.Ledaki I, McIntyre A, Wigfield S, Buffa F, McGowan S, Baban D, Li JL, Harris AL. Carbonic anhydrase IX induction defines a heterogenous cancer cell response to hypoxia and mediates stem cell-like properties and sensitivity to HDAC inhibition. Oncotarget. 2015;6:19413–194127. doi: 10.18632/oncotarget.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McIntyre A, Gilbert D, Goddard N, Looijenga L, Shipley J. Genes, chromosomes and the development of testicular germ cell tumors of adolescents and adults. Genes Chromosomes Cancer. 2008;47:547–557. doi: 10.1002/gcc.20562. [DOI] [PubMed] [Google Scholar]
- 17.Sheikine Y, Gebega E, Melamed J, Lee P, Reuter VE, Ye H. Molecular genetics of testicular germ cell tumors. Am J Cancer Res. 2012;2:153–167. [PMC free article] [PubMed] [Google Scholar]
- 18.Liao SY, Darcy KM, Randall LM, Tian C, Monk BJ, Burger RA, Fruehauf JP, Peters WA, Stock RJ, Stanbridge EJ. Prognostic relevance of carbonic anhydrase-IX in high-risk, early-stage cervical cancer: A gynecologic oncology group study. Gynecol Oncol. 2010;116:452–458. doi: 10.1016/j.ygyno.2009.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choschizick M, Oosterwijk E, Müller V, Simon R, Moch H, Tennstedt P. Overexpression of carbonic anhydrase IX (CAIX) is an independent unfavorable prognostic marker in endometrioid ovarian cancer. Virchows Arch. 2011;459:193–200. doi: 10.1007/s00428-011-1105-y. [DOI] [PubMed] [Google Scholar]
- 20.Kang HJ, Kim IH, Sung CO, Shim JH, Yu E. Expression of carbonic anhydrase 9 is a novel prognostic marker in resectable hepatocellular carcinoma. Virchows Arch. 2015;466:403–413. doi: 10.1007/s00428-014-1709-0. [DOI] [PubMed] [Google Scholar]
- 21.Steward GD, O'Mahony FC, Laird A, Rashid S, Martin SA, Eory L, Lubbock AL, Nanda J, O'Donnell M, Mackay A, et al. Carbonic anhydrase 9 expression increases with vascular endothelial growth factor-targeted therapy and is predictive of outcome in metastatic clear cell renal cancer. Eur Urol. 2014;66:956–963. doi: 10.1016/j.eururo.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Z, Liao G, Li Y, Zhou S, Zou H, Fernando S. Prognostic value of carbonic anhydrase IX immunohistochemical expression in renal cell carcinoma: A metaanalysis of the literature. PLoS One. 2014;9:e114096. doi: 10.1371/journal.pone.0114096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilie M, Mazure NM, Hofman V, Ammadi RE, Ortholan C, Bonnetaud C, Havet K, Venissac N, Mograbi B, Mouroux J, et al. High levels of carbonic anhydrase IX in tumour tissue and plasma are biomarkers of poor prognostic in patients with non-small cell lung cancer. Br J Cancer. 2010;102:1627–1635. doi: 10.1038/sj.bjc.6605690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kock L, Mahner S, Choschzick M, Eulenburg C, Milde-Langosch K, Schwarz J, Jaenicke F, Müller V, Woelber L. Serum carbonic anhydrase IX and its prognostic relevance in vulvar cancer. Int J Gynecol Cancer. 2011;21:141–148. doi: 10.1097/IGC.0b013e318204c34f. [DOI] [PubMed] [Google Scholar]
- 25.Müller V, Riethdorf S, Rack B, Janni W, Fasching PA, Solomayer E, Aktas B, Kasimir-Bauer S, Zeitz J, Pantel K, et al. Prospective evaluation of serum tissue inhibitor of metalloproteinase 1 and carbonic anhydrase IX in correlation to circulating tumor cells in patients with metastatic breast cancer. Breast Cancer Res. 2011;13:R71. doi: 10.1186/bcr2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woelber L, Kress K, Kersten JF, Choschzick M, Kilic E, Herwig U, Lindner C, Schwarz J, Jaenicke F, Mahner S, et al. Carbonic anhydrase IX in tumor tissue and sera of patients with primary cervical cancer. BMC Cancer. 2011;11:12. doi: 10.1186/1471-2407-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang JS, Chen MK, Yang SF, Chang YC, Su SC, Chiou HL, Chien MH, Lin CW. Increased expression of carbonic anhydrase IX in oral submucous fibrosis and oral squamous cell carcinoma. Clin Chem Lab Med. 2014;52:1367–1377. doi: 10.1515/cclm-2014-0129. [DOI] [PubMed] [Google Scholar]
- 28.Zat'ovicová M, Tarábková K, Svastová E, Gibadulinová A, Mucha V, Jakubícková L, Biesová Z, Rafajová M, Gut M Ortova, Parkkila S, et al. Monoclonal antibodies generated in carbonic anhydrase IX-deficient mice recognize different domains of tumour-associated hypoxia-induced carbonic anhydrase IX. J Immunol Methods. 2003;282:117–134. doi: 10.1016/j.jim.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Mostofi FK, Sesterhenn IA. Tumours of the testis and paratesticular tissue. In: Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs (IARC WHO Classification of Tumours) IARC Press; Lyon: 2004. pp. 216–278. [Google Scholar]
- 30.Pastoreková S, Závadová Z, Kostál M, Babusíková O, Závada J. A novel quasi-viral agent, MaTu, is a two-component system. Virology. 1992;187:620–626. doi: 10.1016/0042-6822(92)90464-Z. [DOI] [PubMed] [Google Scholar]
- 31.Takacova M, Bullova P, Simko V, Skvarkova L, Poturnajova M, Feketeova L, Babal P, Kivela AJ, Kuopio T, Kopacek J, et al. Expression pattern of carbonic anhydrase IX in Medullary thyroid carcinoma supports a role for RET-mediated activation of the HIF pathway. Am J Pathol. 2014;184:953–965. doi: 10.1016/j.ajpath.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 32.deWit R, Roberts JT, Wilkinson PM, de Mulder PH, Mead GM, Fossa SD, Cook P, de Prijck L, Stenning S, Collette L. Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3- or 5-day schedule in good-prognosis germ cell cancer: A randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council. J Clin Oncol. 2001;19:1629–1640. doi: 10.1200/JCO.2001.19.6.1629. [DOI] [PubMed] [Google Scholar]
- 33.Bokenmeyer C, Kollmannsberger C, Stenning S, Hartmann JT, Horwich A, Clemm C, Gerl A, Meisner C, Ruckerl P, Schmoll HJ, et al. Metastatic seminoma treated with either single agent carboplatin or cisplatin-based combination chemotherapy: A pooled analysis of two randomised trials. Br J Cancer. 2004;91:683–687. doi: 10.1038/sj.bjc.6602020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Einhorn LH. Treatment of testicular cancer: A new and improved model. J Clin Oncol. 1990;8:1777–1781. doi: 10.1200/JCO.1990.8.11.1777. [DOI] [PubMed] [Google Scholar]
- 35.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 36.Rofstad EK. Microenviroment-induced cancer metastasis. Int J Radiat Biol. 2000;76:589–605. doi: 10.1080/095530000138259. [DOI] [PubMed] [Google Scholar]
- 37.Ljungkvist AS, Bussink J, Kaanders JH, van der Kogel AJ. Dynamics of tumor hypoxia measured with bioreductive hypoxic cell markers. Radiat Res. 2007;167:127–145. doi: 10.1667/RR0719.1. [DOI] [PubMed] [Google Scholar]
- 38.Ostheimer C, Bache M, Güttler A, Kotzsch M, Vordermark D. A pilot study on potential plasma hypoxia markers in the radiotherapy of non-small cell lung cancer. Osteopontin, carbonic anhydrase IX and vascular endothelial growth factor. Strahlenther Onkol. 2014;190:276–282. doi: 10.1007/s00066-013-0484-1. [DOI] [PubMed] [Google Scholar]
- 39.Hyrsl L, Zavada J, Zavadova Z, Kawaciuk I, Vesely S, Skapa P. Soluble form of carbonic anhydrase IX (CAIX) in transitional cell carcinoma of urinary tract. Neoplasma. 2009;56:298–302. doi: 10.4149/neo_2009_04_29. [DOI] [PubMed] [Google Scholar]
- 40.Vranic S, Hes O, Grossmann P, Gatalica Z. Low frequency of HIF-1α overexpression in germ cell tumors of the testis. Appl Immunohistochem Mol Morphol. 2013;21:165–169. doi: 10.1097/PAI.0b013e31825e00b7. [DOI] [PubMed] [Google Scholar]
- 41.Zhou GX, Ireland J, Rayman P, Finke J, Zhou M. Quantification of carbonic anhydrase IX expression in serum and tissue of renal cell carcinoma patients using enzyme-linked immunosorbent assy: Prognostic and diagnostic potentials. Urology. 2010;75:257–261. doi: 10.1016/j.urology.2009.09.052. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki J, Jr, Therrien J, Filion F, Lefebvre R, Goff AK, Smith LC. In vitro culture and somatic cell nuclear transfer affect imprinting of SNRPN gene in pre- and post-implantation stages of development in cattle. BMC Dev Biol. 2009;9:9. doi: 10.1186/1471-213X-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]





