Abstract
Background:
Current electrochemical glucose sensors use a single electrode. Multiple electrodes (redundancy) may enhance sensor performance. We evaluated an electrochemical redundant sensor (ERS) incorporating two working electrodes (WE1 and WE2) onto a single subcutaneous insertion platform with a processing algorithm providing a single real-time continuous glucose measure.
Methods:
Twenty-three adults with type 1 diabetes each wore two ERSs concurrently for 168 hours. Post-insertion a frequent sampling test (FST) was performed with ERS benchmarked against a glucose meter (Bayer Contour Link). Day 4 and 7 FSTs were performed with a standard meal and venous blood collected for reference glucose measurements (YSI and meter). Between visits, ERS was worn with capillary blood glucose testing ≥8 times/day. Sensor glucose data were processed prospectively.
Results:
Mean absolute relative deviation (MARD) for ERS day 1-7 (3,297 paired points with glucose meter) was (mean [SD]) 10.1 [11.5]% versus 11.4 [11.9]% for WE1 and 12.0 [11.9]% for WE2; P < .0001. ERS Clarke A and A+B were 90.2% and 99.8%, respectively. ERS day 4 plus day 7 MARD (1,237 pairs with YSI) was 9.4 [9.5]% versus 9.6 [9.7]% for WE1 and 9.9 [9.7]% for WE2; P = ns. ERS day 1-7 precision absolute relative deviation (PARD) was 9.9 [3.6]% versus 11.5 [6.2]% for WE1 and 10.1 [4.4]% for WE2; P = ns. ERS sensor display time was 97.8 [6.0]% versus 91.0 [22.3]% for WE1 and 94.1 [14.3]% for WE2; P < .05.
Conclusions:
Electrochemical redundancy enhances glucose sensor accuracy and display time compared with each individual sensing element alone. ERS performance compares favorably with ‘best-in-class’ of non-redundant sensors.
Keywords: accuracy, continuous glucose sensor, electrochemical redundant sensor, glucose oxidase, precision, reliability
The cornerstone of glucose homeostasis in healthy individuals is the pancreatic islet β-cell, which continuously senses blood glucose and adjusts insulin production and release accordingly. Type 1 diabetes (T1D) characterized by β-cell loss, mandates lifelong exogenous insulin therapy, the requirements for which can fluctuate greatly. To optimize insulin dosing in people with T1D glucose needs to be measured continuously in real-time.
Continuous glucose monitoring (CGM) which enables near-continuous measurement of interstitial fluid glucose in real-time can improve glycemia in T1D.1-3 Current commercially available CGM sensors incorporate a single electrode utilizing glucose oxidase methodology.4,5 CGM devices, a relatively recent development, do not have the accuracy of the most accurate glucose meters.6 Increased CGM sensor accuracy and reliability could enhance patient trust in these devices and translate into positive clinical outcomes.7 It would also facilitate the development of a closed loop insulin system or ‘artificial pancreas.’ To date, advances in CGM technology have included refinements in structure, electrochemistry and data-processing.
Redundancy in monitoring, defined as ≥2 sensing elements measuring the same output, can be applied to CGM by employing multiple glucose sensing electrodes. The use of multiple redundant electrodes measuring interstitial glucose is feasible.8,9 Importantly, given the burden of wearing multiple devices, and that sensor separation does not impact performance8, discrete sensing elements may be incorporated into a single insertion platform. Also, whilst previous studies incorporating multiple glucose sensors combined the mean or median of individual components, inclusion of an intelligent algorithm processing and combining sensor inputs may further improve measurement accuracy and reliability.
We hypothesize that an electrochemical redundant sensor (ERS) incorporating an intelligent processing algorithm will provide an in vivo CGM platform that compares favorably with nonredundant electrochemical platforms. The study aim was to evaluate the performance of an investigational ERS system in adults with T1D.
Methods
This study, prospectively registered (ACTRN12614000256673), was approved by Human Ethics Research Committees and conducted at St Vincent’s Hospital Melbourne and The Royal Melbourne Hospital. There was no randomization of participants. Participants were masked to glucose data from the ERS. Each participant provided written informed consent.
Investigational Glucose Sensor Design
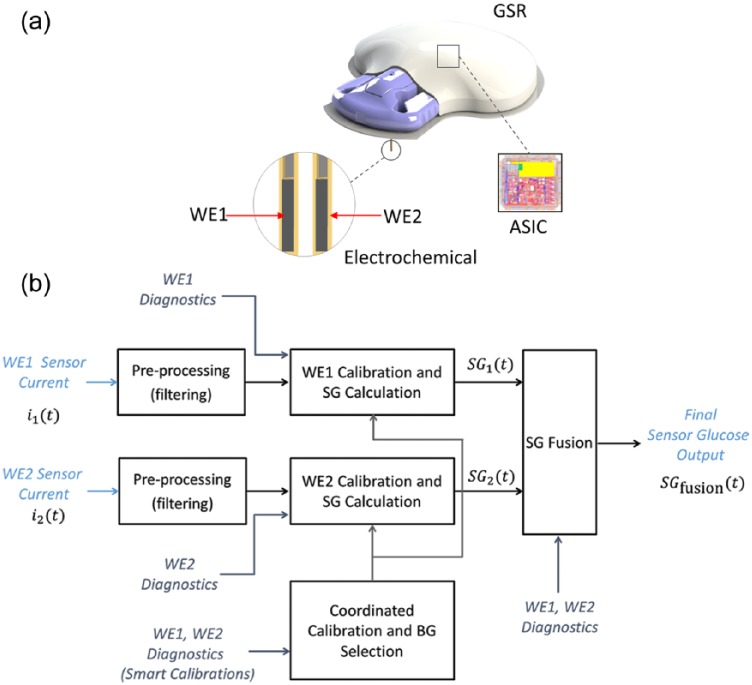
The ERS (Medtronic, Northridge, CA) (Figure 1a) is similar to other Medtronic glucose oxidase based electrochemical sensors available for consumer use (eg, Enlite®), though also includes redundancy and updated sensor design. A single element 0.5 mm wide and 10 mm long is angled at 90 degrees. Each ERS comprises 2 separate working electrodes (WE1 and WE2) which independently quantify glucose levels with both working electrodes inserted through a single 26 gauge needle. This redundancy can mitigate localized performance problems as each WE is housed in a separate flex that measures glucose in opposite directions (ie, 180° rotation, making the ERS a 360° sensor). Localized performance deficiencies are also addressed by the WE geometry, which is distributed across 3 segments on each of the sensor flexes.
Figure 1.
(a) Electrochemical redundant sensor configuration showing working electrode (WE) 1 and 2 and the glucose sensor recorder (GSR) incorporating the application-specific integrated circuit (ASIC); (b) electrochemical redundant sensor signal processing schematic.
Each ERS was linked to a glucose sensor recorder (GSR), from which data were uploaded after sensor removal. An algorithm (Figure 1b) prospectively converted measurements by the application-specific integrated circuit (ASIC) into sensor glucose (SG) values updated every 5 minutes. Algorithm implementation emulates a real-time system because there is no retrospective data processing. To compute SG values, time-dependent current measurements for each working electrode ( and at time) are independently filtered to remove artifact, then converted to 2 SG values ( and, respectively).The sensor is calibrated on an average twice daily using reference blood glucose (BG) measurements to calibrate both working electrodes. Electrochemical impedance spectroscopy (EIS) measurements provide a diagnostic input into the ERS algorithm continuously to assess working electrode health and is used to determine whether can be accurately computed, and whether a new calibration BG is required.
A single SG value,, is computed as
where are time-varying weights determined by the combined state of , ,, as well as the EIS signals of each of the working electrodes. At all times
ERS start-up time was mandated at 60 minutes.
Participants
Adults aged 21-70 years with T1D and prior CGM experience were recruited, with preference given to people with recurrent hypoglycemia. Exclusion criteria were: adhesive allergies, unresolved adverse skin conditions in the area of sensor or device placement and women who were pregnant or planning pregnancy.
Study Protocol
All investigational devices were synchronized and all measurements time stamped.
Day 1 Insertion (visit 1)
Participants attended the clinical trials center (CTC), informed consent was obtained and baseline clinical data collected. Each participant was assigned and educated in the use of the study blood glucose meter. Using a dedicated insertion device two identical ERS were inserted subcutaneously, one into each side of the anterior abdominal wall, and each was paired with a GSR then initialized. In the 3-hours post-insertion a 150-minute frequent sampling test (FST) with 8 capillary glucose measurements by the study glucose meter was performed, commencing 30 minutes post–sensor insertion (+30, 45, 60, 75, 90, 120, 150 and 180 minutes). While a heating box was not used, these studies were performed in a temperature controlled environment. Participants were advised to perform ≥8 capillary blood glucose meter readings using the study meter during their usual activities between CTC visits.
Day 4 post-insertion (visit 2)
Fasted participants returned to the CTC at 7.30 am, ≈72 hours post–sensor insertion. If blood glucose was 72-270 mg/dL and blood ketones <0.6 mmol/L they consumed a standardized test meal containing 65 g carbohydrate, immediately preceded by insulin administered as per their usual regimen. A second FST was performed with an intravenous cannula for blood collection for glucose measurement by the study glucose meter and glucose analyzer (YSI Life Sciences, Yellow Springs, OH) every 15 minutes from 30 minutes prior to until 165 minutes after the test meal. After leaving the CTC participants were advised to continue to perform ≥8 capillary blood glucose readings daily until their final visit.
Day 7 post-insertion (visit 3)
Participants returned to the CTC ≈168 hours post–sensor insertion and the FST protocol and standardized meal undertaken during visit 2 were repeated. At the end of sampling diaries were collected, sensors removed, and GSRs and study meters were returned and uploaded.
Reference Blood Glucose Measurements
YSI glucose analyzer, using glucose oxidase methodology, measured venous plasma glucose levels with CVs of 2.4% at 79 mg/dL and 2.9% at 468 mg/dL.
Bayer Contour Link meter (Bayer Diabetes Care, Whippany, NJ) using glucose oxidase methodology measured capillary blood glucose levels during the day 1 FST, at home and venous glucose levels during the FSTs during meal tests on days 4 and 7. The meter is compliant with ISO 15197:2013 section 6.3 and section 8 accuracy criteria in the laboratory and in the hands of untrained users with diabetes.10
Data Analysis
A descriptive analysis for both aggregate SG results and for individual ERSs was used. SG values were processed prospectively and displayed only when predetermined trace characterization algorithms deemed values to have met criteria qualifying for inclusion. SG values were compared to plasma and capillary glucose levels. Data collection and descriptive analyses included:
Accuracy: Mean absolute relative deviation (MARD) calculated as a percentage of the corresponding reference glucose readings separately for fused and single sensors by correlating glucose measurements against meter glucose and YSI glucose values. Clarke error grid (CEG) analysis was used to quantify the clinical accuracy of CGM versus reference measurements.11 Point accuracy was measured as the proportion of all SG values within 40 mg/dL or 20 mg/dL of the reference value for glucose<80 mg/dL and within 40% or 20% at reference glucose > 80 mg/dL (referred to as %40/40 or %20/20, respectively).
Precision: Precision absolute relative deviation (PARD) was calculated comparing paired glucose measurements from the two ERSs.12 For single electrode performance the paired corresponding single electrode glucose measurement from each of two sensors was compared (ie, WE1 vs WE1 and WE2 vs WE2).
Reliability: Percentage display time was calculated as a proportion of time during which an SG reading was displayed relative to total duration of sensor function. The number of sensors providing glucose data on the final study day (end of day 7) was also determined.
Sensor insertion site appearance: Sites were examined at the time of explant of the sensors on day 7.
Comparisons between individual WEs and fused outputs were performed on parameters relating to accuracy, precision and reliability. Comparison testing was performed using a 2-tailed paired t test for normally distributed, continuous data, a Wilcoxon rank sum test for non-normally distributed continuous data, and a chi-square test for categorical data.
Results
Thirteen female and 10 male T1D participants (mean [SD]: age 53.6 [14.7] years; body mass index 28.8 [5.7] kg/m2; diabetes duration 26 [15] years) participated. Data from 45 ERS sensors worn by 23 participants were available. For one participant one GSR was uncharged and did not record so data were only available from one sensor.
Reference Blood Glucose Meter Performance Versus YSI
Bayer glucose meter readings referenced against YSI measurements (778 paired points) revealed a MARD of 4.3%, and 99.74% of CEG points in Zone A and 0.26% in Zone B.
ERS Accuracy
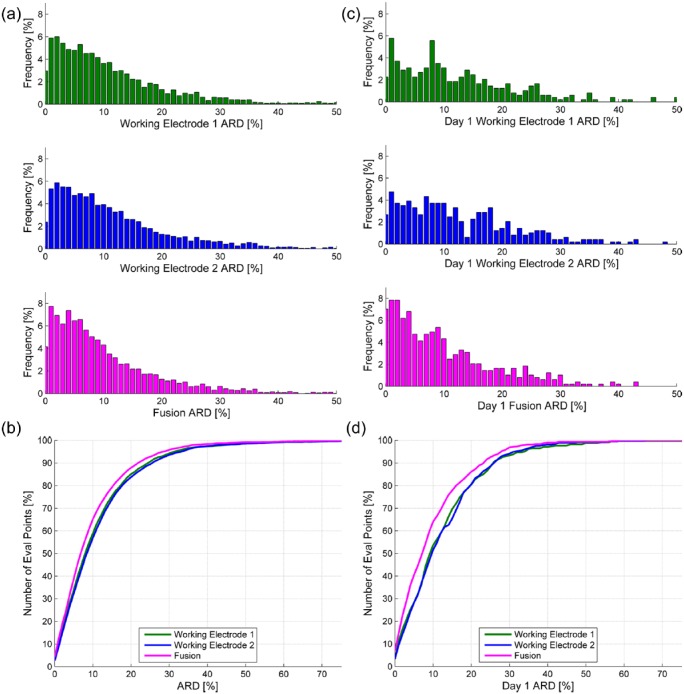
Aggregate performance of the ERS is summarized in Table 1. MARD bench-marked against the Bayer glucose meter with 3,297 paired evaluation points over the 168 hour study duration was 10.1%. Day 1 versus day 1-7 MARD did not differ (mean [SD]: 10.1 [9.5]% vs 10.1 [11.5]%; P = ns). Consensus A alone, Consensus A+B (Figure 2a), 20/20 agreement, 40/40 agreement and ISO accuracy did not significantly differ on day 1 versus the entire study duration. MARD benchmarked against YSI glucose measurements with 1,237 evaluation points on venous blood from the meal FSTs on days 4 and 7 was 9.4 [9.5]% (Table 1), with the corresponding CEG plot in Figure 2b. Frequency distribution and cumulative plots for aggregate day 1-7 and day 1 MARD are shown in Figure 3. The differences observed when comparing single electrode MARD and fused MARD were greatest on day 1 post-insertion.
Table 1.
Aggregate Sensor Performance Data.
| Parameter | Performance day 1 (vs meter) | Performance day 4 and day 7 FST (vs YSI) | Performance days 1-7 (vs meter) |
|---|---|---|---|
| Paired evaluation points (n) | 492 | 1237 | 3297 |
| MARD (%)a | 10.1 (9.5) | 9.4 (9.5) | 10.1 (11.5) |
| Consensus A (%) | 86.4 | 92.7 | 90.2 |
| Consensus A + B (%) | 99.6 | 100.0 | 99.8 |
| % 20/20 mg/dL agreement | 85.6 | 89.3 | 87.9 |
| % 40/40 mg/dL agreement | 99.0 | 98.6 | 98.6 |
| ISO 15197:2013 accuracy (%) | 78.1 | 83.2 | 80.1 |
| 40-80 mg/dL mean absolute difference (mg/dL)a | 12.0 (8.9) | 10.5 (7.9) | 10.3 (8.4) |
| 240-400 mg/dL MARD (%)a | 9.6 (7.8) | 7.3 (5.5) | 8.2 (6.5) |
Mean ± SD.
Figure 2.
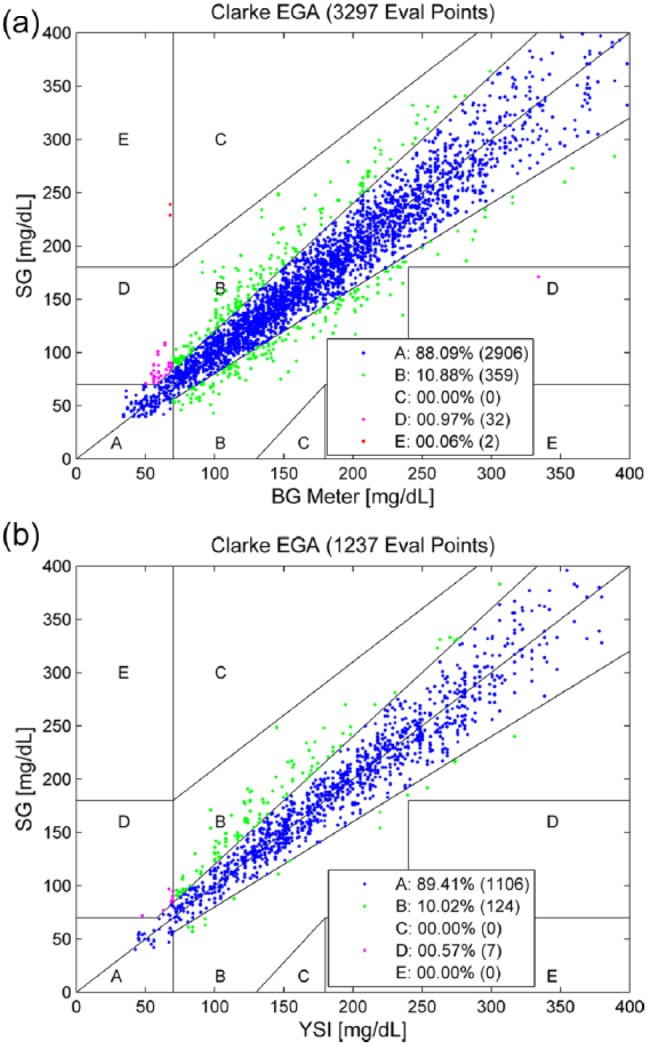
(a) Clarke error grid versus blood glucose meter; (b) YSI.
Figure 3.
(a) Frequency distribution for day 1-7 ARD; (b) cumulative frequency for day 1-7 ARD; (c) frequency distribution for day 1 ARD; (d) cumulative frequency for day 1 ARD.
Accuracy data regarding individual sensor performance with comparisons of component WE1 and WE2 versus fused sensor performance are summarized in Table 2. Differences in day 1-7 MARD and 20/20 agreement favored fused output over individual WEs. Differences in day 1-7 CEG Consensus A were of borderline significance. Figure 4a provides an example where signal processing by the ERS algorithm weighted data from WE1 because of transient noise in the WE2 signal. Figure 4b provides an example where WE1 failed calibration post-insertion and the fusion algorithm at start-up relies almost entirely on WE2. The fused output from ERS improved overall accuracy of individual sensors as reflected by MARD and reduced the number of sensors with outlying MARDs (Figure 5).
Table 2.
Individual Sensor Data.
| Parameter | WE1 (n = 45) | WE2 (n = 45) | Fused output (n = 45) | P (fused output vs WE1 [P1] vs WE2 [P2]) |
|---|---|---|---|---|
| Accuracy | ||||
| Paired evaluation points (n) | 2921 (day 1-7 vs meter) | 2997 day 1-7 vs meter) | 3297 (day 1-7 vs meter) | N/A |
| 349 (day 1 vs meter) | 383 (day 1 vs meter) | 492 (day 1 vs meter) | ||
| 1135 (vs YSI) | 1154 (vs YSI) | 1237 (vs YSI) | ||
| MARD (%) day 1-7 (vs meter)a | 11.4 (11.9) | 12.0 (11.9) | 10.1 (11.5) | P1 < .0001 |
| P2 < .0001 | ||||
| MARD (%) day 1 (vs meter)a | 12.7 (11.7) | 12.9 (11.7) | 10.1 (9.5) | P1 < .0001 |
| P2 < .0001 | ||||
| MARD (%) days 4 and 7 (vs YSI)a | 9.6 (9.7) | 9.9 (9.7) | 9.4 (9.5) | P1 = ns |
| P2 = ns (.09) | ||||
| 20/20 agreement (%) day 1-7 (vs meter) | 84.7 | 83.4 | 87.9 | P1 = .05 |
| P2 = .01 | ||||
| 20/20 agreement (%) day 1 (vs meter) | 79.7 | 79.1 | 85.6 | P1 = ns (.14) |
| P2 = ns (.10) | ||||
| 40/40 agreement (%) day 1-7 (vs meter) | 97.7 | 97.8 | 98.6 | P1 = ns |
| P2 = ns | ||||
| 40/40 agreement (%) day 1 (vs meter) | 97.7 | 98.2 | 99.0 | P1 = ns |
| P2 = ns | ||||
| Consensus A (%) day 1-7 (vs meter) | 88.2 | 87.0 | 90.2 | P1 = ns |
| P2 = .04 | ||||
| Consensus A + B (%) day 1-7 (vs meter) | 99.6 | 99.5 | 99.8 | P1 = ns |
| P2 = ns | ||||
| Precision | ||||
| PARD (%) day 1a | 15.2 (12.2) | 13.8 (5.7) | 13.3 (5.0) | P1 = ns |
| P2 = ns | ||||
| PARD (%) day 1-7a | 11.5 (6.2) | 10.5 (4.4) | 9.9 (3.6) | P1 = ns |
| P2 = ns | ||||
| Reliability | ||||
| Sensor display time (%)a | 91.0 (22.3) | 94.1(14.3) | 97.8 (6.0) | P1 = .04 |
| P2 = ns (.06) | ||||
| Sensors functional to the end of day 7 (%) | 76 | 78 | 87 | P1 = ns |
| P2 = ns | ||||
Mean ± SD.
Figure 4.
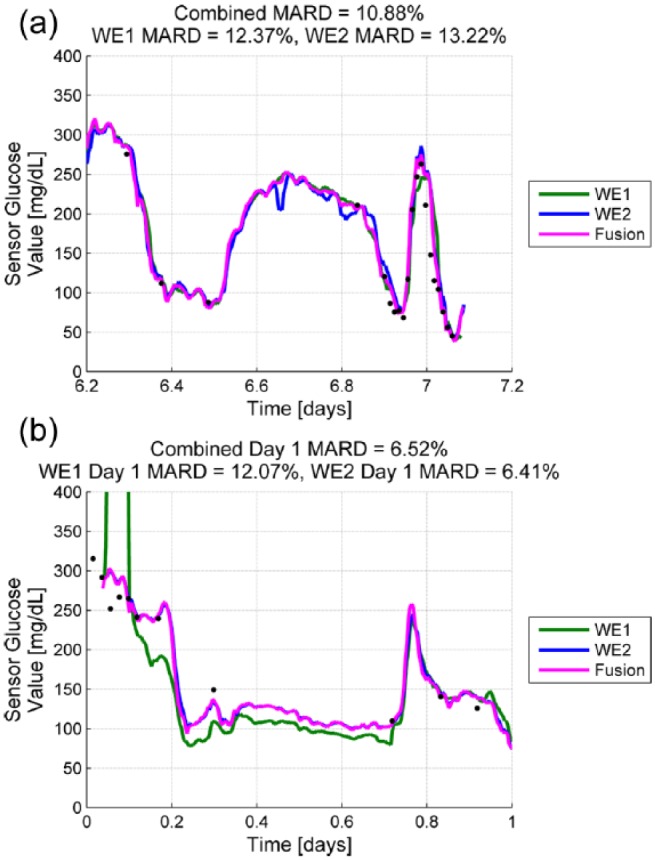
(a) Sample trace demonstrating transient noise in WE2 compensated for by the fusion algorithm which weights WE1 when producing the fusion trace; (b) post-insertion trace where WE1 had not initialized (calibration failed), and the fusion algorithm relies almost entirely on WE2. MARDS provided are for day 1.
Figure 5.
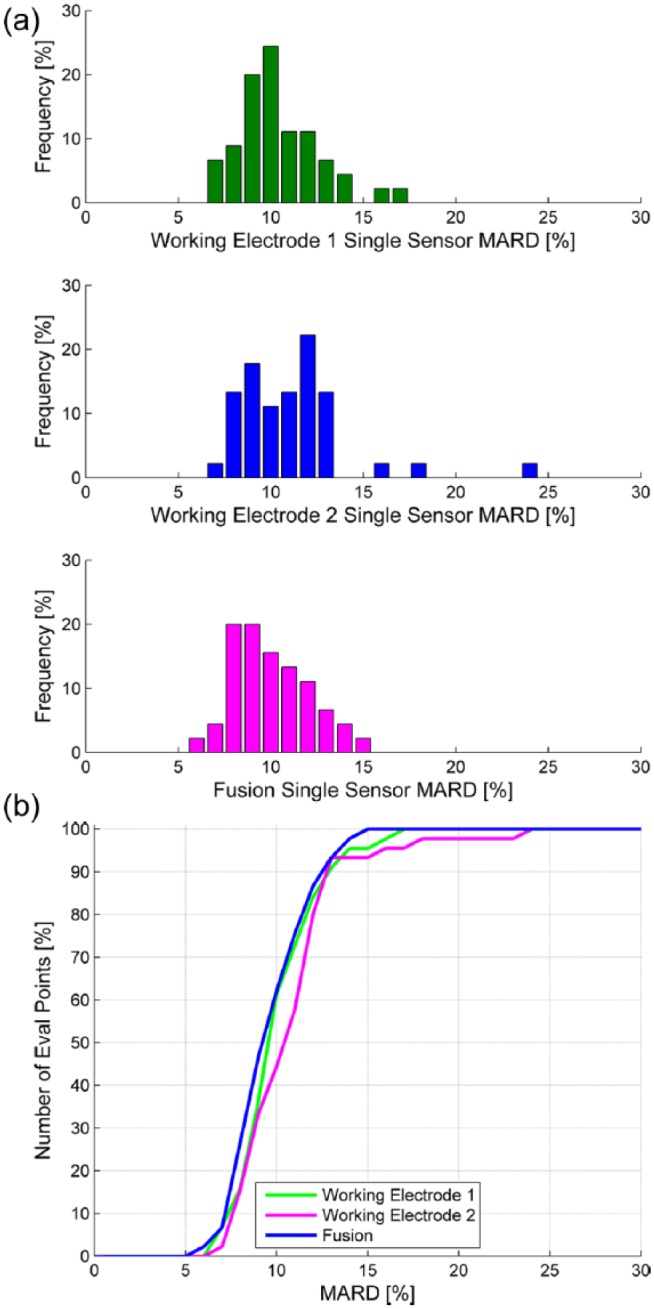
(a) MARD frequency distribution according to individual sensor for WE1, WE2, and fusion; (b) MARD cumulative plot according to individual sensor for WE1, WE2, and fusion.
ERS Precision
ERS day 1 PARD was significantly higher than day 1-7 PARD (13.3 [5.0]% vs 9.9 [3.6]%; P = .003). Day 1-7 PARD or day 1 PARD comparing fused versus component (WE1/WE2) were not significantly different (Table 2).
ERS Reliability
Of the 45 sensors, 6 were terminated by the algorithm before the end of the 7-day study. The reason for their early termination was detection of changes in the sensor’s sensitivity to glucose that, if left unchecked, would compromise the sensor performance. In all cases, the algorithm was able to detect when the sensor performance began to deteriorate before the performance became severely degraded. The average lifetime of the sensors in this study was 6.8 days, with 91% of sensors lasting into day 7, and 100% of sensors lasting into day 6. Sensor % display time was significantly greater with the fused sensor output compared to individual component WEs. The percentage of sensors remaining functional at the end of day 7 while greater was not significantly different (Table 2).
ERS Insertion Site Complications
Qualitative data indicated that the sensors were well-tolerated, and participant acceptance was satisfactory. On day 7 explant sensor sites had no evidence of infection or irritation.
Discussion
This study evaluated a novel glucose sensing system employing redundant electrochemical electrodes linked with an intelligent processing algorithm in a single insertion device. The algorithm combines data from each WE, providing a single output of interstitial fluid glucose levels. Previous approaches to sensor redundancy involved the separate insertion of multiple single-electrode, non-redundant sensors, each with simple nonintelligent processing algorithms.9 Patient acceptability and adherence may be greater for a redundant system requiring a single sensor insertion than multiple insertions. There were no issues with site infection or irritation and devices were well-tolerated. Performance parameters (including accuracy and reliability) for the fused output were superior to those of the individual sensors within the system. Such improved sensor performance would enhance patient management based on decisions made by the patients themselves or by an artificial pancreas. Sensor performance and reliability can impact on patient adherence7,13,14 and enhancing patient trust in the glucose data provided may increase adherence. Given that the clinical benefit of CGM is directly related to the frequency of its use2,3 greater adherence may improve the physical and emotional well-being of the user with diabetes.
ERS accuracy, reflected by a MARD of 10.1% vs meter and 9.4% vs YSI, was comparable to ‘best-in-class’ based on data from an independent assessment of 3 commercially available glucose sensors benchmarked against YSI with reported MARDS of 12.3% for the Navigator™ (Abbott Diabetes Care, Alameda, CA), 10.8% for the Dexcom G4™ (Dexcom, San Diego, CA), 18.3% for Enlite™ (Medtronic, Northridge, CA),5 and 9.0% reported for the modified Dexcom G4 Platinum™ (Dexcom, San Diego, CA) system with an advanced algorithm.15 These commercial CGMs all employ a single working electrochemical electrode. The MARD for the individual single electrodes comprising the ERS averaged 11.7%, better than that previously reported for the Enlite sensor.4 This reduction in MARD in non-redundant Medtronic sensors is presumed due to improved sensor design (including geometry and chemistry) and signal processing, which are independent of sensor redundancy.
The specific benefit of redundancy on sensor accuracy can be gauged from Table 2 data, which compare single WE performance relative to the fused output. The fused output from the WE1 and WE2 components of ERS does not represent the mean of the glucose readings from each WE. When combining data from individual WEs to calculate the sensor glucose reading, the ERS processing algorithm weights input from each WE according to signal quality. This buffer against suboptimal sensor performance would reduce the number of individual ERSs with outlying MARDs. An increase in sensor-to-sensor consistency would likely enhance patient trust in the CGM and promote adherence12 which in turn may improve glycemia.2,3
An overall improvement in accuracy over days 1-7 in the fused output relative to the single WE performance was observed. Our data suggest that a significant contribution to this incremental benefit occurs immediately post–sensor insertion, as evidenced by day 1 statistics. Notably the ERS algorithm mandated sensor start-up 1 hour post-insertion versus 2 hours for current sensors. Day 1 performance post-insertion is a recognized challenge in sensor technology and may relate to time until full sensor hydration which may be addressed by changes in design.15,16 The adverse impact of day 1 on sensor accuracy may be mitigated by redundancy in the ERS algorithm, which can determine viability of each of the WEs and compute weights that deemphasize sensors that have not yet stabilized. This diagnostic process is used in day 1 fusion to gauge this stability, which is used as an input in computation of these weights.
ERS sensor precision was evaluated by computing PARD between the fused glucose outputs of the two concurrently worn ERS sensors.12 Damiano et al5 utilized the standard deviation of 48-hour MARDs to characterize sensor precision for plasma glucose values ranging 70-300 mg/dL in Navigator (9.1 [3.3]%), G4 (8.7 [3.2]%), and Enlite (14.3 [4.5]%). Our ERS data indicate a PARD of 9.9%, in keeping with values for nonredundant sensors detailed above though differences in methodology used for calculating PARD are acknowledged as well as our inclusion of glucose values >300 mg/dL and <70 mg/dL cutoffs utilized in the analysis by Damiano et al,5 which would increase the estimated ERS PARD. As previously reported for nonredundant sensors17 ERS PARD for day 1 was significantly higher than overall PARD, indicating that day 1 precision remains an issue, even with redundancy. Unlike MARD where accuracy was improved with redundancy, the ERS fused output did not significantly increase precision compared with the output from its 2 single component working electrodes.
The differences we observed regarding the relative impact of sensor redundancy on MARD versus PARD may be partly explained by sensor accuracy being more readily assessed (using finger-prick blood glucose meter readings as a reference) during routine sensor use than can precision (comparing glucose measurements from separately inserted sensors).12 Accuracy therefore can be accounted for in determining the relative quality of the glucose information provided by the working electrode, which can be processed by an algorithm in real-time. The method employed to determine sensor precision by Damiano et al5 required single insertion but was a retrospective assessment requiring post-processing of data in 48 hour time blocks. The formal determination of precision in real-time would require insertion of more than one sensor,12 and to account for and adjust for this parameter a third electrode would be required. It is possible that incorporation of a third WE into an ERS may improve precision, but this requires testing.
Sensor display time and survival are both determined by the algorithm assessing sensor signal quality. There is a trade-off between sensor display time and survival versus sensor accuracy. The ERS signal processing algorithm was designed to optimize accuracy at the expense of sensor longevity, the intent being to develop a sensor with sufficient accuracy to be capable of reliably supporting a closed loop system. The reason for early ERS termination was detection by the algorithm of a reduction in the sensor’s sensitivity to glucose that, if left unchecked, would compromise the sensor performance. There is no universally accepted theory as to why sensitivity losses occur in glucose sensors. The consensus is that a foreign body response to the implant is the main underlying factor.18-20 This may result in capsule formation or cellular consumption restricting diffusion of glucose and oxygen to the sensing electrodes,18,21-24 reversible electrode poisoning,25,26 or cause proteins and other molecules to be absorbed into the sensor which may change the basic properties of the chemistry stack.27-29 It should be noted that the strength and time course of the foreign body response varies and sensitivity loss was not observed in the majority of the ERS glucose sensors, with all ERS sensors surviving 5 days and >90% of sensors lasting into the 7th day. Lessons learned in this study will inform further development of the ERS design. This will include refinements aimed at making the sensor more robust to the changes described above to enhance longevity.
Relative to single working electrodes, ERS provided an average 5% increase in sensor display time. Sensor survival to the end of day 7 did not change significantly, though this feasibility study was not powered to detect such a difference. The observed increase in % display time for ERS may translate into ≥1 hour more each day of real-time glucose data, which would be clinically important, particularly in an artificial pancreas. Similarly, if the 10% increment in day 7 survival is confirmed it would also be clinically significant and have a health-economic benefit as premature sensor failure would necessitate replacement.
Conclusions
Our evaluation of a prototype redundant electrochemical CGM sensor indicates that redundancy improved accuracy, consistency and reliability. This is important for patient trust and CGM utilization, which may translate into improved glycemia, reduced diabetes complications, and increased cost-effectiveness. Such qualities are important for artificial pancreas development. Subsequent generations of redundant CGMs may enhance accuracy and reliability even further by incorporating >2 WEs onto a single insertion-platform or by combining electrochemical with an entirely separate glucose sensing methodology such as optical fluorescence.30,31
Acknowledgments
We thank study participants and gratefully acknowledge the assistance of Ms Judith Gooley, Ms Jane Bowden, Ms Jane French, Ms Lee-Anne Lynch, and Ms Marion Jamieson.
Footnotes
Abbreviations: ASIC, application-specific integrated circuit; BG, blood glucose; CEG, Clarke error grid; CGM, continuous glucose monitoring; CTC, clinical trials center; EIS, electrochemical impedance spectroscopy; ERS, electrochemical redundant sensor; GSR, glucose sensor recorder; ISF, interstitial fluid; MARD, mean absolute relative difference; PARD, precision absolute relative deviation; SG, sensor glucose; T1D, type 1 diabetes; YSI, Yellow Springs Instruments.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AV is an employee of Medtronic. JU is and employee of Medtronic. SAM received research support from Medtronic and Roche. AJJ received honoraria from Medtronic, Lily, and Novo-Nordisk. PGC received honoraria from Novo Nordisk. RJM received honoraria, travel, and research support from Novo Nordisk. RS is an employee of Medtronic. DNO received honoraria and research support from Medtronic, Novo Nordisk, Sanofi, and Roche and travel support from Novo Nordisk, Sanofi.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Leona M. and Harry B. Helmsley Charitable Trust, Juvenile Diabetes Research Foundation (JDRF) and St Vincent’s Hospital Melbourne. Material support was provided by Medtronic.
References
- 1. Tamborlane WV, Beck RW, Bode BW, et al. Juvenile Diabetes Research Foundation. Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464-1472. [DOI] [PubMed] [Google Scholar]
- 2. Bergenstal RM, Tamborlane WV, Ahmann A, et al. STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363:311-320. [DOI] [PubMed] [Google Scholar]
- 3. O’Connell MA, Donath S, O’Neal DN, et al. Glycaemic impact of patient-led use of sensor guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia. 2009;52:1250-1257. [DOI] [PubMed] [Google Scholar]
- 4. Mastrototaro JJ. The Minimed Continuous Glucose Monitoring System. Diabetes Technol Ther. 2000;2(suppl 1):13-18. [DOI] [PubMed] [Google Scholar]
- 5. Damiano ER, McKeon K, El-Khatib FH, et al. A comparative effectiveness analysis of three continuous glucose monitors: the Navigator, G4 Platinum, and Enlite. J Diabetes Sci Technol. 2014;8(4):699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Freckmann G, Baumstark A, Schmid C, et al. Evaluation of 12 blood glucose monitoring systems for self-testing: system accuracy and measurement reproducibility. Diabetes Technol Ther. 2014;16(2):113-123. [DOI] [PubMed] [Google Scholar]
- 7. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2009;32(11):1947-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castle JR, Pitts A, Hanavan K, et al. The accuracy benefit of multiple amperometric glucose sensors in people with type 1 diabetes. Diabetes Care. 2012;35:706-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castle JR, Ward WK. Amperometric glucose sensors: sources of error and potential benefit of redundancy. J Diabetes Sci Technol. 2010;4(1):221-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bailey T, Wallace JF, Greene C, et al. Accuracy and user performance evaluation of the Contour® Next Link 2.4 blood glucose monitoring system. Clinica Chimica Acta. 2015;448:139-145. [DOI] [PubMed] [Google Scholar]
- 11. Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10:622-628. [DOI] [PubMed] [Google Scholar]
- 12. Zisser HC, Bailey TS, Schwartz S, Ratner RE, Wise J. Accuracy of the SEVEN continuous glucose monitoring system: comparison with frequently sampled venous glucose measurements. J Diabetes Sci Technol. 2009;3(5):1146-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beck RW, Buckingham B, Miller K, et al. Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2009;32:1947-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hessler DP, Polonsky WH, Bowman F, Price D: The subjective experience of CGM-RT use: comparing current users with ex-users. Diabetes. 2012;61:A215. [Google Scholar]
- 15. Christiansen M, Bailey T, Watkins A, et al. A new-generation continuous glucose monitoring system: improved accuracy and reliability compared with a previous-generation system. Diabetes Technol Ther. 2013;15(10):881-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bailey TS, Chang A, Christiansen M. Clinical accuracy of a continuous glucose monitoring system with an advanced algorithm. J Diabetes Sci Technol. 2015;9(2):209-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Freckman G, Pleus S, Link M, Zschornack E, Klötzer H-M, Haug C. Performance evaluation of three continuous glucose monitoring systems: comparison of six sensors per subject in parallel. J Diabetes Sci Technol. 2013;7(4):842-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gough D, Kumosa L, Routh T, Lin J, Lucisano J. Function of an implanted tissue glucose sensor for more than 1 year in animals. Bioeng Diabetes. 2010;2(42):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Helton K, Ratner B, Wisniewski N. Biomechanics of the sensor-tissue interface—effects of motion, pressure, and design on sensor performance and foreign body response—part I: theoretical framework. J Diabetes Sci Technol. 2011;5(3):632-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Helton K, Ratner B, Wisniewski N. Biomechanics of the sensor-tissue interface—effects of motion, pressure, and design on sensor performance and foreign body response—part II: examples and application. J Diabetes Sci Technol. 2011;5(3):647-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kleuh U, Liu Z, Ouyang T, et al. Blood-induced interference of glucose sensor function in vitro: Implications for in vivo sensor function. J Diabetes Sci Technol. 2007;1(6):842-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kleuh U, Liu Z, Feldman B, et al. Metabolic biofouling of glucose sensors in vivo: Role of tissue microhemorhages. J Diabetes Sci Technol. 2011;5(30):583-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Novak M, Yuan F, Reichert M. Modelling the relative impact of capsular tissue effects on implanted glucose sensor time lag and signal attenuation. Anal Bioanal Chem. 2010;398:1695-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Novak M, Yuan F, Reichert W. Predicting glucose sensor behavior in blood using transport modelling: relative impacts of protein biofouling and cellular metabolic effects. J Diabetes Sci Technol. 2013;7(6):1547-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kharafi F, Saad A, Ateya B, Ghayad I. Electrochemical oxidation of sulfide ions on platinum electrodes. Modern Appl Sci. 2010;4(3):2-11. [Google Scholar]
- 26. Linke B, Kiwit M, Thomas K, Krahwinkel M, Kerner W. Prevention of the decrease in sensitivity of an amperometric glucose sensor in undiluted human serum. Clin Chem. 1999;45(2):283-285. [PubMed] [Google Scholar]
- 27. Chen C, Xie Q, Wang L, et al. Experimental platform to study heavy metal ion-enzyme interactions and amperometric inhibitive assay of Ag+ based on solution state and immobilized glucose oxidase. Anal Chem. 2011;83(7):2660-2666. [DOI] [PubMed] [Google Scholar]
- 28. Gifford R, Kehoe J, Barnes S, Kornilayev B, Alterman M, Wilson G. Protein interactions with subcutaneously implanted biosensors. Biomaterials. 2006;27:2587-2598. [DOI] [PubMed] [Google Scholar]
- 29. Kerner W, Kiwit M, Linke B. The function of a hydrogen peroxide-detecting electroenzymatic glucose electrode is markedly impaired in human sub-cutaneous tissue and plasma. Biosens Bioelec. 1993;8:473-482. [DOI] [PubMed] [Google Scholar]
- 30. Klonoff DC. Overview of fluorescence glucose sensing: a technology with a bright future. J Diabetes Sci Technol. 2012;6(6):1242-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McAuley SA, Bansal A, Horsburgh JC, et al. First evaluation of an orthogonally redundant glucose sensor system in people with type 1 diabetes. Diabetes Technol Ther. 2014;16(S1):A16. [Google Scholar]