Abstract
Nowadays nanotechnology has many applications in products used in various areas of daily life; however, this technology has also an option in modern medicine and pharmacy. Therefore, this technology is also an attractive option for the field of diagnosis and treatment of diabetes. Many people with diabetes measure their blood glucose levels regularly to determine the insulin dose. Ideally glucose values would be measured noninvasively (NI). However, none of all the NI approaches studied in the past decades enabled reliable NI measurements under all daily life conditions. Particularly an unfavorable signal-to-noise ratio turned out to be problematic. Based on the known physical possibilities for NI glucose monitoring the focus of this review is on nanotechnology approaches. Functional prototypes exist for some of these that showed promising results under defined laboratory conditions, indicating a good sensitivity and selectivity for glucose. On the second hand is to optimize the technological process of manufacturing. In view of the rapid progress in micro- and nanoelectronics hopefully NI glucose monitoring systems can be developed in the near future.
Keywords: noninvasive glucose monitoring, physical measurement methods, continuous glucose monitoring, nanotechnology, nanotubes, quantum dots
Brief Introduction Into Nanotechnology
Nanotechnology means the development and production of devices and structures with dimensions <100 nanometers and/or uses characteristic effects and phenomena in this size range (quantum effects).1,2 Nanotechnology will lead to many application fields with products of nanotechnology and procedures, some of which are already mastered. This affects particularly electronics, communication technology, aerospace industry, automobile industry, energy and chemical industry, construction, and of course medical devices and methods.3 Undoubtedly, nanotechnology is one of the key technologies of the 21st century, and it is not surprising that the market for nanotechnology increases rapidly. According to a recent analysis the revenue increased from $185 million in 2005 to $2.7 billion in 2008 and >$20 billion currently.2
In principle certain areas of usage can be distinguished
Nanotechnique, ie, the technology for the production of nanostructures
Nano–building blocks, such as
-Nanoparticles and nanolayers4
-Nanostructures (such as quantum dots)5,6
-Novel types of molecules (eg, carbon nanotubes, fullerenes)7
Nanotools, such as
-Novel microscopes (scanning tunneling microscope, atomic force microscope)
-New technologies in lithography for nanoelectronics
-Self-assembly technologies (eg, DNA synthesis)10
With these tools, there are many different, completely new technological possibilities, such as the production of materials with novel properties (eg, nanosolar cell films, carbon nanotubes as a key material for nanoelectronics), novel devices for nanoelectronics and nanomechanics (eg, nanotube transistors, nanocrystals and detectors, nanobiochips), new method of surface treatment to achieve improved mechanical, thermal, electrical, and chemical properties of surfaces, and also various approaches to medicine.
Nanotechnology in Medicine
In medicine a variety of nanosensors are already used for diagnostic and therapeutic purposes. Given that such sensors are very small in size, they can be implanted directly into the organism as measurement tools or therapeutic tools.2,11,12 Such biochips are made of functional nanoparticles as detector reagents or nanoarrays for chemical, pharmaceutical, biochemical, and genetic tests and can implanted as sensors for continuous monitoring. Some of them will be exemplified:
- New methods for production of biocompatible materials and resulting medical devices, such as
- Nanosensors for medical diagnostics and therapy:
- Novel therapeutic approaches with nanoparticles, eg, the smuggling of super paramagnetic nanoparticles in tumor cells, which vibrate in an alternating electromagnetic field and of the heat kills the cell23
Of all these options the reaction that is of most interest in the field of diabetes technology is the development of glucose sensors for continuous glucose monitoring.
Physical Principles That Can Be Used for Noninvasive Glucose Monitoring
The interaction of glucose molecules with applied energy (radiation, heat, electromagnetic fields, among others) can be used for noninvasive (NI) glucose monitoring. A number of physical principles can be used; for example light absorption, light scattering, polarization of light, fluorescence, Raman Scattering, photoacoustic and impedance measurement. No sample material is required as the applied energy field constitutes directly the measurement probe in a volume of tissue. Every atom or molecule has very specific energetic characteristics (energy levels), which become obvious when energy intake or energy output occurs, that is, so to speak, they provide a “fingerprint” of the corresponding atomic or molecular species. The nature of molecules can be determined qualitatively from the position of spectroscopic signals in a certain measuring range. The concentration can be estimated quantitatively from the intensity of the signals.
For measurement of glucose in a certain tissue volume is it necessary that a specific interaction of the glucose molecules with the applied energy takes place. This implies the difficulty that the concentration of glucose in the human body is relatively low with concentrations in the parts-per-thousand range. Therefore, glucose signals are weak in comparison to other endogenous substances, such as water or albumin that prevail in much higher concentrations, giving rise to stronger signals. The effect of signals nonoriginating from glucose (“noise”) must be compensated and separated from the true glucose signal by use of complex mathematical algorithms.
Even more critical is the fact, that the radiated energy (eg, a beam of light) hits a quite complex structure when it penetrates the skin. Because of the strong anisotropy of skin and tissue, the measurement signal differs considerably, depending, for example, on the penetration depth, making it difficult to accurately measure glucose. Physical methods are therefore touching the limits of measurability of glucose. The crucial question is whether the accuracy and precision required for diabetes treatment can be reached by employing such methods. Taking as precondition the accuracy of glucose meters used by patients today for self-measurement of glucose in capillary blood samples, about 95% of all measurement results should be located within ±15 mg/dL (0.83 mmol/L) with glucose levels <100 mg/dL (5.6 mmol/L) or ±15% with values >100 mg/dL.28
Examples of Noninvasive Glucose Monitoring Systems
Basically, it is irrelevant whether the sensors used for glucose monitoring provide discrete results, as with blood glucose self-monitoring, or continuous data as with continuous glucose monitoring (CGM) in the interstitial fluid. In principle, both approaches can be realized NI with the same measurement approach. To date most approaches for NI glucose monitoring involved measuring methods in which “light” (= not necessarily in the visible range) is absorbed or scattered. Back in the 1990s patients were offered various underdeveloped products to purchase, which did not meet the requirements needed from a clinical and regulatory point of view, and thus frustrated the patients and diabetologists (eg, DiaSensor 1000, Diasense UK Ltd; GluControl, Arithmed GmbH; TouchTrakPRO 200, Samsung Inc).
Under highly controlled conditions in the laboratory glucose can be measured in the human skin by absorption spectroscopy. However, besides a high technical effort, the measurement accuracy is not sufficient and the reproducibility of the results as well. Despite various efforts of a number of companies and academic institutions, all such approaches failed. One example is the Sensys GTS™, a device developed by Sensys Medical Inc, a US company. This measurement system employed light absorption in the near infrared range (NIR). The company had initially built a compact desktop unit, which then was developed into a patient’s device for every day’s use. To assure reproducible measurements, the measuring point at the skin has to be prepared by shaving, application of a hydrogel, washing and finally drying to create defined skin moisture.29 If the preparation and measurement was conducted by a technician, 79.6% of paired measurement results were in the zone A and 20.1% of the values in the still acceptable zone B, making reference to the error grid analysis (EGA). This analysis compares the results obtained with the test device with those obtained with a laboratory device under clinical aspects of accuracy. However, if preparation and measurements were made by patients themselves, only 37.5% of the values were in zone A and 45.8% in zone B.30 Such a high influence of external conditions is not acceptable under real-life applications and explains why such devices were not successful.
Another example is a device which was under development by Grove Instruments. They also rely on absorption of light in the NIR for glucose measurement and presented results, in which 96.3% of the measurement results were in zone A of the EGA.31 However, the measurements were performed without blinded experimenters and subsequent data analysis was performed by the company. Thus, no independent evaluation of the measurement performance of this device was performed.
The physical principle of Raman scattering uses the incident light of a specific wavelength (monochromatic light) that is scattered inelastically by the glucose molecules, thus it is shifted in frequency. With different molecules, the scattering occurs differently, producing spectral bands at different frequencies of the spectrum (the term “spectrum” refers to the intensity of lines over a frequency range). The glucose concentration is determined by measuring the intensity of the characteristic bands for glucose.32
The problem in evaluation of such spectra is that a variety of other molecules also generate spectral peaks which have to be separated from the glucose signals. For example in a sample with 2 sugar molecules, glucose and galactose, it is difficult to distinguish both molecules in the Raman spectrum because spectral lines, although at different frequencies (or wave numbers), are broad and overlap. Follow is an inadequate measurement accuracy from a clinical point of view.32,33
In the year 2010, the company C8 MediSensors from California presented a NI glucose measurement system based on Raman spectroscopy. No data obtained in clinical studies with the system were ever published in journals with a peer review process. In 2012, this system received a CE mark which in principle enables the company to market their device in Europe. The company had a notable presence in the industry exhibition area of different congresses. Nevertheless, in 2013 C8 MediSensors had to file bankruptcy as it becomes obvious that the measurement quality of the system was insufficient.
The negative assessment of the 2 optical methods used in these 2 devices does not mean that such approaches cannot be used in principle; however, a massive improvement in the signal-to-noise ratio is required. This will hopefully be possible by better receivers (photo receiver, etc), a development which is linked to the improvements seen with modern microelectronics/nanotechnology (see below).
A different approach was used by the company PreciSense. A glucose sensor is implanted into the upper region of subcutaneous adipose tissue. For measurement of glucose a fluorescent light is applied to the skin. Fluorescence occurs when an atom after its excitation (energy supply by irradiation with light, for example) returns to the ground energy state.34,35 The released energy is usually emitted, for example, as light and can be measured. However, a direct measurement inside the tissue is almost impossible. Therefore, the PreciSense approach is based on the measurement of the glucose-dependent fluorescence using the affinity principle: 2 components, dextran and a glucose-binding material with a specific fluorescence, are brought together (concanavalin A labeled with a fluorescent substance). If no glucose is prevailing in the measurement sample, a characteristic fluorescence light is emitted after excitation with laser light. This light can be measured with an optical sensor. However, if glucose is added, the dextran is displaced from the bonds of the second component (glucose possesses a higher affinity to it). The newly formed bonds change the fluorescence signal. This change depends on the change in the glucose concentration and shows a shift in the wavelength (color) of light.34
Another NI glucose monitoring device is GlucoTrack®, developed by the company Integrity Inc. based in Israel. This device, which has a CE mark, combines 3 physical principles for glucose measurement: ultra-sound, application of electromagnetic waves and calorimetry. Each individual method has a certain sensitivity and specificity for glucose. Individual calibration is requires to be performed prior to conducting measurement. This calibration is valid for 6 months. Unfortunately, no full publications presenting data from clinical studies are available; however, data were presented at various congresses. Data from 1 recent study with >200 patients showed that measurement results were in 95.3 ± 1.6% of the cases in the clinically acceptable A+B zones of the EGA. The MARD (mean absolute relative difference) was 30.8 ± 2.2%.36
The issue of insufficient signal to noise ratio which can be mainly attributed to the skin can be bypassed if the glucose sensors are implanted either directly in the vascular system (= blood glucose measurement) or under the skin in the subcutaneous adipose tissue to provide access to the interstitial fluid. However, implantation means that at least the surgical insertion procedure is not NI. Nevertheless, if the glucose sensor has a long shelf life, that is, it can be used for months or even years, then an implantable system might be an attractive alternative. An example for such a system is a device that is currently under clinical development by the US-based company Senseonics.37 This system measures glucose by means of laser induced fluorescence.
A thin cylinder shaped sensor (diameter: 3 mm, length: 14 mm) is implanted in the subcutaneous tissue. The obtained measurement results with this approach in the clinical studies performed that far showed that the results are as often in zone A of the error grid plot as with currently available CGM systems (about 80% in A, 16-19% in B).
Opportunities for glucose sensors with nanotechnology
In view of the limitations and disadvantages of the currently applied technologies for glucose monitoring other approaches are of high interest.
Nanotechnology opens up completely new possibilities for the development of glucose sensors. In nanostructures characteristic effects take place, which are not found in bulk materials. Also the ratio of surface area to volume is very large. This leads to a number of physical and chemical effects such as interfacial phenomena, altered reactivity, charge carrier effects and quantum mechanical effects38,39 as well as enhanced optical properties (for example quantum dot fluorescence). In miniaturized glucose sensors, such nanoscale properties can have several advantages, including higher surface areas (yielding larger currents and faster responses) and improved catalytic activities. This should result in an improved sensitivity of the glucose sensor, a lower signal-to-noise ratio and a higher selectivity of the measurement.
Nanofabrication techniques can generate glucose sensors with very small dimensions (for example by laser ablation, chemical vapor deposition or arc discharge [Nanotubs, Fullerene etc]).40 Such small sensors can be easily implanted or would be injectable in sense of “glucose measurement tattoos.”41,42 In addition, they could potentially avoid the foreign body response of the immune system answer due to their small size and, consequently, have longer lifetimes. Finally, micro- and nanoelectronic technologies offer the possibility of a cost-effective mass production. As the costs of such sensors depend massively on their widespread usage, diabetes therapy might be revolutionized if low-cost glucose sensors based on nanotechnology become available.
Examples of Nanomaterials and Nanostructures in Experimental Glucose Sensors
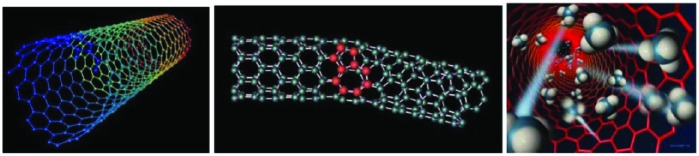
Micro- and nanoelectronics offer new solutions for glucose sensors with a high signal-to-noise ratio. Well-studied nanomaterials are nanotubes, especially carbon nanotubes. Nanotubes can be classified as single-walled nanotubes and multiwalled nanotubes. Typically, the diameter of a nanotube is few nanometers, the tube wall can be up to 0.3 nanometers thick (Figure 1). Such molecular assemblies of carbon atoms were presented for the first time in the Russian physical literature as early as in the late 50s,43 then again in the Anglo-Saxon literature 3 decades later.44 During the production process at very high temperatures, specific substances can be introduced into these nanotubes. These react with glucose (for example: glucose oxidase → bound to a fluorescent dye).
Figure 1.
Nanotubes approximately few nanometers in diameter composed of carbon atoms. Each point represents a carbon atom.
In such a way, the nanotube functions as a light amplifier. The fluorescence signal can be measured and reflect the glucose concentration. It is envisioned that such nanotubes are wrapped in a dialysis fiber and transplanted under the skin. For the glucose measurement, the skin area is irradiated with a laser and the glucose concentration measured via the fluorescent light induced. The thinner the nanotubes are, the larger the band gap and the greater the energy absorption and the fluorescence signal.45 On basic of single-wall-nanotubes enzyme-based optical glucose sensors were investigated.46,47 In combination with enzymes for the catalysis of glucose reaction (eg, immobilized glucose oxidase [GOx]), the decisive advantage of such nanostructures is the very large surface area and the efficient electron transfer from enzyme to electrode.48 The carbon nanotube (CNT) fiber, 28 µm in diameter, was made of bundles of double walled CNTs concentrically compacted into multiple layers forming a nanoporous network structure. Cyclic voltammetry study revealed a superior electrocatalytic activity for CNT fiber compared to the traditional Pt–Ir coil electrode. The electrode end tip of the CNT fiber was freeze-fractured to obtain a unique brush-like nanostructure resembling a scaled-down electrical “flex,” where GOx enzyme was immobilized using glutaraldehyde cross-linking in the presence of bovine serum albumin. An outer epoxy-polyurethane layer was used as semipermeable membrane. The sensor function was tested against a standard reference electrode. The sensitivities, linear detection range and linearity for detecting glucose for the miniature CNT fiber electrode were better than that reported for a Pt-Ir coil electrode. Thermal annealing of the CNT fiber at 250°C for 30 minutes prior to fabrication of the sensor resulted in a 7.5-fold increase in glucose sensitivity. The CNT fiber based glucose biosensor was shown to be stable for up to 70 days.48
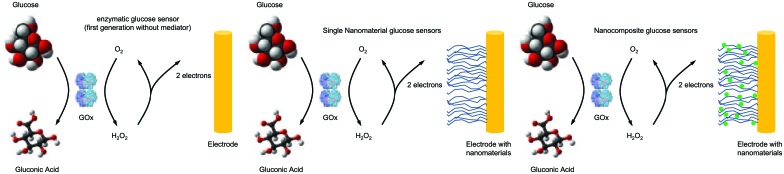
The use of nanostructured materials in glucose sensors in comparison to currently used GOx sensors of first generation is shown in Figure 2.49
Figure 2.
Nanostructured materials used in glucose sensors. Left: Presently used electrochemical glucose sensors use glucose oxidase (GOx) for chemical process and generate an electrochemical signal. This signal is transferred through O2 reduction to H2O2 (or can reduce another chemical mediator). Nanomaterials can be incorporated into such sensors to increase surface area, improve catalytic action, modify operating parameters, and improve electron transfer from the enzyme to the electrode. This can be accomplished through the use of single types of nanomaterials (middle) such as carbon nanotubes or nanocomposites consisting of multiple nanomaterials working together (right) (modified from Cash and Clark49).
Similar to ordinary glucose sensors based on GOx, it is possible to improve electron transfer between the enzyme and the electrode by using an electrochemical mediator. This is reduced by the GOx reaction and transfers its electrons to the electrode. In this sense, an approach is to modify the nanotubes with an electrochemical mediator such as ferrocene,50 ferricyanide,51 or another. A further improvement in sensor performance is made possible by the combination of CNTs with various nanoparticles, for example, noble metals (silver, gold, platinum) or silica, titanium dioxide, and others (Figure 2, right). This combination of nanomaterials improves the catalytic activity and following the sensitivity of measurement.
A further example is a combination of dextran coated nanotube with the concanavalin A (Con A) to a Protein Affinity-Based Optical Glucose Sensors.52 In presence of Con A the nanotube with dextran aggregates and the fluorescence signal decreases. In presence of glucose the aggregation between the (dextran)-nanotube and Con A dissolves and the fluorescence signal increases.
Options for Nonelectrochemical Measurement Methods With Nanotechnology
An attractive option for glucose monitoring are biocompatible polymeric nanosensors implanted under the skin (like a tattoo). The fluorescence properties of such sensors change in response to changes in the glucose concentration in the interstitial fluid. Such changes can be read out using optical interrogation through the skin after light excitation with a laser beam. Such sensors can based on polymeric nanosensors incorporating boronic acid derivatives to recognize glucose. Nanospheres based on N-isopropylacrylamide containing a covalently bound phenyl-boronic acid derivative as well as 2 attached fluorophores have been synthesized.53 In case of low glucose concentration the nanospheres are small and holding the fluorophores close together. The consequence is an efficient resonance energy transfer. In case of higher glucose concentration the glucose binding to the boronic acid is reduced, subsequently the polymer swells which increases the average distance between fluorophores. Therefore this decreases resonance energy transfer, which increases the donor fluorescence and decreases the acceptor fluorescence. Special fluoresphores can be CNT that showed a glucose controlled aggregation onto concanavalin A. As the aggregates have different fluorescence than free CNTs, detection of glucose is possible through measuring the CNT fluorescence change.54
An interesting nanostructure is quantum dots, usually made of semiconductor material (eg, CdSe, InGaAs, or GaInP/InP). Quantum dots can be prepared by molecular beam epitaxy or lithographically processes in semiconductor layer system (nanolayers with a few atomic layers), for example, with an electron beam and following dry etching procedure. A quantum dot is small enough to exhibit quantum mechanical properties. Typically, a quantum dot contains about 10,000 atoms. Charge carriers (electrons, holes) in a quantum dot are so far limited in their mobility in all 3 spatial directions that their energy no longer continuous, but only discrete values may assume. Quantum dots thus behave like atoms, but can their shape, size or the number of electrons are influenced in them. These electronic and optical properties of quantum dots can be tailored.40 This enables production of quantum dots that have favorable optical properties for use in different sensors. As this sensor itself do not interact with glucose molecules directly, an attachment for example of GOx to a quantum dot is necessary. In this case the luminescence of quantum dots can be quenched by hydrogen peroxide. In the presence of glucose, the enzyme generates hydrogen peroxide, which quenches the quantum dots, providing an optical signal change proportional to glucose concentrations. For enhancement of GOx activity the assembling of a complex with quantum dots of different compounds is possible, for example CdTe.55
Outlook
Currently no NI glucose system is available that has documented its usability under all conditions in daily life; however, numerous academic institutions and companies are working on respective developments.
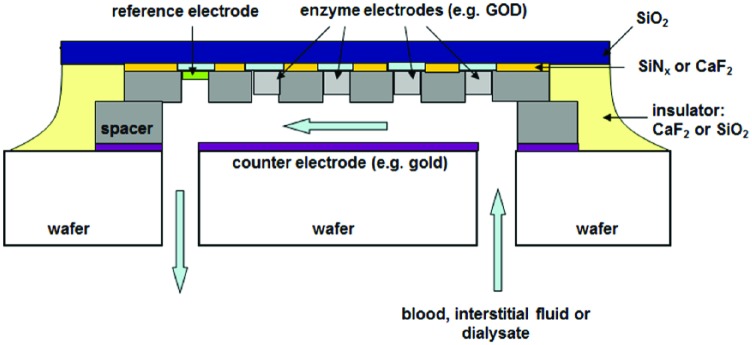
The ongoing miniaturization of semiconductor integrated circuits since its first introduction nearly half a century ago, has finally led to nanotechnological solutions which enable completely new approaches for glucose sensing.39,44 It is already possible with existing technology to produce glucose sensors as integrated components, like integrated circuits (see Figure 3 as an example). With such sensor architecture it is also conceivable that on a sensor chip not only enzymes for glucose measurement are placed, but additional enzymes for other relevant metabolic products, such as ketone bodies or lactate. This would allow supervising the metabolic processes of the organism in a complex fashion.
Figure 3.
Proposed structure of a glucose sensor based on a biochemical to enzymatically catalyzed reaction. Such sensor is produced by the common microelectronics technology as a microcircuit. Supplementary enzymes could be integrated to monitor further metabolic products of the organism.37
Nanotechnology will most probably enable development of glucose sensors that offer a number of advantages over known CGM systems, for example such sensors would be injectable under the skin. A NI measurement of glucose with a sufficient accuracy might be possible by using novel physical effects that occur in nanomaterial.56 Thus, glucose monitoring systems based on nanotechnology might represent the beginning of a revolution in glucose sensing.
Footnotes
Abbreviations: CGM, continuous glucose monitoring; CNT, carbon nanotube; EGA, error grid analysis; GOx, glucose oxidase; H2O2, hydrogen peroxide; LED, light-emitting diode; MARD, mean absolute relative difference; NIR, near infrared range.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AT is head of science at Medtronic, Germany, Diabetes Division, a manufacturer and distributor of insulin pumps and glucose sensors. LH is chair of the Working Group for Diabetic Technology of the German Diabetes Association (AGDT). He is a consultant for companies like Roche Diagnostics, Medtronic and Senseonics for the development of new diagnostic approaches in diabetes. AR is coworker in Laboratorio de Nanotrónica, Benemerita Universidad Autonoma de Puebla, Mexico with no commercial interest in medical devices. AZ is head of Laboratorio de Nanotrónica, Benemerita Universidad Autonoma de Puebla, Mexico with no commercial interest in medical devices.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Chopra N, Gavalas VG, Bachas LG, Hinds BJ, Bachas LG. Functional one-dimensional nanomaterials: applications in nanoscale biosensors. Anal Lett. 2007;40:2067-2096. [Google Scholar]
- 2. n-tech. Available at: http://nanomarkets.net. Accessed March 2015.
- 3. Thomas A. Nanotecnología, sensores y su aplicación a la medición de la glucosa en pacientes con diabetes mellitus. Nanociencia et Molectronica. 2006;4(2):682-694. [Google Scholar]
- 4. Yu MF, Files BS, Arepalli S, Ruoff RS. Tensile loading of ropes of single wall carbon nanotubes and their mechanical properties. Phys Rev Lett. 2000;84:5552. [DOI] [PubMed] [Google Scholar]
- 5. Ekimov AI, Efros AL, Onushchenko AA. Quantum size effect in semiconuctor microcrystals. Solid State Com. 1985;56(11):921-924. [Google Scholar]
- 6. Soutter W. Continuous flow synthesis method for fluorescent quantum dots. Available at: http://www.azonano.com/article.aspx?ArticleID=3473. Accessed August 2015.
- 7. Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE. C60: Buckminsterfullerene. Nature. 1985;318:162-163. [Google Scholar]
- 8. Pompe W, Rodel G, Weiss HJ, Mertig M. Bio-nanomaterials: Designing Materials Inspired by Nature. New York, NY: Wiley; 2013. [Google Scholar]
- 9. Liao S, Seeman NC. Translation of DNA signals into polymer assembly instructions. Science. 2004;306(5704):2072-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campus news. Available at: http://www.berkeley.edu/news/media/releases/2000/07/27_nano.html. Accessed August 2015.
- 11. Zehe A. La Nanotecnologia como fuerza economica: aplicaciones y productos mercantiles. Nanociencia et Molectronica. 2007;5(2):1015-1034. [Google Scholar]
- 12. Saini R, Saini S, Sharma S. Nanotechnology: the future medicine. J Cutan Aesthet Surg. 2010;3(1):32-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moro T, Takatori Y, Ishihara K, et al. Surface grafting of artificial joints with a biocompatible polymer for preventing periprosthetic osteolysis. Nat Mat. 2004;3:829-836. [DOI] [PubMed] [Google Scholar]
- 14. Rajagopalan M, Oh IK. Fullerenol-based electroactive artificial muscles utilizing biocompatible polyetherimide. ACS Nano. 2011;22;5(3):2248-2256. [DOI] [PubMed] [Google Scholar]
- 15. Ghanbari H, de Mel A, Seifalian AM. Cardiovascular application of polyhedral oligomeric silesquioxano nanomaterials: a glimpse into prospective horizons. Int J Nanomed. 2011;6:775-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu H, Webster TJ. Nanomedicine for implants: a review of studies and necessary experimental tools. Biomaterials. 2007;28(2):354-369. [DOI] [PubMed] [Google Scholar]
- 17. Wan ACA, Ying JY. Nanomaterials for in situ cell delivery and tissue regeneration. Adv Drug Deliv Rev. 2010;62(7-8):731-740. [DOI] [PubMed] [Google Scholar]
- 18. Solanki A, Kim JD, Lee KB. Nanotechnology for regenerative medicine: nanomaterials for stem cell imaging. Nanomedicine. 2008;3(4):567-578. [DOI] [PubMed] [Google Scholar]
- 19. Dinh TV, Kasili P. Fiber-optic nanosensors for single-cell monitoring. Anal Bioanal Chem. 2005;382(4):918-925. [DOI] [PubMed] [Google Scholar]
- 20. Lee YEK, Kopelman R, Smith R. Nanoparticle PEBBLE sensors in live cells and in vivo. Annu Rev Anal Chem. 2009;2(6):57-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mohanty P, Yu C, Wang X, Hong MK, Rosenberg CL, Weaver DT. Field effect transistor nanosensor for breast cancer diagnostics. In: Herold KH, Rasooly A, eds. Invited Review in Biosensors and Molecular Technologies for Cancer Diagnostics. Boca Raton, FL: CRC Press; 2012: 1-19. [Google Scholar]
- 22. Dragonieri S, Annema JT, Schot R, et al. An electronic nose in the discrimination of patients with non-small cell lung cancer and COPD. Lung Cancer 2009;64(2):166-170. [DOI] [PubMed] [Google Scholar]
- 23. Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161-171. [DOI] [PubMed] [Google Scholar]
- 24. Ruckh TT, Clark HA. Implantable nanosensors: toward continuous physiologic monitoring. Anal Chem. 2014;86(3):1314-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Etheridge ML, Cambell SA, Erdmann AG, Haynes CL, Wolf SM, McCullough J. The big picture on nanomedicine: The state of investigational and approved nanomedicine products. Nanomed Nanotech Biol Med. 2013;9:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Billingsley K, Balaconis MK, Dubach JM, Zhang N, Lim E, Francis KP, Clark HA. Fluorescent nano-optodes for glucose detection. Anal Chem. 2010;82(9):3707-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown JQ, Srivastava R, McShane MJ. Encapsulation of glucose oxidase and an oxygen-quenched fluorophore in polyelectrolyte-coated calcium alginate microspheres as optical glucose sensor systems. Biosens Bioelec. 2006;21:212-216. [DOI] [PubMed] [Google Scholar]
- 28. ISO 15197:2013. In vitro diagnostic test systems—requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. (ISO/DIS 15197:2013). Available at: http://www.iso.org/iso/catalogue_detail?csnumber=54976. Accessed March 2015.
- 29. Mattu M, Makarawicz MR, Blank TB, Lorenz AD, Monfre L, Az C. Evaluation of a guideless non-invasive glucose sensor. Diabetes. 2008;57(suppl 1):A117. [Google Scholar]
- 30. Jovanovich L, Ahmann A, Edelman S, et al. Human factors assessment for measuring glucose non-invasively. Diabetes. 2007;56(suppl 1):A9. [Google Scholar]
- 31. Combs AH, Harjunmaa HI, Kun S, et al. Optical noninvasive glucometer achieves ISO required clinical accuracy in pilot study. Diabetes Technol Ther. 2013;15(suppl 1):A7. [Google Scholar]
- 32. Lyandres O, Yuen JM, Shah NC, VanDuyne RP, Walsh JT, Glucksberg MR. Progress toward an in vivo surface-enhanced raman spectroscopy glucose sensor. Diabetes Technol Ther. 2008;10(4):257-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lipson J, Bernhardt J, Block U, et al. Requirements for calibration in noninvasive glucose monitoring by raman spectroscopy. J Diabetes Sci Technol. 2009;3:233-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kristensen JS. Trans-cutaneous fluorescence lifetime based continuous glucose reading for long term interrogation. Diabetologia. 2005;48(suppl 1):A49. [Google Scholar]
- 35. Nielsen JK, Christiansen JS, Kristensen JS, et al. Clinical evaluation of a transcutaneous interrogated fluorescence lifetime-based microsensor for continuous glucose reading. J Diabetes Sci Technol. 2009;3(1):98-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gal A, Harman-Boehm I, Drexler A, et al. Enabling frequent blood glucose monitoring at home using a truly non-invasive device. Diabetes Technol Ther. 2015;17(suppl 1):A77. [Google Scholar]
- 37. DeHennis AD, Tankiewicz S, Raisoni B, Long C, Whitehurst T, Colvin A. An integrated wireless Fluorimeter for a long term implantable, continuous glucose monitoring system. Diabetes Technol Ther. 2013;15(suppl 1):A58. [Google Scholar]
- 38. Allhoff F, Lin P, Moore D. The basics of nanotechnology. In: What Is Nanotechnology and Why Does It Matter? From Science to Ethics. New York, NY: John Wiley; 2010:1-19. [Google Scholar]
- 39. Zehe A, Thomas A. Tecnología Epitaxial de Silicio. Norderstedt, Germany: eBook; 2000. [Google Scholar]
- 40. Thomas A, Torres Tapia E, Ramírez A, Zehe A. Las nanopartículas—Nanomateriales de tantas aplicaciones asombrosas en nanomedicina y nanotecnología biomédica, Internet Electron. J Nanocs Moletrón. 2015;13(1):2315-2326. [Google Scholar]
- 41. Brown JQ, McShane MJ. Modeling of spherical fluorescent glucose microsensor systems: design of enzymatic smart tattoos. Biosens Bioelectron. 2006;21(9):1760-1769. [DOI] [PubMed] [Google Scholar]
- 42. Stein EW, Grant PS, Zhu H, McShane MJ. Microscale enzymatic optical biosensors using mass transport limiting nanofilms. 1. Fabrication and characterization using glucose as a model analyte. Anal Chem. 2007;79(4):1339-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Radushkewitsch LK, Lukjanowitsch VM. O struktura ugleroda, obrazujucegosja pri termitscheskom razlozenii okisi uglerodana zeleznom kontakte. J Fis Chim. 1952;26:88-95. [Google Scholar]
- 44. Iijima S, Ichihashi T. Single shell carbon nanotubes of 1 nm diameter. Nature. 1993;363:603-605. [Google Scholar]
- 45. Strano M. The chemistry of single walled carbon nanotubes. Applications to nanotube separation and biomolecule detection. Paper presented at: 2nd International Conference on Advanced Technology and Treatments for Diabetes; 2009. [Google Scholar]
- 46. Bachilo SM, Strano MS, Kittrell C, Hauge RH, Smalley RE, Weisman RB. Structure-assigned optical spectra of single-walled carbon nanotubes. Science. 2002;298(5602):2361-2366. [DOI] [PubMed] [Google Scholar]
- 47. Barone PW, Strano MS. Single walled carbon nanotubes as reporters for the optical detection of glucose. J Diabetes Sci Technol. 2009;3(2):242-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhu Z, Song W, Burugapalli K, Moussy F, Li Y-L, Zhong X-H. Nano-yarn carbon nanotube fiber based enzymatic glucose biosensor. Nanotechnology. 2010;21(16):165501. [DOI] [PubMed] [Google Scholar]
- 49. Cash KJ, Clark HA. Nanosensors and nanomaterials for monitoring glucose in diabetes. Trends Mol Med. 2010;16(12):584-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Qiu JD, Zhou WM, Guo J, Wang R, Liang RP. Amperometric sensor based on ferrocene-modified multiwalled carbon nanotube nanocomposites as electron mediator for the determination of glucose. Anal Biochem. 2009;385:264-269. [DOI] [PubMed] [Google Scholar]
- 51. Barone PW, Baik S, Heller DA, Strano MS. Near-infrared optical sensors based on single-walled carbon nanotubes. Nat Mat. 2005;4:86-92. [DOI] [PubMed] [Google Scholar]
- 52. Cella LN, Chen W, Myung NV, Mulchandani A. Single-walled carbon nanotube-based chemiresistive affinity biosensors for small molecules: ultrasensitive glucose detection. J Am Chem Soc. 2010;132:5024-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zenkl G, Mayr T, Klimant I. Sugar-responsive fluorescent nanospheres. Macromol Biosci. 2008;8:146-152. [DOI] [PubMed] [Google Scholar]
- 54. Barone PW, Strano MS. Reversible control of carbon nanotube aggregation for a glucose affinity sensor. Angew Chem Int Ed. 2006;45:8138-8141. [DOI] [PubMed] [Google Scholar]
- 55. Cao LH, Ye J, Tong L, Tang B. A new route to the considerable enhancement of glucose oxidase (GOx) activity: the simple assembly of a complex from CdTe quantum dots and GOx, and its glucose sensing. Chemistry. 2008;14:9633-9640. [DOI] [PubMed] [Google Scholar]
- 56. Zehe A. El crepúsculo de la nanobiotecnología. J Nanociencias et Moletronica. 2003;1(2):86-92. [Google Scholar]