Abstract
Background:
The speed of insulin absorption after subcutaneous delivery is highly variable. Incorrect assumptions about insulin pharmacokinetics compromise effective glycemic regulation. Our ultimate goal is to develop a system to monitor insulin levels in vivo continuously, allowing pharmacokinetic parameters to be calculated in real time. We hypothesize that a bead-based detection system can be run on a flow-through microfluidic platform to measure insulin in subcutaneous fluid sampled via microdialysis. As a first step in development, we focused on microsphere-based measurement of insulin.
Methods:
Polystyrene microspheres coated with an anti-insulin monoclonal antibody were exposed to insulin-containing solutions, and after addition of a fluorescently labeled anti-insulin monoclonal antibody with a distinct epitope, bead-associated fluorescence was detected by fluorescence microscopy in 96-well plates or in a flow-through, microfluidic platform.
Results:
The bead detection system in plates had a linear range in buffer for regular human insulin (RHI), insulin lispro, and insulin aspart of 15-1115 µIU/ml, 14-976 µIU/ml, and 25-836 µIU/ml, respectively. Measurement on plasma samples demonstrated proportionality between basal and peak insulin levels similar to the laboratory reference method. Preliminary results in a polydimethylsiloxane-based, flow-through, microfluidic platform showed a strong signal at peak insulin levels.
Conclusions:
We have developed a microsphere-based system to rapidly measure levels of insulin and insulin analogs. We have further demonstrated proof of concept that this bead detection system can be implemented in a lab-on-a-chip format, which will be further developed and combined with microdialysis for real-time monitoring of insulin in vivo.
Keywords: insulin, insulin analogs, microsphere, microfluidic, pharmacokinetics
Diabetes mellitus type 1 (T1D) is a disorder of glucose regulation characterized by autoimmune destruction of the insulin-producing pancreatic beta cells and the need for lifelong insulin replacement therapy. Achieving and maintaining near-normal blood glucose (BG) concentrations is critical for successful long term care of patients with diabetes mellitus and prevention of diabetic complications.1-7 However, the therapy required to achieve this goal is extremely demanding. Despite state-of-the-art insulin therapy using rapid-acting insulin analogs, hyperglycemia and hypoglycemia are common in most people with T1D.5-7
The availability of insulin pumps and continuous glucose monitors (CGMs) have made possible the development of systems that automatically deliver insulin in response to real time glucose measurements.8-13 These bionic pancreas (or artificial pancreas) devices measure BG frequently, estimate the proper insulin dose, and deliver insulin. However, development of control algorithms capable of appropriately regulating insulin dosing has been challenging due to the slow and unpredictable absorption of insulin that is delivered subcutaneously.12-14 Since automatic delivery systems give insulin doses frequently, there is a risk of giving excess insulin if the pending effect of previously administered insulin is underestimated. This is referred to as “stacking” of insulin, and leads to hypoglycemia.12-14 Development of a wearable device to monitor interstitial insulin levels in real time would allow the pharmacokinetic characteristics of insulin to be calculated and fed back to the bionic pancreas to optimize insulin delivery. It would also allow early detection of failures in insulin delivery, such as due to an insulin infusion set becoming occluded or pulled out of the skin.
Currently, pharmacokinetic characterization of insulin is performed by measuring the concentration of insulin in blood samples obtained at intervals after injection using methods that are time consuming and must be performed in a laboratory, making them impractical to enable dynamic adjustment of bionic pancreas algorithm parameters.15-17 Other analytical sensing systems are being developed, but none of them can yet be applied to continuous insulin monitoring due to inadequate sensitivity,18-20 as well as challenges to integrating them into a mobile, real-time measurement device.21 As an alternative to these approaches, we have chosen to develop a microfluidic platform using microsphere beads to detect insulin. Microsphere assays are suitable for rapid detection of low levels of analyte. They can be used in multiplex analysis of multiple target analytes in very small sample volumes.22-25 Their high surface area-to-volume ratio enhances capture of the analyte, which enhances their sensitivity.26,27 Our hypothesis is that a monoclonal antibody based microsphere detection system can be developed into a system capable of quantitatively measuring insulin in a microfluidic device in real time. Ultimately, we intend to apply this system to insulin measurements in subcutaneous interstitial fluid (ISF), which is in equilibrium with the blood plasma and can be obtained using microdialysis.
The goal of this study was to develop a bead-based detection system compatible with a microfluidic processing capable of measuring expected levels of insulin in the ISF.
We present in this work calibration data using a bead-based detection system for regular human insulin (RHI) and insulin analogs at concentrations covering the expected clinically effective concentration range in T1D patients. We further compared the bead detection system to a laboratory reference method using plasma samples from human subjects after insulin injections, thereby testing the system in a matrix more complex than the ISF. Finally, we demonstrated proof of concept that the bead-based detection system can be implemented in a lab-on-a-chip format.
Methods
Monoclonal Antibodies
Hybridomas producing monoclonal antibodies HTB-124 and HTB-125 against human insulin were obtained from the ATCC Hybridoma Bank. Both antibodies are from the IgG subclass and have high affinity for RHI, with Kds of 2×10-8 and 3×10-9 for HTB-124 and HTB-125, respectively.28 Immunoglobulin production was performed by Precision Antibody (Columbia, MD). They were purified from hybridoma supernatants using protein G columns and stored at 4⁰C in phosphate buffered saline (PBS).
Preparation of Insulin Standard
Purified RHI (zinc human insulin crystals, a gift from Eli Lilly and Company, Indianapolis, IN), insulin lispro powder (Humalog, also a gift from Eli Lilly), or insulin aspart powder (Novolog, a gift from Novo Nordisk, Bagsværd, Denmark) were diluted in 0.1M HCl to a concentration of 1 mg/ml. Human serum was heated in a thermostat water bath at 60°C for 1 hour to destroy endogenous insulin. Insulin calibrators were prepared in the heat-treated serum and diluted to a concentration of 0.1 mg/ml. Serial dilutions of these stock solutions were then performed in 1% (w/v) blocking buffer to produce standards.
Reference Measurements
Reference insulin measurements were performed with the Architect insulin assay (Abbott Laboratories, Green Oaks, IL), which uses chemiluminescent microparticle immunoassay technology.
Source of Human Plasma Samples Containing RHI and Insulin Aspart
To evaluate the detection system’s clinical performance, we used stored plasma samples. Samples containing human insulin were collected at intervals after a fasted nondiabetic volunteer consumed a breakfast meal. Samples containing insulin aspart were collected at intervals after a fasting volunteer with type 1 received a 5 unit bolus of insulin aspart along with a small breakfast meal. The clinicaltrials.gov number for the study that produced these samples is NCT00811317.
Microsphere Preparation
Antibody HTB-125 was biotinylated with EZ-Link NHS-PEG4-Biotin (Thermo Fischer, Cambridge, MA) according to the manufacturer’s protocol. The purified antibody was applied to 10 µm streptavidin conjugated polystyrene microspheres (Bangs Laboratories Inc, Fishers, IN), which were diluted to a final concentration of 0.5 mg/ml in PBS with 0.005% (w/v) Tween-20 (Sigma Aldrich, Boston, MA), at a ratio of 20 μg of IgG per mg particles (~14-fold excess over the estimated binding capacity of the beads). The mixture was shaken at RT for 90 min. Unbound active sites were blocked with BlockAid (B-10710, Invitrogen/Thermo Fisher, Cambridge, MA) for 1 hour. Finally, the microspheres were washed in PBS (Sigma Aldrich) with 0.5% (w/v) bovine serum albumin (Sigma Aldrich), diluted to a final concentration of 0.5 mg/ml, and stored at 4°C. Antibody HTB-124 was labeled with Alexa Fluor 488 (Invitrogen/Thermo Fisher) according to the manufacturer’s protocol and stored at 4°C.
Detecting System Procedure
The microsphere detecting system was performed at room temperature. Conjugated microsphere (30 μl, 0.5 μg/μl) and 30 μl of target insulin analyte were shaken for 30 min. Thereafter, the secondary labeled immunoglobulin was added to a final concentration of 1μg/ml and the tube, protected from light, was shaken for an additional 30 min, followed by a wash with 60 μl PBS. Conjugated microspheres were prepared to run 3 calibration curves for RHI (2 different batches), 2 calibration curves for insulin lispro (2 different batches), and 2 calibration curves for insulin aspart (2 different batches), with similar results. Data for each point in each curve were derived from a tube containing ~2.5×104 beads. The beads from each tube were added to a flat-bottomed well of a 96-well plate and were allowed to settle. They were then imaged with an inverted fluorescent microscope (Zeiss 200 Axiovert, Carl Zeiss, Oberkochen, Germany).
Image Analysis
Fluorescence images were captured on a Zeiss 200 Axiovert microscope using an AxioCAM MRm digital camera and AxioVision 4.8 software (Carl Zeiss) from samples based on AlexaFluor488 fluorescence (excitation 494 nm/emission 519 nm). Up to 6 images from each well were captured at 10× magnification. Each image was subdivided into 4 subimages. The number of subimages, each containing ~40 beads that were analyzed for each calibration point was between 8 and 24. For the images used for the measurement of insulin in human plasma, the edges of the images were cropped to remove areas of poor image quality before processing. ImageJ (ImageJ.net), and an algorithm implemented in Matlab software (MathWorks, Natick, MA) were used for the image processing and quantization of fluorescence associated with microspheres. The fluorescence associated with the microsphere was measured using the following steps:
Subtract from each pixel in the image the median signal for the whole image (background)
Calculate the standard deviation of the remaining signal
Consider each pixel with intensity larger than 1.3 times the STD as signal above background
Find connectivity components of pixels (“clusters”)
Define beads as clusters with the area between 60 and 350 pixels
Sum the intensity of all beads in each image and divide by the number of beads to calculate the mean intensity per bead
Calibration curves were derived using a range of insulin concentrations ranging from 500 pg/ml-50 ng/ml (86 pM-8620 pM) and generated using a regression model with Origin Pro 8 (OriginLab Corporation, Northampton, MA). The detection limit was defined as the minimal detectable concentration distinguishable from the blank. Analytical sensitivity = (3 standard deviations of the zero mean response) × (Lowest calibrator dose) / (Mean lowest calibrator response – Mean zero response). The regression model was applied using the log-log transformed data using an equation of the form Y = B+A(X), where X is the dilution of the insulin calibrator and Y is the corresponding response signal obtained.
Microfluidic Device Fabrication
Microfluidic flow chambers were fabricated using soft lithography. Negative photoresist SU-8 2100 (MicroChem, Newton, MA) was deposited onto clean silicon wafers to a thickness of 150 μm, and patterned by exposure to UV light through a transparency photomask (CAD/Art Services, Bandon, OR). The Sylgard 184 poly(dimethylsiloxane) (PDMS) (Dow Corning, Midland, MI) was mixed with cross-linker (ratio 10:1), poured onto the SU8 mold, degassed thoroughly and cured for 12 hours at 65°C. Next, the PDMS devices were peeled off the wafer and bonded to glass slides after oxygen-plasma activation of both surfaces. Prior to the experiments, the microfluidic channels were treated with 0.25% pluronic acid F-127 by filling the channels with the solution and then flushing them with PBS and air. Tygon Micro Bore PVC Tubing 100f, 0.010” ID, 0.030” OD, 0.010” Wall (Small Parts Inc, Logansport, IN) was connected to the channels and to the syringes. Syringe pumps (Harvard Apparatus, Holliston, MA) were used to control the flow of solutions through the device.
In microfluidic experiments, the flow through of the microfluidic device was temporarily halted during image acquisition.
Results
Microsphere-Based RHI Detection System
The microsphere-based insulin detection system is depicted as a conceptual scheme in Figure 1A, and fluorescent images of the beads are shown in Figure 1B. A representative calibration curve derived for RHI is shown in Figure 2A. A log-log transformation of the data was linear from the limit of detection of 110 pM to 8000 pM (15.3-1115 µIU/ml) (r2 = 0.98) (Table 1). Fasting insulin levels in patients with type 1 diabetes typically range from 50-180 pM (7-25 µIU/ml) and peak postprandial insulin concentrations are usually less than 1800 pM (250 µIU/ml).13 The microsphere-based detection system can therefore be fitted across most of the clinical range of insulin concentrations, and is linear in the expected range of peak insulin levels. The mean intraassay CV for this system was 16%.
Figure 1.
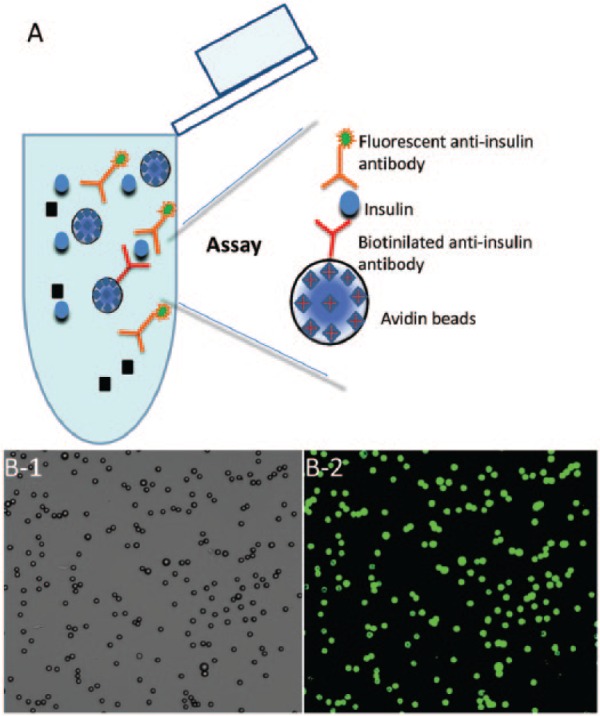
The microsphere insulin detection system. (A) Streptavidin-coated microspheres, at approximately 10 µm in diameter, were conjugated with biotin labeled capture anti-insulin antibodies. Then microsphere sensors were exposed to a solution containing insulin, and thereafter a fluorescent secondary antibody was added, followed by a wash step. (B) A typical image of microspheres, mostly found in singlets, is shown on the left as seen either under phase contrast, or on the right as imaged with fluorescence microscopy (excitation 494 nm, emission 519 nm).
Figure 2.
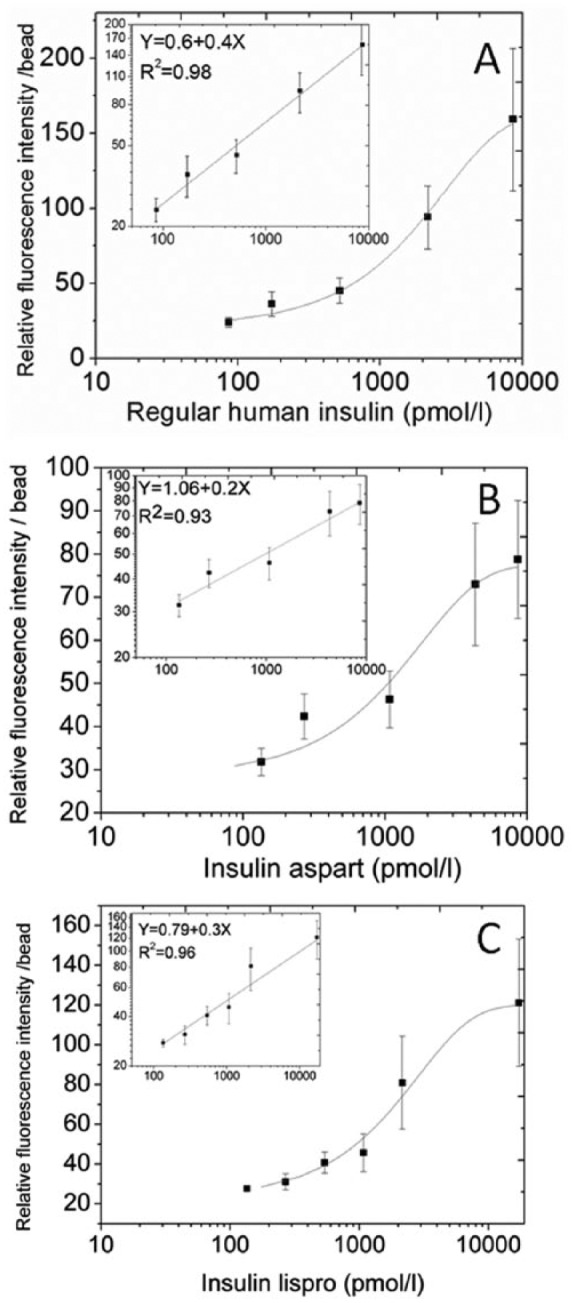
Representative calibration curves of the microsphere based detection system. Each data point represents the mean ± standard deviation of the mean. (A) A calibration curve was obtained after preparation of a regular human insulin stock solution in a pool of heat inactivated serum and dilution of the stock in blocking buffer. Both a linear-log curve as well as a linear regression on a log-log curve (inset) are presented (B and C). Corresponding calibration curves obtained for (B) insulin aspart and (C) insulin lispro.
Table 1.
Mathematical Parameters and Performance.
| Characteristics | Regular human insulin | Insulin lispro | Insulin aspart |
|---|---|---|---|
| Linear parameters | |||
| a (slope) | 0.4 | 0.3 | 0.2 |
| b | 0.6 | 0.79 | 1.06 |
| r2 | .98 | .96 | .93 |
| Performance criteria | |||
| Linear range (pM) | 110-8000 | 101-7000 | 180-6000 |
| Lower detection limit (pM) | 110 | 101 | 180 |
Microsphere-Based Detection System for Insulin Analogs
We next evaluated the system’s performance with the rapid-acting insulin analogs most commonly used by patients with diabetes mellitus, insulin aspart and insulin lispro. A calibration curve was derived using calibrators that ranged in the concentration from 0.78 to 100 ng/ml (134.5-17 240 pmol/L). Representative standard curves for the insulin analogs are shown in Figures 2B and 2C and mathematical parameters are reported in Table 1. The insulin lispro detecting system was linear from the limit of detection of 101 pM to 7000 pM (14-976 µIU/ml), while the insulin aspart detecting system was linear from the limit of detection of 180 pM to 6000 pM (25-836 µIU/ml). The lower sensitivity for insulin aspart could be due to reduced affinity of 1 of the antibodies, which were raised against RHI, for insulin aspart. The mean intraassay CVs for insulin aspart and insulin lispro were 14% and 19%, respectively.
Quantization of Endogenous RHI in Plasma
To evaluate the detecting system in a clinical context, and in the more complex matrix of human plasma, we used samples obtained at intervals from a volunteer with normal glucose metabolism after ingestion of a meal. As shown is Figure 3A, the results of the microsphere detection system followed the same pattern as the reference measurements obtained with the Abbott Architect assay, showing insulin levels rising from basal levels to peak and then clearing. The time to peak was the same as measured by both systems.
Figure 3.
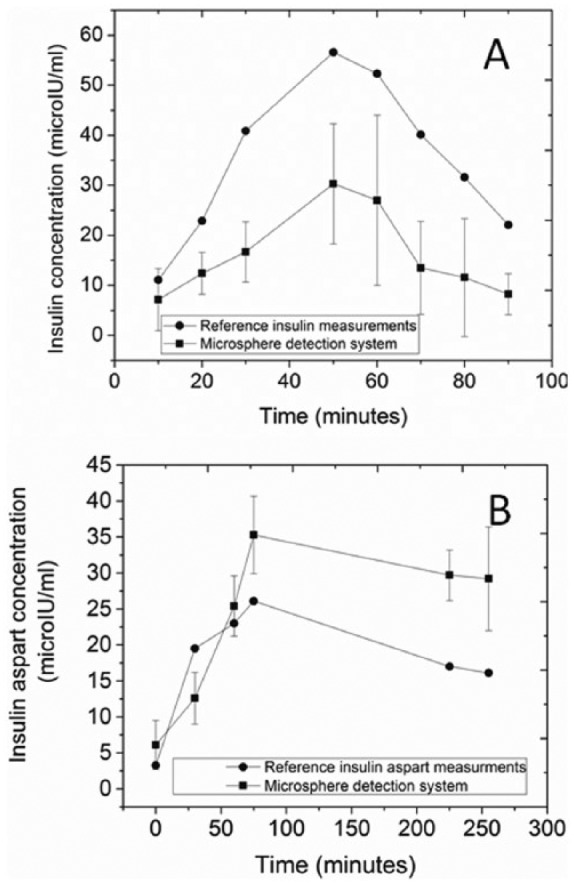
Measurements of insulin in human plasma samples. Each data point represents the mean ± SD. (A) Regular human insulin detected in a subject without diabetes after consuming a high carbohydrate meal at time zero. Samples were collected every 10 minutes. Reference values (Abbot Architect insulin assay, solid circles) are compared to the microsphere detection system (solid squares). Each data point represents the mean ± SD for 6-9 subimages. (B) Insulin aspart detected in a subject with type 1 diabetes and no endogenous insulin production (C-peptide negative in a mixed-meal tolerance test) that received 5 units of insulin aspart at time zero. Reference values (Abbot Architect insulin assay, solid circles) are compared to the microsphere detection system (solid squares). Each data point represents the mean ± SD for 8-16 subimages.
Quantitation of Insulin Aspart in Plasma
Most patients with type 1 diabetes use a rapid-acting insulin analog such as insulin aspart. We therefore tested the system on plasma samples obtained after injecting a volunteer with type 1 diabetes and no endogenous insulin secretion, with 5 units of insulin aspart. Figure 3B represents a time series of results of the microsphere detection system compared with results obtained with the Abbott Architect assay, which is reported to have approximately 60-70% cross-reactivity with insulin aspart.29,30 The measured change in insulin levels with time was similar to that measured with the reference method, and the time-to-peak was the same as measured by both systems.
Dynamic Monitoring of Insulin Levels in a Flow-Through Microfluidic Platform
To obtain real-time information about insulin levels, we developed a first generation device with the potential to continuously report insulin levels in a continuous stream of analyte. A prototype PDMS microfluidic device was designed as a flow-through device. Figure 4A shows the device injected with yellow and blue dyes to illustrate the mixing of input fluids, while Figure 4B schematically illustrates the flow-through detection system. A solution containing insulin is introduced into the microfluidic device through 1 inlet port and a suspension of microspheres conjugated to biotinylated HTB-125 anti-insulin monoclonal antibody is introduced into another. The insulin containing solution and the microsphere suspension mix by diffusion in a laminar flow region and then more thoroughly as they flow through a serpentine channel in a turbulent flow region. Once mixing occurs the target insulin is captured by the antibody-coated microspheres. A solution containing the fluorescently labeled HTB-124 (Figure 4C) anti-insulin monoclonal antibody is introduced into a third inlet port, followed by a second serpentine channel that enables a thorough mixing and labeling of insulin captured on the coated beads. The fluorophore at the surface of the microspheres then produces a localized fluorescent signal when illuminated by an excitation radiation (Figure 4D). After quantitation of bead-associated fluorescence, the materials flow to a waste collection reservoir.
Figure 4.
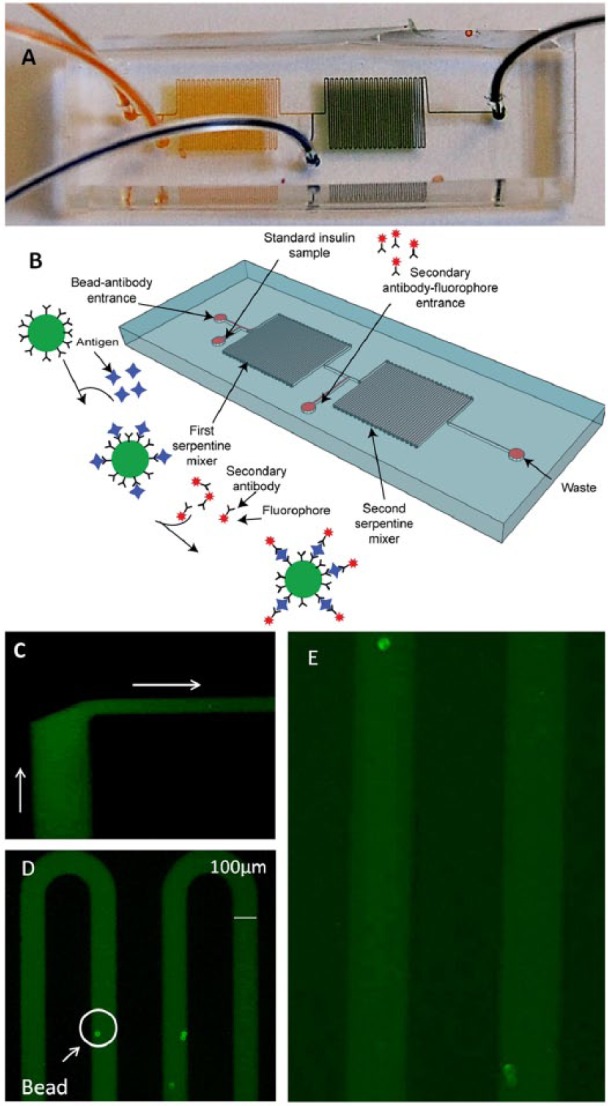
Microfluidic device for detection of insulin. (A) Dye was injected into the microfluidic device to demonstrate the fluid path and mixing of the inputs; the yellow and the blue solutions mix to make green. (B) The sequence of events is as follows: microspheres conjugated with anti-insulin antibody and solution containing insulin are both introduced into the device through separate ports and allowed to mix in the first serpentine segment. Anti-insulin fluorescently labeled antibody is introduced between the first and second serpentine segments. The conjugated microspheres are imaged as they pass through the detection region. The products of the reaction are collected at the waste outlet port. (C) Introduction of fluorescent labeled antibody solution (from the bottom of the image) into the stream of bead-containing fluid (from the left of the image). The 2 fluids do not mix extensively until the beginning of the serpentine segment (20× magnification). (D) Detection of insulin by the appearance of fluorescence at the surface of the insulin antibody-conjugated microspheres in the second serpentine segment (10× magnification). 12.5 ng/ml (300 µIU/ml) of insulin was detected as a proof of concept (n = 19 beads). (E) The device was also used to detect 6.25 ng/ml (150 µIU/ml) of insulin (n = 14 beads) shown at higher magnification (~30×).
Typical results after introduction of RHI at a high and medium concentration of 300 µIU/ml and 150 µIU/ml are shown in Figure 4. A strong fluorescent signal above background was produced on the beads within 1-4 min from the time the first beads reached the second serpentine channel (Figures 4D and 4E).
Discussion
We have developed a microsphere-based detection system capable of measuring insulin and insulin analogs. We have shown that the linear range of the detection system encompasses most of the dynamic range of insulin levels in patients with T1D treated with exogenous insulin. Previous studies have shown that insulin levels in the ISF are approximately 40% lower than in plasma.31,32 Therefore, basal insulin levels in the ISF of patients with type 1 diabetes are expected to range from 4-15 µIU/ml, whereas the lower limit of detection of the detection system is ~15 µIU/ml for RHI and insulin lispro, and ~25 µIU/ml for insulin aspart, Thus, the expected insulin concentrations in the ISF when plasma insulin is at basal (fasted) levels in the patients with type 1 diabetes are below the detection limit of our system in its current form. However, peak postprandial insulin concentrations are usually in the range of 50-250 µIU/ml,13 and the detection systems in their current form are linear from their limit of detection of ~15-25 µIU/ml to at least 800 µIU/ml. Therefore, when an insulin bolus is of sufficient size to raise the plasma insulin into the postprandial range, we will be able to detect and identify the peak despite the sensitivity limitations of the current system, and this should be sufficient to achieve our goal of identifying insulin peaks after a bolus injection to determine PK parameters. The sensitivity of our system is inferior to that of commercially available ELISA and RIA assays. However, these assays require several hours for completion, and are not compatible with detection of insulin in a flow through apparatus. Rapid measurement in a flow-through configuration is crucial to characterize the pharmacokinetics of insulin in real time with a wearable device. We are currently working on reducing background and increasing sensitivity by optimizing the preparation of the beads and the microfluidic device itself. Although the anti-insulin monoclonal antibodies we used in the system were high affinity (Kds of 2×10-8 and 3×10-9), different anti-insulin antibodies with higher affinity and specificity could improve the performance of the assay. The system has a higher detection limit and has less sensitivity for insulin aspart than for RHI and insulin lispro. It is possible that sequence differences between insulin aspart, lispro, and RHI are responsible for reduced affinity of 1 or both of the antibodies for insulin aspart relative to the other 2 insulins. We were not able to detect insulin glulisine at all with the antibody pair used in these experiments.
We demonstrated that bead-based detection system detected peaks of similar shape to the reference method in plasma samples in 2 patients. Plasma is a much more complex matrix than the buffers we used to dilute samples for determination of detection system performance.
Plasma is likewise much more complex than the microdialysis fluid that will ultimately be the input to the detection system. Many patients with T1D have detectable titers of anti-insulin antibodies, and the presence of these antibodies in the matrix may negatively affect the accuracy of insulin immunoassays. We plan to use 100 000 Dalton cutoff microdialysis membrane to capture microdialysis fluid. Therefore antibodies (~150 000 Daltons) will be excluded, eliminating this potential source of confounding in those patients with high antibody titers. We do not know whether our antibodies recognize proinsulin. If so, different antibodies would be needed for use in patients who produce significant quantities of insulin endogenously, such as patients with type 2 diabetes.
Another limitation of our system is the variability of the signal from bead to bead. Despite this variability, we were able to clearly distinguish different levels of insulin by measuring the fluorescence of multiple beads. In the intended application, this variability will be managed by measuring the fluorescence of large numbers of beads per unit time, which will be facilitated by a continuous, flow-through format. Furthermore, the main goal is to determine the pharmacokinetic parameters of insulin in real time, not to determine absolute levels of insulin at any given time. Therefore, as long as the detection system is able to clearly distinguish peaks, it will meet the requirements for its intended use.
The next steps in the development of this technology for continuous insulin monitoring are to validate the microfluidic flow-through system and to develop a detection approach that allows quantization of the fluorescence associated with each bead without stopping the flow of analytes through the device. Once real time flow-through measurement is achieved, we will connect the inlet of the microfluidic chip to the outlet of a microdialysis catheter inserted into the subcutaneous tissue for real time measurement of insulin levels in the ISF. This application will require characterization of the recovery of insulin from the ISF using microdialysis catheters and analysis of the consistency of the time lag between changes in insulin levels in the plasma and in the ISF.
Conclusions
We have demonstrated that a simple bead-based detection system has adequate sensitivity for the detection system of all but fasting or basal insulin levels, and an appropriate linear dynamic range for the detection of postprandial peak levels of insulin in clinical samples. We further demonstrated proof of principle that it can be adapted to a flow-through microfluidic format without requiring any washing of the beads. This detection system is therefore suitable for further development into a method for continuous insulin monitoring.
Acknowledgments
The authors would like to thank Eli Lilly and Company for providing purified zinc human insulin crystals and insulin lispro powder and Novo Nordisk for providing insulin aspart powder. They would like to thank Dr Patrick Sluss for his scientific advice.
Footnotes
Abbreviations: BG, blood glucose; CGM, continuous glucose monitor; ELISA, enzyme-linked immunosorbent assay; ISF, interstitial fluid; PBS, phosphate buffered saline; PDMS, polydimethylsiloxane; PK, pharmacokinetic; RHI, regular human insulin; RIA, radioimmunoassay; T1D, type 1 diabetes mellitus.
Author Contributions: LK: design, performance of experiments, data analysis, writing manuscript. ES: designed the flow through devices. RSM and MLY: critically reviewing manuscript. TK: design, development of the microsphere based detection systems in the continuous flow based device, critically reviewing manuscript. SJR: design, data analysis, writing manuscript, critically reviewing manuscript.
Disclosures: MLY, TK, and SJR have a patent pending on microfluidic bead detection systems. TK and SJR are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Juvenile Diabetes Research Foundation award 17-2013-485 (to SJR).
References
- 1. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [DOI] [PubMed] [Google Scholar]
- 2. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the micro-vascular complications of type 1 diabetes mellitus. JAMMA. 2002;287:2563-2569. [Google Scholar]
- 4. American Diabetes Associations. Standards of medical care in diabetes. Diabetes Care. 2009;32:S13-S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diabetes Control and Complications Trial Research Group. Hypoglycemia in the diabetes control and complications trial. Diabetes. 1997;46:271-286. [PubMed] [Google Scholar]
- 6. Leese GP, Wang J, Broomhall J, et al. DARTS/MEMO Collaboration: frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care. 2003;26:1176-1180. [DOI] [PubMed] [Google Scholar]
- 7. Petitti DB, Klingensmith GJ, Bell RA, et al. Glycemic control in youth with diabetes: The SEARCH for Diabetes in Youth Study. J Pediatr. 2009;155:668-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doyle FJ, Huyett LM, Lee JB, Zisser HC, Dassau E. Closed-loop artificial pancreas systems: engineering the algorithms. Diabetes Care. 2014;37:1191-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peyser T, Dassau E, Breton M, Skyler JS. The artificial pancreas: current status and future prospects in the management of diabetes. Ann NY Acad Sci. 2014;1311:102-123. [DOI] [PubMed] [Google Scholar]
- 10. Leelarathna L, Dellweg S, Mader JK, et al. Day and night home closed-loop insulin delivery in adults with type 1 diabetes: three-center randomized crossover study. Diabetes Care. 2014;37:1931-1937. [DOI] [PubMed] [Google Scholar]
- 11. Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. New Engl J Med. 2014;371:313-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55:3344-3350. [DOI] [PubMed] [Google Scholar]
- 13. El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type one diabetes. Sci Transl Med. 2010;2(27):27ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Russell SJ, El-Khatib FH, Nathan DM, Damiano ER. Efficacy determinants of subcutaneous microdose glucagon during closed-loop control. J Diabetes Sci Technol. 2010;4:1288-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chevenne D, Trivin F, Porquet D. Insulin assays and reference values. Diabetes Metab. 1999;25:459-476. [PubMed] [Google Scholar]
- 16. Deng B, Liu Z, Luo G, Ma H, Duan M. Rapid quantitative determination and assessment of insulin in oil formulation by micellar electrokinetic capillary chromatography. J Pharm Biomed Anal. 2002;27:73-80. [DOI] [PubMed] [Google Scholar]
- 17. Andersen L, Jørgensen PN, Jensen LB, Walsh D. A new insulin immunoassay specific for the rapid acting insulin analog, insulin aspart, suitable for bioavailability, bioequivalence, and pharmacokinetic studies. Clin Biochem. 2000;33:627-633. [DOI] [PubMed] [Google Scholar]
- 18. Wang J, Zhang X. Needle type dual microsensor for the simultaneous monitoring of glucose and insulin. Anal Chem. 2001;73:844-847. [DOI] [PubMed] [Google Scholar]
- 19. Roper MG, Shackman JG, Dahlgren GM, Kennedy RT. Microfluidic chip for continuous monitoring of hormone secretion from live cells using an electrophoresis-based immunoassay. Anal Chem. 2003;75:4711-4717. [DOI] [PubMed] [Google Scholar]
- 20. Cheng L, Pacey GE, Cox JA. Carbon electrodes modified with ruthenium metallodendrimer multilayers for the mediated oxidation of methionine and insulin at physiological pH. Anal Chem. 2001;73:5607-5610. [DOI] [PubMed] [Google Scholar]
- 21. Xu M, Luo X, Davis JJ. The label free picomolar detection of insulin in blood serum. Biosens Bioelectron. 2013;39:21-5. [DOI] [PubMed] [Google Scholar]
- 22. Konry T, Bale SS, Bushman A, et al. Particles and microfluidics merged: perspectives of highly sensitive diagnostic detection. Microchimia Acta. 2012;176:251-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Verpoorte E. Beads and chips: new recipes for analysis. Lab Chip. 2003;3:60-68. [DOI] [PubMed] [Google Scholar]
- 24. Derveaux S, Stubbe BG, Braeckmans K, et al. Synergism between particle-based multiplexing and microfluidics technologies may bring diagnostics closer to the patient. Anal Bioanal Chem. 2008;391:2453-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Konry T, Hayman RB, Walt DR. Microsphere-based rolling circle amplification microarray for the detection of DNA and proteins in a single assay. Anal Chem. 2009;81:5777-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chou J, Wong J, Christodoulides N, Floriano PN, Shanchez X, McDevitt J. Porous bead based diagnostic platforms: bridging the gaps in healthcare. Sensors. 2012;12:15467-15499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Golberg A, Linshiz G, Kravets I, et al. Cloud-enabled microscopy and droplet microfluidic platform for specific detection of escherichia coli in water. PLOS ONE. 2014;9:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schroer JA, Bender T, Feldmann RJ, Kim KJ. Mapping epitopes on the insulin molecule using monoclonal antibodies. Eur J Immunol. 1983;13:693-700. [DOI] [PubMed] [Google Scholar]
- 29. Moriyama M, Hayashi N, Ohyabu C, Mukai M, Kawano S, Kumagai S. Performance evaluation and cross reactivity from insulin analogs with the ARCHITECT insulin assay. Clin Chem. 2006;52:1423-1426. [DOI] [PubMed] [Google Scholar]
- 30. Heurtault B, Reix N, Meyer N, et al. Extensive study of human insulin immunoassays: promises and pitfalls for insulin analogues detection and quantification. Clin Chem Lab Med. 2014;52:355-362. [DOI] [PubMed] [Google Scholar]
- 31. Jansson PA, Fowelin JP, Von Schenck HP, Smith UP, Lonnroth PN. Measurement by microdialysis of the insulin concentration in subcutaneous interstitial fluid. Diabetes. 1993;42:1469-1473. [DOI] [PubMed] [Google Scholar]
- 32. Sjostrand M, Holmang A, Lonnroth P. Measurement of interstitial insulin in human muscle. Am J Physiol. 1999;276:E151-E154. [DOI] [PubMed] [Google Scholar]


