Abstract
Background:
Efficacy and safety of the Medtronic Hybrid Closed-Loop (HCL) system were tested in subjects with type 1 diabetes in a supervised outpatient setting.
Methods:
The HCL system is a prototype research platform that includes a sensor-augmented insulin pump in communication with a control algorithm housed on an Android-based cellular device. Nine subjects with type 1 diabetes (5 female, mean age 53.3 years, mean A1C 7.2%) underwent 9 studies totaling 571 hours of closed-loop control using either default or personalized parameters. The system required meal announcements with estimates of carbohydrate (CHO) intake that were based on metabolic kitchen quantification (MK), dietician estimates (D), or subject estimates (Control). Postprandial glycemia was compared for MK, D, and Control meals.
Results:
The overall sensor glucose mean was 145 ± 43, the overall percentage time in the range 70-180 mg/dL was 80%, the overall percentage time <70 mg/dL was 0.79%. Compared to intervals of default parameter use (225 hours), intervals of personalized parameter use (346 hours), sensor glucose mean was 158 ± 49 and 137 ± 37 mg/dL (P < .001), respectively, and included more time in range (87% vs 68%) and less time below range (0.54% vs 1.18%). Most subjects underestimated the CHO content of meals, but postprandial glycemia was not significantly different between MK and matched Control meals (P = .16) or between D and matched Control meals (P = .76). There were no episodes of severe hypoglycemia.
Conclusions:
The HCL system was efficacious and safe during this study. Personally adapted HCL parameters were associated with more time in range and less time below range than default parameters. Accurate estimates of meal CHO did not contribute to improved postprandial glycemia.
Keywords: artificial pancreas, diabetes, insulin pump therapy, human trials
The commercialization of systems for automated titration of insulin, referred to as artificial pancreas (AP) systems, for people with type 1 DM is close to becoming a reality due to the collaborative efforts of investigators in academia, funding agencies, and industry. The components of the AP system are subcutaneous insulin delivery pumps and continuous glucose sensors (CGM) and an algorithm. The AP system calculates the amount of insulin to be delivered at distinct time intervals using past and present CGM measurements and insulin delivery parameters. The AP systems should be designed to be safe and effective with consideration of the current devices’ accuracy and reliability. Furthermore, the AP system should perform well despite relatively slow insulin action and the relatively fast effect of meal disturbances. The algorithm must account for device accuracy and reliability, the relatively slow pharmacodynamics of insulin, and the relatively fast effects of carbohydrate ingestion.
Currently, there are numbers of publications from human trials demonstrating that AP systems can be deemed safe and effective for home use. The majority of the conducted AP trials were overnight studies,1-7 with fewer full-day AP studies.8-14 The CGM to insulin feedback titration algorithm generally implements either modifications of the proportional-integral-derivative (PID)15 controller, the model predictive controller (MPC)16 or fuzzy-logic17 control. Most of the AP systems are based solely on insulin titration, however, some AP systems utilize 2 hormones—insulin and glucagon18,19—or even multihormonal combinations.20
There is increasing evidence suggesting that the AP systems, to achieve tight glycemic control with current subcutaneous insulin pharmacokinetic/pharmacodynamics (PK/PD) profiles, require a meal announcement.21 This is mainly the result of the fact that the glucose response to a meal intake is faster than glucose response to insulin delivery by the subcutaneous route. It is expected that newer, faster insulin analogs may improve the ability of AP systems to deal with unannounced meals, if there is a significant enhancement of insulin PK/PD profiles.
Large uncertainties in inter- and intra day meal responses and daily and periodic variability in insulin action require AP systems to be equipped with sophisticated and personalized safety mechanisms that titrate insulin efficiently, adjust insulin titration, and reduce or eliminate insulin over-delivery. Medtronic’s Hybrid Closed-Loop (HCL) system is equipped with a number of safety modules and adaptable personalized insulin delivery rates that should enable it to be a commercial product in the near future.
Methods
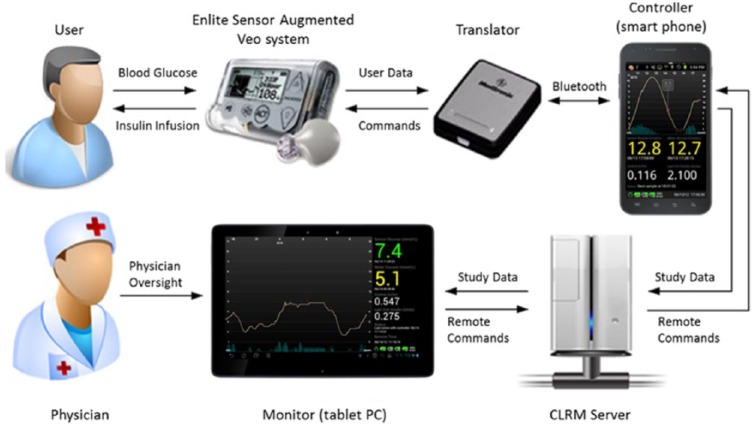
The system is schematically depicted in Figure 1. This study was conducted in a protected home setting at the Ch. Sheba Medical Center, Israel, that was equipped with a closed-circuit television (CCTV), and that transmitted to a monitoring room located 200 meters away. The protected home is part of a small neighborhood that is physically disconnected from any hospital building. A health care provider, trained in the management of diabetes-related emergencies, was continuously supervising the experiment by watching the CCTV. In addition, the Android-based HCL system enabled remote monitoring of sensor glucose, insulin delivery, and blood glucose levels obtained with a glucose meter
Figure 1.
Medtronic’s Android-based HCL system. The HCL software was embedded in an Android cellular device that communicated/controlled the sensor augmented pump (Paradigm® Veo™ and Enlite Glucose Sensor) via the translator. The Android cellular device constantly uploaded the study data to the CareLink Remote Monitor (CLRM) server by the Internet. The CLRM server constantly delivered the study data to an Android-based monitor with the ability to send commands back to the controller via the Internet.
The participants spent 4 days and 3 nights under free living conditions. They were allowed to move while escorted within the medical center which includes 2 shopping areas and restaurants, a 3.5 km circular walking route, and a gym.
Food was freely accessible, and both homemade and dining-out was available. All meals were photographed for evaluation of constituents. We studied SG values during 3 hours following meals, for which the preprandial bolus was calculated based on carbohydrate (CHO) content that was either 1. known precisely by virtue of preparation in a metabolic kitchen preparation (MK), 2. estimated by a dietician (D), or 3. estimated by the subject (Control). MK and D meals were each matched to their own Control meals according to time and CHO content, as assessed by meal photographs. All Control meals were consumed first so subjects were not able to deduce the CHO content of the Control meals from the MK or D CHO values.
The protocol was approved by the local Institutional Review Board, and written informed consents were obtained from each patient. Recruitment criteria included age 18-70, type 1 DM for at least 1 year, use of an insulin pump for at least 6 months. Exclusion criteria included current or possible pregnancy, and diabetic ketoacidosis and severe hypoglycemia with seizure or loss of consciousness in the prior 12 months.
The controller of the HCL system included a relatively large number of interconnected modules that were designed to increase patient safety. The algorithm at the core of the HCL system is the PID-IFB that is described in Steil et al.15 The HCL system evaluated sensor integrity continuously. Every midnight, the parameters of the system, including the limit of the amount of insulin that could be delivered, were adapted. The system delivered an estimated safe correction bolus if the user provided a BG measurement. The user was instructed to estimate the amount of CHO intake in grams and, based on the user defined carbohydrate to insulin ratio, the system delivered a meal bolus. If the system detected a sensor fault, missed sensor transmission, or insulin delivery exceeded a predetermined maximum over a predefined period of time, the system reverted to a safe basal insulin delivery mode.
Personalized parameters were based on recent insulin deliveries and SG/BG measurements; when these were not available, the system used default parameters that were based on subjects’ total daily insulin requirements. Medtronic’s Android-based HCL system adaptation algorithm to calculate personalized HCL parameters was triggered only if a few criteria were met that included insulin delivery, and SG and BG measurements. As a result, part of the trial was conducted with the HCL default values that were based only on patient’s total daily insulin dose (TDD). The primary objective of the reported trial was to assess the closed-loop system in supervised outpatient conditions. The system adaptation efficacy was an interesting outcome that was retrospectively analyzed.
Results
Data are presented from 9 studies (5 female). Subjects’ mean (± SD) age was 53.3 ± 15.7 years, duration of diabetes was 22.6 ± 11.9 years, and baseline HbA1c was 7.2 ± 0.8%.
Table 1 presents the glycemic parameters obtained during the closed-loop control period. The overall sensor glucose mean was 145 ± 43 mg/dL, the overall percentage of time spent between 70-180 mg/dL was 80% and the overall percentage of time below 70 mg/dL was 0.79%. The sensor glucose mean was 137 ± 37 mg/dL vs 158 ± 49 mg/dL (P < .001) with the personalized HCL parameters versus the default HCL parameters. The percentage of time spent between 70-180 mg/dL and below 70 mg/dL with the personalized HCL parameters versus the default HCL parameters were 87% versus 68% and 0.54% versus 1.18%, respectively. The percentages of time during the overnight period that was between 70-150 mg/dL were compared for the overall study, the personalized HCL parameter time period, and the default HCL parameter time period. Results showed percentages that were 66%, 78%, and 62%, respectively. Time spent above 250 mg/dL for the overall study, the adapted CL parameters, and the default CL parameters were 2%, 1%, and 4%, respectively.
Table 1.
Summary of the Android-Based HCL System.
| Overall |
Personalized parameters |
Default parameters |
||||
|---|---|---|---|---|---|---|
| 24 hours | 00:00-06:00 | 24 hours | 00:00-06:00 | 24 hours | 00:00-06:00 | |
| Mean SG | 145 | 140 | 137 | 130 | 158 | 145 |
| SD SG | 43 | 40 | 37 | 29 | 49 | 43 |
| % time spent at glucose level | ||||||
| 70-180 mg/dL | 80 | 83 | 87 | 93 | 68 | 80 |
| 70-150 mg/dL | 62 | 66 | 72 | 78 | 47 | 62 |
| >180 mg/dL | 20 | 17 | 12 | 7 | 31 | 20 |
| >250 mg/dL | 2 | 1 | 1 | 0 | 4 | 2 |
| <70 mg/dL | 0.79 | 0.77 | 0.54 | 0 | 1.18 | 0.79 |
| <60 mg/dL | 0.12 | 0.30 | 0 | 0 | 0.31 | 0.12 |
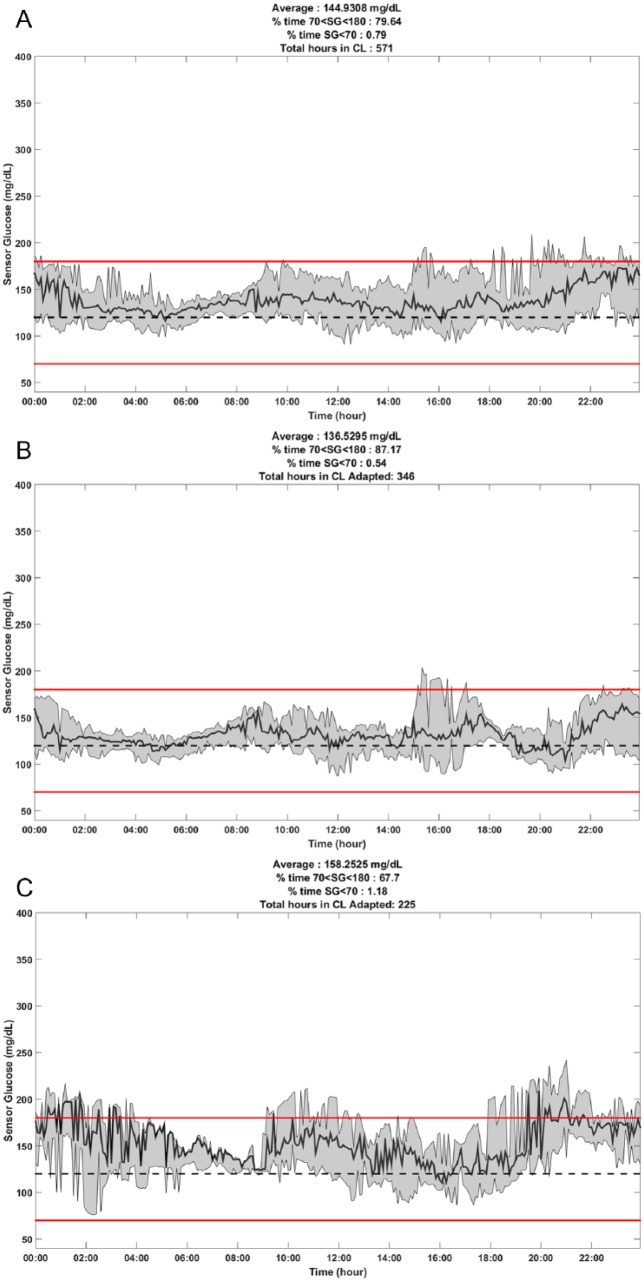
Figure 2 shows plots of the median and interquartile ranges (IQR) of SG values obtained during the 571 hours of HCL use (Panel A), during the 346 hours of use of the personalized parameters (Panel B), and during the 225 hours of use of the default parameters (Panel C).
Figure 2.
Diurnal pattern of median and interquartile range of sensor glucose values under various conditions. (A) Data from 571 hours of HCL control in 9 experiments. (B) Data from 346 hours of HCL control with personalized parameters. (C) Data from 225 hours of HCL control with default parameters. Red lines at 70 and 180 mg/dL indicate boundaries of the target range.
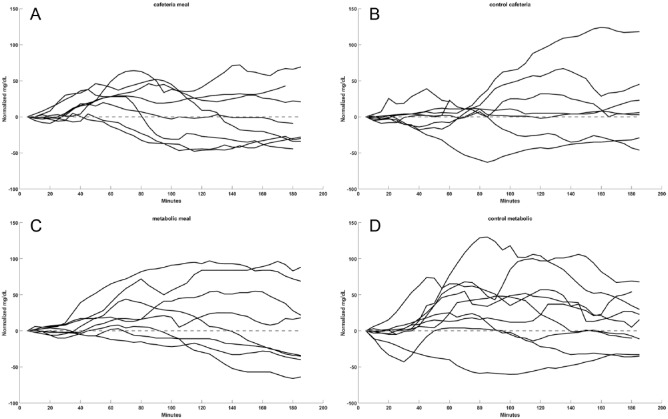
Figure 3 depicts the aggregated 3 hours postprandial SG profiles when the insulin boluses for the meals used (A) D CHO counts and (B) matched Control CHO counts; and (C) MK CHO counts and (D) matched Control CHO counts.
Figure 3.
Glycemia following meals according to the source of CHO estimates. (A) Estimates provided by dieticians (n = 8). (B) Estimates provided by subjects for time- and CHO-matched controls (n = 6). (C) Estimates provided by metabolic kitchen (n = 8). (D) Estimates provided by subjects for time- and CHO-matched meals (n = 9). The mean ± SD difference between baseline and 3-hour postprandial SG values for each of the meal types in panels A-D was 6.8 ± 28.3, 6.0 ± 31.5, 13.9 ± 36.0, and 18.1 ± 39.1 mg/dL, respectively.
Subjects tended to underestimate the CHO content of their meals; their mean estimates were 53 ± 45% of the values provided by dieticians and 72 ± 21% of the values provided by the metabolic kitchen (P < .01 for each). Subjects underestimated the CHO content in 13 of the 14 meals for which it was estimated by a dietician or the metabolic kitchen.
Differences in SG values from meal start to 3 hours postprandial with Control versus D CHO estimation were 6.0 ± 31.5 mg/dL and 6.8 ± 28.3 mg/dL (P = .76), and for Control versus MK were 13.9 ± 36.0 mg/dL and 18.1 ± 39.2 mg/dL, respectively (P = .16).
Discussion
The Medtronic Android-based HCL system was evaluated for 4 days and 3 nights in a protected outpatient setting that resembled free living conditions, while providing safety that is essential when testing new AP systems.
Comparison of the 3 hours postprandial glycemic profiles obtained with 3 types of meal bolus estimations did not show any significant differences in the postprandial SG increments with CHO estimations made by the dietician, the research subject and in a metabolic kitchen. Although, the CHO content of the meals were systematically underestimated by subjects, the data suggests that the precise counting of CHO does not significantly influence postprandial SG excursions with the Android-based HCL system.
Medtronic Minimed developed an advanced method that is using the insulin delivery rates combined with sensor readings, BG meter readings and meal announcements to calculate the upper safe limit of the insulin delivery rate. However, when the adaptation with the discussed Android system did not met the predefined criteria then the system used a default value that is calculated solely by the daily total insulin delivery. The results showed that the adaptation is highly effective by comparing the periods of time the system was delivering insulin with default and adapted maximum delivery rates. The sensor glucose mean was 137 ± 38 mg/dL vs 158 ± 49 mg/dL (P = 9e-93) with the personalized HCL parameters versus the default HCL parameters.
The overall percentage of time with SG values between 70-180 mg/dL was 79.5%. Time in this target range was much higher when personalized parameters were used (86.1%) than when default parameters were used (67.7%). Intervals of personalized parameter use were also characterized by less time with SG values <70 mg/dL than intervals of default parameter use (0.75% vs 1.18% of the time).
Although different studies were conducted in different conditions we still can compare the outcomes to evaluate the potential of the discussed closed-loop system. The strength of the presented results is reinforced when they are compared to published ones with closed-loop systems over 24 hours. Thabit et al14 reported in a 12-week free-living home condition study a mean of 67.7% time in the range 70-180 mg/dL. Kovatchev et al11 reported in a 40-hour study with 18 subjects the percentage of time in the range 70-180 mg/dL was 66.1%. Russell et al,13 using a dual hormonal system, reported 76-80% time in the range 70-180 mg/dL. In this study we did not include a control arm, and therefore we cannot claim that the closed-loop system improved the glycemic control of the specific group of subjects. However, the results of the study indicate the potential of the discussed closed-loop system in effectively managing the glycemic control with low occurrence of hypoglycemia in people with type 1 diabetes
Conclusions
In conclusion, the Medtronic Android-based HCL system showed high efficacy in automatically regulating the glucose levels, while significantly reducing the risk of hypoglycemia. Even though a precise CHO count may improve the overall AP control, it was found that the adaptation module of this system was effective in compensating for incorrect carbohydrate estimation. Results of this study will be used to continue to improve the Medtronic HCL algorithm and system.
Footnotes
Abbreviations: AP, artificial pancreas; CCTV, closed-circuit television; CGM, continuous glucose sensors; CHO, carbohydrate; D, dietician; HCL, Hybrid-Closed Loop; IQR, interquartile ranges; MK, kitchen quantification; MPC, model predictive controller; PID, proportional-integral-derivative; PK/PD, pharmacokinetic/pharmacodynamics; TDD, total daily insulin dose
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: BG, AR, NK, DW, NP, GV, RG, FK and OC are Medtronic employees.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Support was received from the JDRF (1-SRA-2014-240-M-R).
References
- 1. Hovorka R, Elleri D, Thabit H, et al. Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2014;37:1204-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thabit H, Elleri D, Leelarathna L, et al. Unsupervised overnight closed loop insulin delivery during free living: analysis of randomised cross-over home studies in adults and adolescents with type 1 diabetes. Lancet. 2015;385(suppl 1):S96. [DOI] [PubMed] [Google Scholar]
- 3. Del Favero S, Place J, Kropff J, et al. Multicenter outpatient dinner/overnight reduction of hypoglycemia and increased time of glucose in target with a wearable artificial pancreas using modular model predictive control in adults with type 1 diabetes. Diabetes Obes Metab. 2015;17:468-476. [DOI] [PubMed] [Google Scholar]
- 4. Brown SA, Kovatchev BP, Breton MD, et al. Multinight “bedside” closed-loop control for patients with type 1 diabetes. Diabetes Technol Ther. 2015;17:203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thabit H, Elleri D, Leelarathna L, et al. Unsupervised home use of an overnight closed-loop system over 3-4 weeks: a pooled analysis of randomized controlled studies in adults and adolescents with type 1 diabetes. Diabetes Obes Metab. 2015;17:452-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nimri R, Muller I, Atlas E, et al. MD-Logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes Care. 2014;37:3025-3032. [DOI] [PubMed] [Google Scholar]
- 7. Kropff J, Del Favero S, Place J, et al. 2 month evening and night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: a randomised crossover trial. Lancet Diabetes Endocrinol. 2015;3:939-947. [DOI] [PubMed] [Google Scholar]
- 8. Ly TT, Roy A, Grosman B, et al. Day and night closed-loop control using the integrated Medtronic hybrid closed-loop system in type 1 diabetes at diabetes camp. Diabetes Care. 2015;38:1205-1211. [DOI] [PubMed] [Google Scholar]
- 9. Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371:313-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Del Favero S, Bruttomesso D, Di Palma F, et al. First use of model predictive control in outpatient wearable artificial pancreas. Diabetes Care. 2014;37:1212-1215. [DOI] [PubMed] [Google Scholar]
- 11. Kovatchev BP, Renard E, Cobelli C, et al. Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes Care. 2014;37:1789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leelarathna L, Dellweg S, Mader JK, et al. Day and night home closed-loop insulin delivery in adults with type 1 diabetes: three-center randomized crossover study. Diabetes Care. 2014;37:1931-1937. [DOI] [PubMed] [Google Scholar]
- 13. Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371:313-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373:2129-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steil GM, Palerm CC, Kurtz N, et al. The effect of insulin feedback on closed loop glucose control. J Clin Endocrinol Metab. 2011;96:1402-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parker RS, Doyle FJ, Peppas NA. A model-based algorithm for blood glucose control in Type I diabetic patients. IEEE Trans Biomed Engineer. 1999;46:148-157. [DOI] [PubMed] [Google Scholar]
- 17. Atlas E, Nimri R, Miller S, Grunberg EA, Phillip M. MD-logic artificial pancreas system: a pilot study in adults with type 1 diabetes. Diabetes Care. 2010;33:1072-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Russell SJ, El-Khatib FH, Nathan DM, Magyar KL, Jiang J, Damiano ER. Blood glucose control in type 1 diabetes with a bihormonal bionic endocrine pancreas. Diabetes Care. 2012;35:2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haidar A, Legault L, Dallaire M, et al. Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. CMAJ. 2013;185:297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weinzimer SA, Sherr JL, Cengiz E, et al. Effect of pramlintide on prandial glycemic excursions during closed-loop control in adolescents and young adults with type 1 diabetes. Diabetes Care. 2012;35:1994-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elleri D, Dunger D, Hovorka R. Closed-loop insulin delivery for treatment of type 1 diabetes. BMC Med. 2011;9:120. [DOI] [PMC free article] [PubMed] [Google Scholar]