Abstract
The original continuous glucose monitors (CGMs) were limited to 3-day, blinded observation periods where glucose data was hidden from patients and later retrospectively analyzed by a provider to help guide the management of diabetes. Unblinded CGM, released several years later, allows patients to view their glucose data in real-time amidst their daily routines, enabling them to better understand how variables such as activity, nutrition, and medications affect glucose levels. Research studies consistently demonstrate improved glycemic control and reduced hypoglycemia in children and adults with type 1 and type 2 diabetes while using unblinded CGM.1-4 As such, we believe that all CGM usage in clinical practice should be in real-time, unblinded mode for short-term and long-term wear periods.
Keywords: CGM, continuous glucose monitor, glucose monitoring, glucose sensor
Self-monitoring of blood glucose (SMBG) with glucose meters remains the mainstay of glycemic monitoring in type 1 and type 2 diabetes, but has major shortcomings such as the inability to provide glucose trend information and the potential to miss silent glucose excursions.
In contrast, continuous glucose monitors (CGMs) sample interstitial glucose every 5 minutes, providing detailed trend information and analytics. Technological advances have lowered the MARD of current generation CGMs (eg, G4 Platinum with Software 505, Dexcom, San Diego, CA) to under 10%, clinically matching that of capillary glucose meters.5
The first CGM (MiniMed CGMS, Medtronic, Northridge, CA) was exclusively used for short-term, blinded use. After a 3-day wear period during which BG measurements were hidden from the patient, the data would be reviewed retrospectively by a clinician. The first unblinded (real-time) CGM devices, the MiniMed Guardian RT-System and Dexcom STS-CGM, arrived over 5 years later. Such real-time CGMs are now available for both short-term “professional” trials and long-term “personal” use.
Real-time CGM use has been repeatedly shown to improve glycemic control in both children and adults, while concurrently reducing the incidence of hypoglycemia.1-4 Therefore, real-time CGM is now endorsed by the AACE,6 the Endocrine Society,7 and ADA8 as a component of standard of care management for diabetes. However, providers continue to use blinded CGM during short-term wear periods despite inconclusive evidence.9-11
This article highlights why the advent of real-time, unblinded CGM obviates the need for blinded CGM in all settings other than a formal clinical research study.
The Myth of a “Regular Day”
Perhaps the most common argument for using blinded CGM is to better capture a patient’s “regular day,” where behavior is uninfluenced by knowledge of BG data.
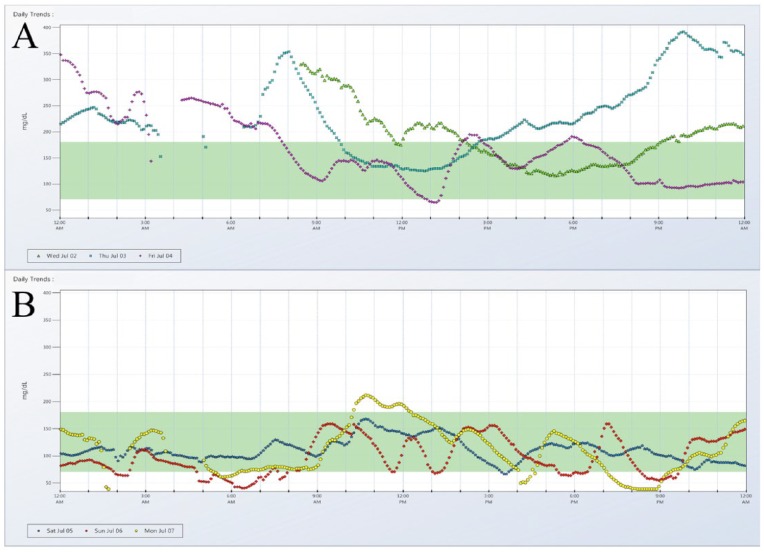
Unfortunately, there is no such thing as a “regular day” of glucose in type 1 diabetes. A limited, 3- to 7-day sample size can easily span both workweek and weekend days, each with different schedules of physical activity and nutrition. Figure 1 demonstrates an example of such variability and how even a routine procedure such as an insulin pump site change can alter glucose patterns. Figure 1A shows the 72 hours of glucose data preceding the site change, and Figure 1B visualizes the following 72 hours. The 2 consecutive 3-day graphs differ markedly, and each individual tracing lacks any consistent pattern, even within the same panel.
Figure 1.
CGM tracings of 3 days leading up to insulin pump site change (A) and 3 days following insulin pump site change (B).
Even if it were possible to reproduce identical behaviors on consecutive days, subcutaneously injected insulin has been shown to have a day to day coefficient of variation between 27% and 59% for basal insulins12 and 20 to 30% for short-acting insulins13,14 within the same patient. Such fluctuations in insulin action would undoubtedly affect a “regular day” of CGM readings.
In addition, the Hawthorne effect, where individuals modify their behavior in response to their awareness of being observed, suggests that it would be naïve to blind a CGM and expect to capture a “regular day” within a time period as short as 3 days.
This lack of consistency was perhaps best summarized by Kerssen et al,15 who found high day-to-day glucose variability when using CGM for 2 consecutive days on pregnant women with type 1 diabetes. Depending on which day was analyzed, physician recommendations for insulin dose adjustments varied by 29% to 48%.
Real-Time Monitoring Is an Intervention
Rather than simply observation, the utility of CGM lies in its ability to uniquely empower and educate patients in real time, a goal that can effectively improve long-term glucose control.
A multicenter, randomized controlled crossover study of 153 children and adults with type 1 diabetes using insulin pump therapy (CSI) compared glycemic control during periods with access to CGM data (Sensor On arm) and without access to CGM data (Sensor Off arm).16 The mean difference in HbA1c was –0.43% (–4.74 mmol/mol) in favor of the Sensor On arm (8.04% [64.34 mmol/mol] vs 8.47% [69.08 mmol/mol]; 95% CI –0.32%, –0.55% [–3.50, –6.01 mmol/mol]; P < .001). When real-time data was available to the subjects, less time was spent in hypoglycemia (19 vs 31 min/day, P = .009), and the insulin pump features were used more frequently, in both number of daily insulin boluses (6.8 ± 2.5 vs 5.8 ± 1.9, P < .0001) and temporary basal rate events (0.75 ± 1.11 vs 0.26 ± 0.47, P < .0001). This study shows that unblinded CGM enables patients to interact more effectively with their disease in real-time and make meaningful adjustments.
A recent survey of 222 successful CGM users (mean HbA1c of 6.9% with minimal hypoglycemia) with type 1 diabetes detailed how the real-time display of glucose information such as BG and trend arrows provides actionable insights.17 Consistent with the previously discussed crossover study, a majority of survey respondents reported more frequent insulin boluses or injections per day after starting CGM. Respondents also reported that CGM data led to adjustments in the timing and quantity of insulin doses before meals and at times when they were correcting for elevated BG.
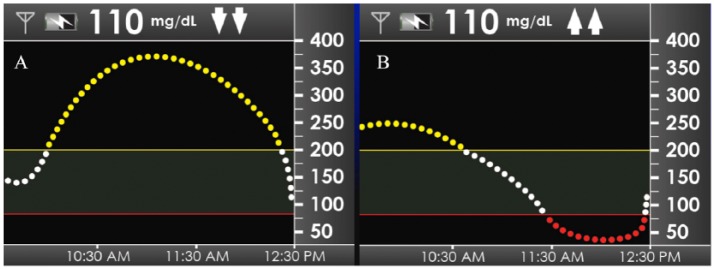
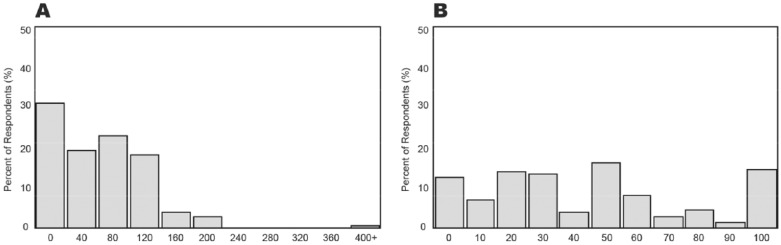
The survey quantified these adjustments by asking respondents to describe how they would adjust their insulin dose based on the 2 CGM tracings illustrated in Figure 2, each pairing the same euglycemic BG of 110 mg/dL with a different glucose curve and trend arrows. Figure 3A shows that over 70% of respondents would increase their dose in response to Figure 2A (2 up arrows), and Figure 3B shows that nearly 90% would decrease their dose in response to Figure 2B (2 down arrows). These results demonstrate that patients have learned to regard the glucose direction as equally (or perhaps more) important as the glucose value itself. This invaluable educational opportunity would have been lost in the setting of blinded CGM.
Figure 2.
Simulated CGM displays at mealtime with euglycemia (110 mg/dL), presented in a survey to experienced CGM users. Panel A shows 2 trend arrows down; panel B shows 2 trend arrows up.
Figure 3.
Impact of the direction and rate of glucose change on a mealtime insulin dose at euglycemia (110 mg/dL). Panel A indicates the percentage of respondents who increased their insulin dosages 0 to 400% when 2 up arrows were displayed. Panel B indicates the percentage of respondents who decreased their insulin dosages 0 to 100% when 2 down arrows were displayed.
Unlike real-time CGM, blinded CGM has not been convincingly proven to improve glycemic control. A study enrolled 102 patients with type 1 and type 2 diabetes into a 3-day blinded CGM trial with the iPRO (Medtronic, Northridge, CA) and did not find significant improvement in HbA1c up to 7 months after the CGM was worn.11 Another study did not find a significant difference in HbA1c levels in patients with type 1 diabetes when comparing those using SMBG and those using a 72-hour blinded CGM trial.9
In addition to trend arrows and direction, real-time CGM users have the benefit of instantly interpreting their reading with all the appropriate contextual information, such as recent meals, exercise, and stressors. Just as structured capillary glucose testing better facilitates patient understanding and engagement,18 the accuracy of integrating behaviors in real-time with CGM data far exceeds that of any written diary or memory recall.
Revealingly, the aforementioned survey found that over 80% of respondents believed real-time analysis of their CGM data was more useful than retrospective analysis. Of those surveyed, 19% never or rarely downloaded their CGM data during clinician office visits. If the overwhelming benefit to CGM wearers lies in real-time analysis, why should we restrict this feature? And similarly, if downloading the data to reveal patterns is of utmost importance, then why are so few providers and patients doing it?
The Perils of Blinded CGM: Dead in Bed
We concede that cost concerns and access issues may necessitate short-term wear periods of CGM, but blinded use offers no clear advantage over real-time use, even in 3- to 7-day wear periods. In fact, blinded CGM incurs additional risk and potential harm.
A case report documents a 23-year-old with type 1 diabetes who suffered a lethal hypoglycemic event in his sleep while undergoing an observation period with blinded, short-term professional CGM to investigate recurrent severe hypoglycemia.19 On the first evening of his trial, he exercised at the gym after dinner and then went to bed. At approximately 9 am the next morning, he was discovered by his family to be dead in bed. He unfortunately did not respond to glucagon or resuscitative efforts by paramedics.
Postmortem analysis of his insulin pump and blinded CGM device revealed severe hypoglycemia (BG below 50 mg/dL), highlighted by the arrows on Figure 4, for at least 3 hours prior to his death. Had he been wearing an unblinded CGM, a hypoglycemic alarm would have sounded, potentially triggering a life-saving intervention by the patient himself or a nearby relative.
Figure 4.
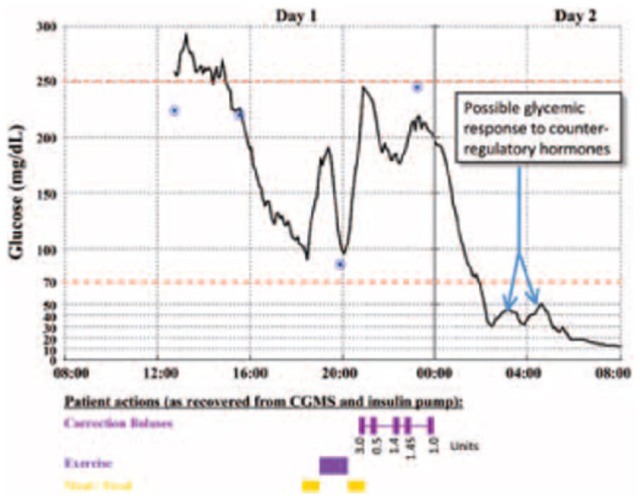
Glucose levels captured by the retrospective CGM for the evening before and the morning of the patient’s death. The calibrations measured and entered by the patient are represented by the 4 circles. The timings of the patient’s meals, exercise, and correction insulin boluses are represented by the bars along the bottom of the graph. The precipitous decrease in glucose level after the correction doses can be observed to start just after midnight, and possible counterregulatory efforts are noted once the glucose level declined to below 30 mg/dL shortly after 2 am.
This case tragically exposes a dangerous vulnerability of blinded CGM. Unblinded CGM would have equally accomplished the provider’s original purpose of identifying severe hypoglycemia, yet somehow the belief remains that having the patient see this information could be a disadvantage. The undeniable reality is that blinding the patient to his CGM information ultimately contributed to his death.
While admittedly a worst-case scenario, deaths resulting from severe hypoglycemia occur regularly. Recent studies suggest that 4% to 10% of deaths in people with type 1 diabetes are due to hypoglycemia.20-23 Even nonlethal severe hypoglycemic events during a blinded CGM wear period incur financial and emotional costs. Meanwhile, real-time CGM has been consistently proven to reduce frequency and time spent in hypoglycemia.1-4
Conclusion: The Value of Patient Empowerment
We believe that many of the underlying motivations for using blinded professional CGM could be rooted in the paternalistic and anachronistic perspective that the practitioner always knows best. We believe that the most valuable and sustaining interventions empower and educate the patients as they deal with their disease day in and day out. As such, except for research studies, all usage of CGM should be unblinded.
We ultimately need to consider what we are trying to accomplish or avoid by blinding patients to their information. Are we concerned that they will react to the information and cause hypoglycemia? Real-time CGM has been clearly shown to lower hypoglycemia, therefore blinded CGM increases their risk. Are we concerned that patients will modify their behavior so we cannot “catch them in the act”? We believe that it is more beneficial for patients, rather than providers, to “catch themselves in the act.” Perhaps it is difficult for us as practitioners to “let go” and concede that patients should be more involved in the decision-making process.
Think practically about patients you have started on real-time CGM for a moment. When starting them on the device, do you teach them how to adjust insulin based on trend arrows? Probably not, but patients usually figure this out; as studies repeatedly demonstrate, their HbA1c improves, their time in range improves, and/or their rate of hypoglycemia goes down.1-4 These benefits arise from what patients have learned themselves about their disease, and not solely from a doctor’s instructions. This realization may be a hard pill to swallow for providers, but it clearly suggests that patients have earned a seat at the table when it comes to making management decisions.
For type 1 diabetes, we strongly advocate for the use of long-term unblinded over short-term blinded CGM usage. In fact, we would go further to say that the use of blinded CGM in a clinical setting is not ethical and not indicated, period. If circumstances require the use of short-term CGM, then unblinded CGM should be selected. Real-time availability of hypoglycemic alarms and trend arrows add exponentially more value than a static capillary glucose meter reading. This information can be long-lasting and complements any insights the caregiver gleans from retrospective analysis. We believe that combining both the real-time and retrospective data gathered by CGM is the most appropriate and impactful way to utilize these devices. This statement is also supported by the aforementioned literature.
For type 2 diabetes, both short and long-term unblinded CGM usage can be quite impactful as shown by Vigersky et al,24 and we assert that CGM should always be unblinded in this patient population as well. In our experience working with patients, we often see dramatic, lasting improvement during and after unblinded short-term CGM trials because real-time BG provide an eye-opening and immediate feedback loop that reinforces and/or dissuades positive and negative behaviors related to diet, exercise, and medications.
CGM offers a revolutionary way for patients to better monitor BG and reduce acute complications such as hypoglycemia by allowing patients to actively engage with their own information. In conclusion, we would like to ask one last question. Would you ever elect to blind your patient from their capillary glucose meter readings? If the answer is no, which we believe is likely, then why does this debate exist for CGM?
Footnotes
Abbreviations: BG, blood glucose; CGM, continuous glucose monitor; CSI, continuous subcutaneous insulin; HbA1c, hemoglobin A1c; SMBG, self-monitoring of blood glucose.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JP has served as a consultant for Dexcom Inc. SE has served as a consultant and advisory board member for and has received research funding from Dexcom Inc.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Tamborlane WV, Beck RW, Bode BW, et al. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464-1476. [DOI] [PubMed] [Google Scholar]
- 2. Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Riveline JP, Jollois FX, Messaoudi N, et al. Insulin-pump use in everyday practice: data from an exhaustive regional registry in France. Diabetes Metab. 2008;34:132-139. [DOI] [PubMed] [Google Scholar]
- 4. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363:311-320. [DOI] [PubMed] [Google Scholar]
- 5. Bailey TS, Chang A, Christiansen M. Clinical accuracy of a continuous glucose monitoring system with an advanced algorithm. J Diabetes Sci Technol. 2015;9:209-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blevins TC, Bode BW, Garg SK, et al. Statement by the American Association of Clinical Endocrinologists Consensus Panel on continuous glucose monitoring. Endocr Pract. 2010;16:730-745. [DOI] [PubMed] [Google Scholar]
- 7. Klonoff DC, Buckingham B, Christiansen JS, et al. Continuous glucose monitoring: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96:2968-2979. [DOI] [PubMed] [Google Scholar]
- 8. American Diabetes Association. Glycemic targets. Sec. 6 in Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S33-S40. [DOI] [PubMed] [Google Scholar]
- 9. Tanenberg R, Bode B, Lane W, et al. Use of the continuous glucose monitoring system to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc. 2004;79:1521-1526. [DOI] [PubMed] [Google Scholar]
- 10. Allen NA, Fain JA, Braun B, Chipkin SR. Continuous glucose monitoring counseling improves physical activity behaviors of individuals with type 2 diabetes: a randomized clinical trial. Diabetes Res Clin Pract. 2008;80:371-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pepper GM, Steinsapir J, Reynolds K. Effect of short-term iPRO continuous glucose monitoring on hemoglobin A1c levels in clinical practice. Diabetes Technol Ther. 2012;14:654-657. [DOI] [PubMed] [Google Scholar]
- 12. Heise T, Nosek L, Ronn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614-1620. [DOI] [PubMed] [Google Scholar]
- 13. Ziel FH, Davidson MB, Harris MD, Rosenberg CS. The variability in the action of unmodified insulin is more dependent on changes in tissue insulin sensitivity than on insulin absorption. Diabet Med. 1988;5:662-666. [DOI] [PubMed] [Google Scholar]
- 14. Heinemann L, Weyer C, Rauhaus M, Heinrichs S, Heise T. Variability of the metabolic effect of soluble insulin and the rapid- acting insulin analog insulin aspart. Diabetes Care. 1998;21:1910-1914. [DOI] [PubMed] [Google Scholar]
- 15. Kerssen A, deValk HW, Visser GHA. Day-to-day glucose variability during pregnancy in women with type 1 diabetes mellitus: glucose profiles measured with the continuous glucose monitoring. BJOG. 2004;11:919-924. [DOI] [PubMed] [Google Scholar]
- 16. Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pettus J, Price D, Edelman SV. How patients with type 1 diabetes translate continuous glucose monitoring data into diabetes management decisions. Endocr Pract. 2015;21(6):613-620. [DOI] [PubMed] [Google Scholar]
- 18. Polonsky WH, Fisher L, Schikman CH, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program Study. Diabetes Care. 2011;34(2):262-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanenberg RJ, Newton CA, Drake AJ. Confirmation of hypoglycemia in the “dead-in-bed” syndrome, as captured by a retrospective continuous glucose monitoring system. Endocr Pract. 2010;16:244-248. [DOI] [PubMed] [Google Scholar]
- 20. Patterson CC, Dahlquist G, Harjutsalo V, et al. Early mortality in EURODIAB population-based cohorts of type 1 diabetes diagnosed in childhood since 1989. Diabetologia. 2007;50:2439-2442. [DOI] [PubMed] [Google Scholar]
- 21. Jacobson AM, Musen G, Ryan CM, et al. , Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feltbower RG, Bodansky HJ, Patterson CC, et al. Acute complications and drug misuse are important causes of death for children and young adults with type 1 diabetes: results from the Yorkshire Register of Diabetes in Children and Young Adults. Diabetes Care. 2008;31:922-926. [DOI] [PubMed] [Google Scholar]
- 23. Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia. 2006;49:298-305. [DOI] [PubMed] [Google Scholar]
- 24. Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]