Abstract
Background:
Time-varying dynamics is one of the main issues for achieving safe blood glucose control in type 1 diabetes mellitus (T1DM) patients. In addition, the typical disturbances considered for controller design are meals, which increase the glucose level, and physical activity (PA), which increases the subject’s sensitivity to insulin. In previous works the authors have applied a linear parameter-varying (LPV) control technique to manage unannounced meals.
Methods:
A switched LPV controller that switches between 3 LPV controllers, each with a different level of aggressiveness, is designed to further cope with both unannounced meals and postprandial PA. Thus, the proposed control strategy has a “standard” mode, an “aggressive” mode, and a “conservative” mode. The “standard” mode is designed to be applied most of the time, while the “aggressive” mode is designed to deal only with hyperglycemia situations. On the other hand, the “conservative” mode is focused on postprandial PA control.
Results:
An ad hoc simulator has been developed to test the proposed controller. This simulator is based on the distribution version of the UVA/Padova model and includes the effect of PA based on Schiavon.1 The test results obtained when using this simulator indicate that the proposed control law substantially reduces the risk of hypoglycemia with the conservative strategy, while the risk of hyperglycemia is scarcely affected.
Conclusions:
It is demonstrated that the announcement, or anticipation, of exercise is indispensable for letting a mono-hormonal artificial pancreas deal with the consequences of postprandial PA. In view of this the proposed controller allows switching into a conservative mode when notified of PA by the user.
Keywords: linear parameter-varying control, postprandial physical activity, switched systems, type 1 diabetes mellitus
There has been an intense research activity in the last decades to produce an artificial pancreas (AP) that may help type 1 diabetes mellitus (T1DM) patients. Automatic feedback control of blood glucose levels has been an active research topic since the 1970s,2 and with the development of the continuous glucose monitoring (CGM),3 it has gained momentum only in recent years.4-7 In addition, very recently it has been tested in an outpatient setting.8-12 An important issue to be considered here is the possibility that an AP could help patients in their everyday life by reducing the need for self-management strategies.
The typical disturbances considered for controller design are meals, which increase the glucose level, and physical activity (PA), which increases the subject’s sensitivity to insulin. Recently, models of PA have been developed in Schiavon,1 Breton,13 Dalla Man et al,14 Jacobs et al,15 Ben Brahim et al,16 Schiavon et al,17 and references therein. These models could be included in a simulator to test the performance of different control algorithms under PA. The general idea is to produce similar results with this disturbance in the glucose-insulin regulation, that is, eliminate the need of PA anticipation. In this respect, an automatic insulin adjustment via continuous subcutaneous insulin infusion (CSII) at the start of PA seems to be a reasonable decision. Therefore, several works focused on detecting PA have been presented.18-20 Furthermore, multiple clinical studies have shown that the chances of experiencing hypoglycemia decrease when adjusting the insulin injection at the beginning of PA.21-23 However, it is worth mentioning that none of them refer to postprandial PA, which is the main topic of this article. PA approximately 2 hours after a meal seems to be a worst case situation, because even if the insulin infusion is reduced, or the pump is switched off, there is still high insulin on board (IOB), which under PA (that increases insulin sensitivity) will increase the risk of hypoglycemia. In Turksoy et al,24 an adaptive control with unannounced meals and exercise is presented. The PA in some of those cases could be considered as postprandial, but either the PA was initiated too soon or far after the meal, or the patient had low IOB during exercise, or he/she ate after completion of PA. In those situations where the PA was performed approximately 2 hours after a meal and with an insulin bolus injected at the mealtime, the patient had to be rescued with a snack. As stated in Riddell et al,25 one of the factors affecting blood glucose fluctuations during PA are the amount of insulin and food in the body at the time of the activity.
Previous work by the authors using time-varying controllers, that have been tested in silico, were presented in Colmegna et al.26 In addition, in Colmegna and Sánchez-Peña,27 similar results were achieved in a linear parameter-varying (LPV) controller framework. An extension of this latter proposal is an approach that switches between a selection of multiple LPV controllers that have been designed for different objectives. In Colmegna et al,28 the authors have applied that strategy to regulate the blood glucose level in response to unannounced meals by switching between 2 LPV controllers. One controller is dedicated to dealing with large and persistent hyperglycemic excursions as in the postprandial stage, and the second controller is responsible for glucose control at all other times. The switch is triggered via an estimator that detects persistent high glucose values. This is similar to the proposal in Gondhalekar et al.29
In this article, the strategy is to extend these results to patients performing late postprandial PA by generating a third LPV controller that is more conservative. Here, late postprandial is defined as approximately 2 hours after a meal. The possibility of estimating the appearance of PA is also explored, so that exercise anticipation is unnecessary. Unfortunately, as is demonstrated later, this is not possible, at least in mono-hormonal (insulin) control and under postprandial PA. PA affects glucose differently and may induce either hyperglycemia or hypoglycemia. The type of PA is a key (aerobic or anaerobic) and the amount of available insulin and its delayed peak of action is the other major contribution. Most of the time after the meal the patient has a larger amount of “free” insulin that allows glucose uptake by the muscles. No matter how fast the estimator detects the presence of PA, a hypoglycemia episode is likely to occur in mono-hormonal control. This happens when the exercise is initiated in the (late) postprandial period and the controller reacts according to the estimator output, or, in an open-loop setting, with no basal attenuation or pump suspension approximately 1 hour prior to the PA.17,30 It is worth noting that hypoglycemia episodes have been detected even in healthy subjects during postprandial exercise.31 Hence, (late) postprandial PA needs to be anticipated to avoid PA induced hypoglycemia. Despite this limitation, the notion of switched LPV control can still be used, but with a controller purposefully designed for such a situation and triggered by the user.
The article is organized as follows. The next section presents the methods based on the PA model, details the inherent problems which appear in postprandial PA, and also includes the controller design based on a previous work by the authors. Numerical simulations with the new switched LPV controller are provided in the third section. A discussion of these results and future research directions are presented in the fourth section, and conclusions are drawn in the fifth section.
Methods
PA Model
Although there are several works that aim toward providing better understanding of the effect of exercise on glucose physiology, modeling PA is still an open problem. Glucose excursions during and after exercise do not only depend on the type of PA, but also on many other factors such as duration of diabetes, gender and fitness level. In Breton13 and Dalla Man et al,14 a mathematical model that links heart rate (HR) with PA was developed. However, in van Bon et al,30 no correlation could be demonstrated between the increase in HR and the decrease in glucose concentrations during moderate-intensity exercise.
A well-known effect related to PA is the increase in insulin sensitivity. In Schiavon et al,31 that effect was quantified during moderate exercise in 4 periods of 15 minutes each separated by a 5-minute rest, in the postprandial state. In this work, that result is included in the FDA-accepted metabolic simulator as reported in Schiavon1 and Schiavon et al.17 Therefore, the model parameter that represents the insulin sensitivity is modified as follows:
where
with , the subject’s sensitivity to insulin at rest, and the exercise start time.
As indicated in Schiavon et al17 and Schiavon et al,31 this method has some limitations. First, it assumes a nonphysiological step increase in at . Furthermore, it only describes the effect of exercise on insulin-dependent glucose utilization, although insulin-independent effects are also known to be triggered due to the PA. Despite the aforementioned drawbacks, it is worth noting that the results presented in Schiavon et al31 were obtained using the triple-tracer technique for the first time to measure the PA effect on glucose metabolism in the postprandial period. In addition, although with this modeling framework we can only simulate moderate PA in the (late) postprandial period, we understand that, as mentioned in the Introduction, this is one of the most difficult control situations due to the large increase in the subject’s sensitivity to insulin at the time he/she still has high IOB. Finally, this physiological effect can be incorporated into the FDA-accepted UVA/Padova T1DM simulator in a straightforward way.1,17
Control Problems in Postprandial PA
As stated in Schiavon et al,17 there are 2 important questions regarding the inclusion of postprandial PA in closed-loop control. Here, we add a third one.
Is it necessary to anticipate the incoming PA to avoid postprandial hypoglycemia?
In case the answer is yes, which is the best closed-loop strategy?
Can bihormonal control help in this regard and avoid the necessity of anticipating PA?
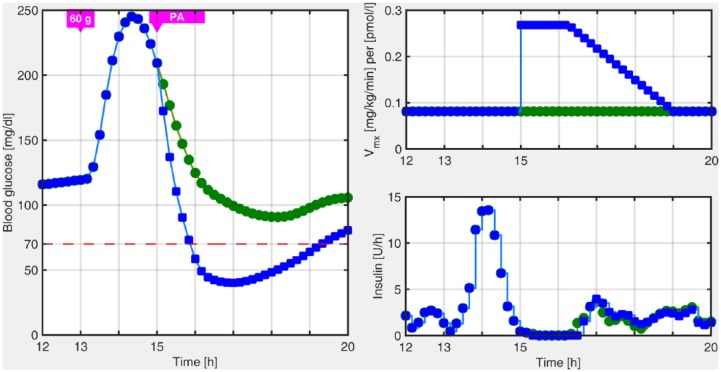
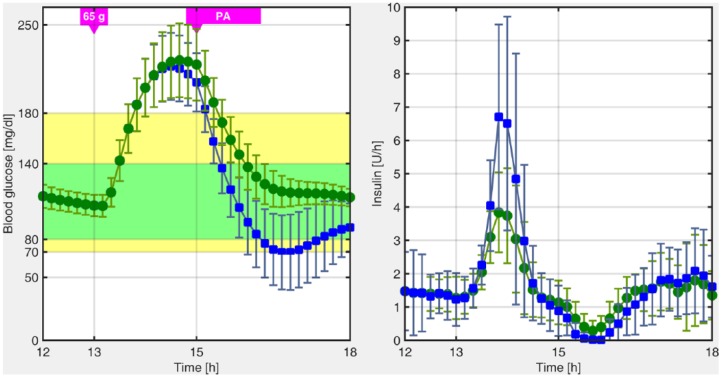
In previous works in the LPV framework, we have focused on unannounced meal perturbations. This was handled by means of an estimator that detects persistent high glucose values, and by switching to a more aggressive LPV controller, which was previously designed. Specifically, the controller switches to a hyperglycemia operating mode when high and rising glucose values are detected, for example, after a meal. The LPV controller in this region is more aggressive because it is focused on reducing the hyperglycemia peak. However, if the patient is involved in PA in the postprandial state, hypoglycemia, which occurs when the blood glucose level drops below 70 mg/dl, could not be prevented, as is depicted in Figure 1.
Figure 1.
Postprandial blood glucose excursion obtained with the switched-LPV strategy with (blue squares) and without (green circles) unannounced PA.
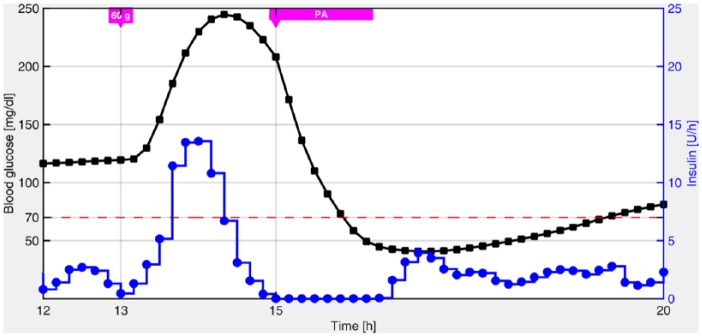
The same idea applied to unannounced meals could be attempted with PA. The goal would be to have a similar “exercise estimator” that could anticipate this perturbation, and thereby, avoid the user’s involvement, for example, unannounced PA. However, it can be shown by means of in silico simulations, that even if we have a perfect PA estimator, the postprandial hypoglycemia may not be avoided. In fact, in Figure 2, it is shown that the strategy of cutting the total insulin infusion to zero at the instant when PA is initiated is still futile, at least with a mono-hormonal control strategy. The latter was also detected experimentally in van Bon et al.30 Basically, the problem is that the amount of insulin injected at meal times could be excessive under the effect of PA. Unfortunately, considering that PA can be detected, at the earliest, at the time it is initiated, it is impossible to make a decision at meal times which involves postprandial PA, before PA has been detected. In addition, only the patient can anticipate PA before it happens.
Figure 2.
Blood glucose response (black squares) obtained with the autonomous switched-LPV strategy when insulin administration (blue circles) is interrupted during PA.
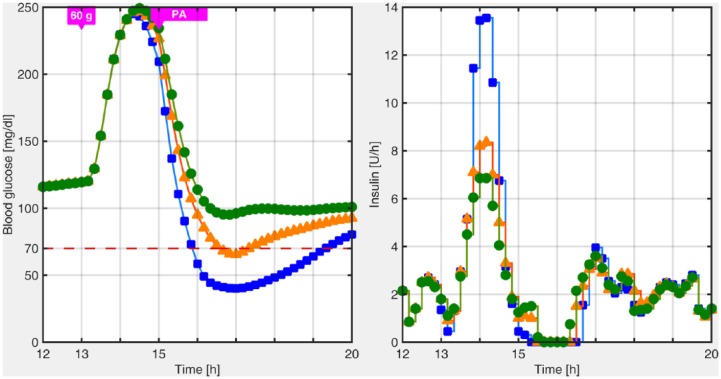
Once we agree with the fact that PA anticipation is necessary, the best strategy can rely on another LPV controller that may be designed for PA situations, that is, a third, more conservative controller, in the switched-LPV control approach. The switching signal in this case would be triggered by the patient 30 minutes before the meal, because there is no way to anticipate this information in a real situation by CGM feedback. The conservative region will be active during 5 hours for it to remain active during almost the whole postprandial period, and thereafter, the other LPV controllers will automatically take over the insulin delivery. Similar postprandial periods have been defined in Colmegna et al,26 Colmegna et al,28 Monnier and Colette,32 Nimri et al,33 and Breton et al.34 The comparison between this new, more conservative approach, that will be detailed in the next section, and the previous presented in Colmegna et al28 is depicted in Figure 3. As shown in that figure, even without switching to the most aggressive controller at mealtime, the postprandial hypoglycemia due to PA cannot be avoided.
Figure 3.
Postprandial blood glucose excursion when the control law switches to the most aggressive controller at meal time (blue squares), when the control law does not switch to the most aggressive controller at meal time (orange triangles), and when the control law switches to the conservative controller 30 minutes before the meal (green circles).
Controller Design
The following is a very brief summary of work presented in Colmegna et al.28 In that work, for each in silico Adult of the UVA/Padova simulator, 2 LPV controllers with were synthesized. Controller was designed to control most of the time, while was applied only when high and rising glucose values were detected, for example, after a meal.
The model structure presented in van Heusden et al,35 and slightly adapted in Colmegna et al,26 was considered for design purposes. The main advantage of such a model structure is that it is a simple third-order model with a personalized gain based solely on a priori clinical information: total daily insulin (TDI), carbohydrate ratio (CR), and body weight (BW). Therefore, for each Adult , an individualized transfer function , from the insulin delivery input (pmol/min) to the glucose concentration output (mg/dl), can be obtained. Note that depends on both indexes: and . This is because the gain of the transfer function is intentionally smaller than the gain of to obtain a more aggressive control law when large and persistent hyperglycemic excursions are detected.
In this work, a third LPV controller is included to manage postprandial PA. To that end, the gain of the corresponding transfer function is purposefully defined to be greater than the gain of . In this way, is associated with a more conservative model, and therefore, with a less aggressive control law.
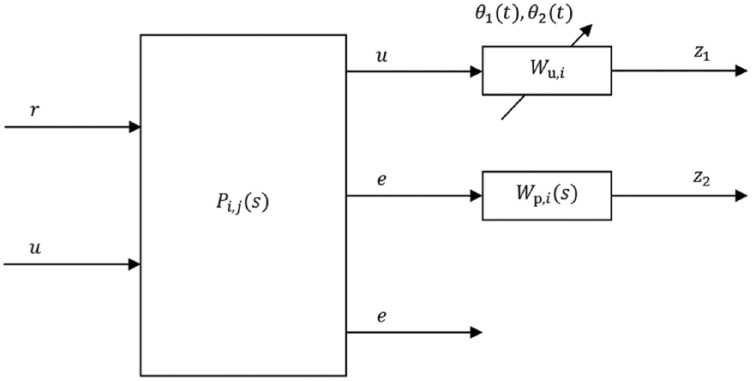
The augmented continuous-time model for controller design is depicted in Figure 4, where:
Figure 4.
Augmented model for controller design.
and are, respectively, the reference and error signals, is the control action, and and are the design weights. As shown in the same figure, 2 parameters have been included in each augmented model to adapt the controller during the closed-loop implementation. The time-varying parameters are and . The first parameter is real-time measurable and depends on the glucose level measured by the CGM. The second parameter depends on , which are the estimated current and basal plasma insulin levels, respectively. The estimation is performed through the subcutaneous insulin model proposed in Dalla Man et al,36 considering its mean population values. In the case of , the input to the model is the current injected insulin, and in the case of , the basal insulin dosage. Note that can be obtained off-line, before the simulation.
The performance and actuator weights and are designed such that the open-loop model matrices depend affinely on the parameters , . Also, the parameter regions are convex polytopes with a finite number of vertices, that are known. Hence, the optimization problem related to the LPV controller synthesis can be stated in terms of a finite number of linear matrix inequalities (LMIs). Specifically, for each LPV controller, the problem is solved in terms of LMIs, that is, a common single quadratic Lyapunov function for each set of vertices. Note that the vertex controllers can be synthesized off-line.
During the implementation phase, the LPV controllers for can be computed as follows:
where are the polytopic coordinates of the measured parameter , and are the vertices of the parameter regions.
The LPV “fast poles” problem can be solved by defining a convenient LMI region to keep the poles of each “frozen” linear time invariant closed-loop system (holding the parameter fixed) in a prescribed region of the complex plane.37 These pole region is selected at least 10 times slower than the controller sampling time minutes.
As previously mentioned, automatically takes over the insulin delivery when high and rising glucose values are detected. In that way, a hyperglycemia detection algorithm has been implemented based on and an estimation of its rate of change, which is obtained by a causal fourth-order Savitzky-Golay filter.38,39 On the other hand, is applied manually by user notification.
Results
The glucose-insulin model presented in Dalla Man et al40 was modified to include the effect of PA in the postprandial state. All 11 in silico adults (one is an average patient) of the distribution version of the UVA/Padova metabolic simulator are considered for simulations, using CGM as the sensor and a generic CSII pump.
Due to the fact that closed-loop performance of the switched-LPV strategy with several unannounced meals has already been tested in Colmegna et al,28 here, simulations are exclusively focused on postprandial PA. Therefore, the protocol has only a duration of 6 hours. It starts at noon with the switched-LPV controller closing the loop, and the conservative LPV controller is triggered manually at 12:30 pm. Then, a meal of 65 g carbohydrates is ingested at 1 pm, and thereafter, a moderate-intensity exercise commences at 3 pm. Finally, the controller automatically leaves the conservative mode, that is, , at 5:30 pm and returns to the autonomous mode, that is, . The case where PA is not announced is also tested for comparison purposes.
The average time responses obtained with and without postprandial PA announcement are depicted in Figure 5. As shown in that figure, if exercise is not announced, a large insulin spike appears, because the controller triggers into an aggressive mode when the meal is detected. In that way, subsequent PA makes postprandial hypoglycemia unavoidable. On the other hand, as can be seen from Figure 5, if the controller is triggered into the conservative mode 30 minutes before the meal, postprandial hypoglycemia does not occur.
Figure 5.
Average closed-loop responses for all the in silico adults of the distribution version of the UVA/Padova simulator with (green circles) and without (blue squares) PA anticipation. The lines with markers are the mean values, and the mean STD values are represented by vertical bars, every 10 minutes. The filled yellow and green regions represent the 70-180 mg/dl and 80-140 mg/dl ranges, respectively.
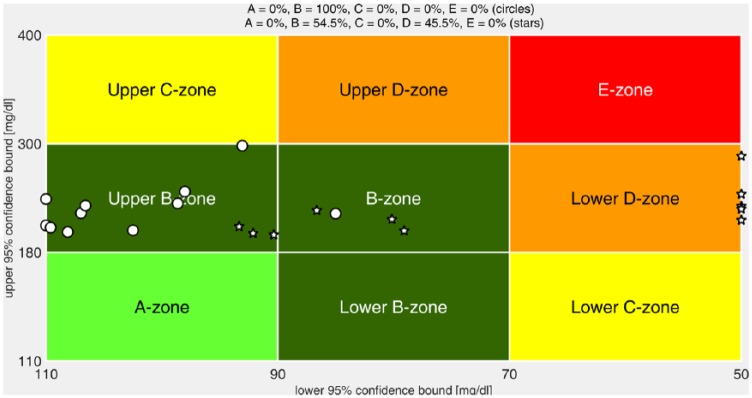
The control variability grid analysis (CVGA) plots41 and the average results for announced and unannounced PA are presented in Figure 6 and Table 1, respectively. Both the CVGA plots, as well as the average results, are computed based on the 5-hour time interval following the start of the meal, considering a 95% confidence interval. It is noteworthy that although the risk of hypoglycemia is substantially reduced with the conservative strategy, the risk of hyperglycemia is scarcely affected. In fact, hyperglycemia is slightly reduced (HBGI = 5.8 to 5.3) comparing with the case of standard autonomous switching from normal () mode to aggressive () mode and no exercise.
Figure 6.
CVGA plots of the closed-loop responses of all the in silico adults of the distribution version of the UVA/Padova simulator with (circles) and without (stars) PA anticipation. The CVGA categories represent different levels of glucose control, as follows: accurate (A zone), benign deviation into hypo/hyperglycemia (lower/upper B zones), benign control (B zone), overcorrection of hypo/hyperglycemia (upper/lower C zone), failure to manage hypo/hyperglycemia (lower/upper D zone), and erroneous control (E zone).41
Table 1.
Comparison Between the Average Results for All the in Silico Adults of the Distribution Version of the UVA/Padova Simulator Obtained With and Without PA Anticipation.
| PA anticipation |
Yes |
No |
||
|---|---|---|---|---|
| Postprandial PA | Yes | No | Yes | No |
| Max BG (mg/dl) | 224 | 224 | 219 | 219 |
| Min BG (mg/dl) | 104 | 107 | 64 | 105 |
| % time in (70 180) mg/dl | 68.6 | 50.4 | 60.0 | 65.4 |
| % time > 300 mg/dl | 0.0 | 0.0 | 0.0 | 0.0 |
| % time > 180 mg/dl | 31.4 | 49.6 | 26.1 | 34.6 |
| % time < 70 mg/dl | 0.0 | 0.0 | 14.3 | 0.0 |
| % time < 50 mg/dl | 0.0 | 0.0 | 8.5 | 0.0 |
| # < 80 mg/dl | 0 | 0 | 6 | 0 |
| # < 70 mg/dl | 0 | 0 | 5 | 0 |
| # < 50 mg/dl | 0 | 0 | 5 | 0 |
| LBGI | 0 | 0 | 4.5 | 0 |
| HBGI | 5.3 | 8.6 | 4.2 | 5.8 |
HBGI, high blood glucose index; LBGI, low blood glucose index.
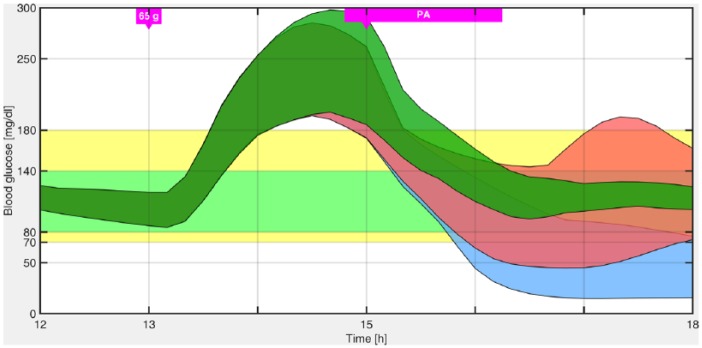
Figure 7 illustrates that reducing the insulin infusion to the basal rate before the PA and up to its completion produces a high risk of hypoglycemia. Furthermore, in that figure it is shown that even switching off the pump during that period of time, hypoglycemia could not be avoided in all patients, and, in addition, increase glucose variability. One of the reasons is that 30 minutes before the PA the controller already injected the insulin “bolus” in response to the meal. Hence, even if the insulin infusion is reduced, or the pump is switched off, there is still a large amount of free insulin, which under PA (that increases insulin sensitivity) will increase the risk of hypoglycemia. This is the reason why, as stated above, it is switched to the most conservative LPV control region 30 minutes previous to the meal, so that the controller is less aggressive in response to the CHO ingestion, and as a consequence, when PA begins there is less IOB. Finally, in Table 2 outcomes for different meal sizes (and therefore different IOB at the start of PA) using the switched LPV controller with and without PA anticipation are compared.
Figure 7.
Envelopes of the minimum and maximum responses for all the in silico adults of the distribution version of the UVA/Padova simulator with the conservative LPV strategy (filled green region), pump suspension (filled red region), and insulin infusion reduction to the basal rate (filled light blue region).
Table 2.
Comparison Between the Average Results for All the in Silico Adults of the Distribution Version of the UVA/Padova Simulator to Different Unannounced Meal Sizes Obtained Using the Switched LPV Controller With and Without PA Anticipation.
| Meal size (gCHO) |
55 |
60 |
65 |
70 |
75 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| PA anticipation | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No |
| IOB at tex (U) | 3.1 | 3.9 | 3.2 | 4.1 | 3.3 | 4.3 | 3.3 | 4.4 | 3.4 | 4.7 |
| Max BG (mg/dl) | 205 | 201 | 214 | 210 | 224 | 219 | 233 | 228 | 243 | 236 |
| Min BG (mg/dl) | 104 | 69 | 104 | 66 | 104 | 64 | 103 | 61 | 102 | 57 |
| % time in (70 180) mg/dl | 76.6 | 67.7 | 71.9 | 62.9 | 68.6 | 60.0 | 66.0 | 57.1 | 64.0 | 53.9 |
| % time > 300 mg/dl | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.6 | 1.1 |
| % time > 180 mg/dl | 23.4 | 19.3 | 28.1 | 23.3 | 31.4 | 26.1 | 34.0 | 28.1 | 36.0 | 29.4 |
| % time < 70 mg/dl | 0.0 | 13.0 | 0.0 | 13.8 | 0.0 | 14.3 | 0.0 | 14.8 | 0.0 | 16.7 |
| % time < 50 mg/dl | 0.0 | 6.5 | 0.0 | 7.5 | 0.0 | 8.5 | 0.0 | 10.0 | 0.0 | 11.7 |
| # < 80 mg/dl | 0 | 6 | 0 | 6 | 0 | 6 | 0 | 7 | 1 | 8 |
| # < 70 mg/dl | 0 | 5 | 0 | 5 | 0 | 5 | 0 | 5 | 0 | 7 |
| # < 50 mg/dl | 0 | 5 | 0 | 5 | 0 | 5 | 0 | 5 | 0 | 5 |
| LBGI | 0 | 3.5 | 0 | 4.1 | 0 | 4.5 | 0.1 | 5.1 | 0.1 | 6.4 |
| HBGI | 3.9 | 3.2 | 4.6 | 3.6 | 5.3 | 4.2 | 6.0 | 4.7 | 6.8 | 5.2 |
gCHO, grams of carbohydrates; HBGI, high blood glucose index; LBGI, low blood glucose index.
Discussion
Here, the questions posed in Schiavon et al17 regarding the inclusion of PA in closed-loop control are analyzed.
Is it necessary to anticipate the incoming PA to avoid postprandial hypoglycemia?
In case the answer is yes, which is the best closed-loop strategy?
The answer to the first one is yes, and the solution presented here involves a switched LPV controller based on current research by the authors described above, with the addition of a third more conservative LPV region used in the case of PA.
Furthermore, a third question, whether a bihormonal control could allow the patient to not be part of the control loop, is also considered. Although this is not yet reliable from the technological point of view,42 it is worth exploring and will be the matter of future research. In that case, an estimator of PA should be included as part of the control so that the patient would not have to trigger a signal before a meal. Such a controller would increase glucagon infusion a short time after the PA has been detected to compensate and avoid a hypoglycemia event. In (late) postprandial PA, where the IOB is high and glucose is decreasing, injecting glucagon as soon as possible is recommended to avoid hypoglycemia.42,43 In addition, it is important to emphasize that in nondiabetics there is a rise in glucagon and a reduction in insulin release at the onset of mild to moderate aerobic PA.24 Hence, increasing the dose of glucagon as soon as PA is detected is a prudent decision.
Several articles indicate the plausibility of bihormonal control in the presence of PA.44-48 In particular, in the last 2 articles, patients receive a lunch at approximately 12:30 pm and PA starts at 4 pm (at 6 pm they receive another meal). At that time (4 pm), a peak in the infusion of glucagon can be observed (Figure 1 in Russell et al47 and Figures 2 and 3 in El-Khatib et al48). In van Bon et al,44 patients receive 40 g of CHO during lunch, 2 hours later they start moderate PA and 1.5 hours later they receive a 60 g CHO meal. The presence of that meal near to the PA has an influence, as mentioned in that article. Nevertheless, it concludes that subcutaneous glucagon administration was almost always effective to prevent hypoglycemia when glucose was falling rapidly after exercise.
Conclusions
A switched-LPV control strategy was presented to deal with unannounced meals and (late) postprandial PA. It is clear that a more detailed description of PA is necessary to include both insulin-dependent and insulin-independent effects on glucose absorption during exercise. The actual simulator used here only describes the worst case scenario, that is, postprandial PA, and includes only the insulin-dependent effects.
From this work, it was concluded that, at least under mono-hormonal (insulin) control, patients may need to announce at least 30 minutes before meals the intention to perform PA in the (late) postprandial stage so that no hypoglycemia is induced. In this case, the user simply “pushes a button” and this signal triggers a more conservative LPV controller. This is much easier than in meal announcement strategies where the insulin bolus needs to be computed beforehand based on the meal carbohydrate content.
The switched-LPV technique can be seen as a generalization of previous results obtained by the authors. It can be used both in an unannounced (meals) and announced (PA) fashion, and allows both automatic and user-defined switches for other possible situations, for example, stress. The results are promising and will be tested against the complete UVA/Padova simulator when this includes a suitable exercise model.
Footnotes
Abbreviations: AP, artificial pancreas; BW, body weight; CGM, continuous glucose monitoring; CR, carbohydrate ratio; CSII, continuous subcutaneous insulin infusion; CVGA, control variability grid analysis; HR, heart rate; IOB, insulin on board; LMI, linear matrix inequality; LPV, linear parameter-varying; PA, physical activity; TDI, total daily insulin; T1DM, type 1 diabetes mellitus.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: PHC and RSSP were supported by the Nuria (Argentina) and Cellex (Spain) Foundations. RG, ED, and FJD were supported by the following grants: NIH DP3DK094331 and NIH R01DK085628.
References
- 1. Schiavon M. Modeling the effect of physical activity on postprandial effect. PhD dissertation, University of Padova; 2014. [Google Scholar]
- 2. Clements AH, Chang PH, Myers RW. The development of Biostator, a glucose controlled insulin infusion system (GCIIS). Horm Metab Res. 1977;7:23-33. [PubMed] [Google Scholar]
- 3. Hovorka R. Continuous glucose monitoring and closed-loop systems. Diabet Med. 2006;23(1):1-12. [DOI] [PubMed] [Google Scholar]
- 4. Cobelli C, Dalla Man C, Sparacino G, Magni L, De Nicolao G, Kovatchev BP. Diabetes: models, signals and control. IEEE Rev Biomed Eng. 2009;2:54-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harvey R, Wang Y, Grosman B, et al. Quest for the artificial pancreas. IEEE Eng Med Biol Mag. 2010;29(2):53-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cobelli C, Renard E, Kovatchev BP. Artificial pancreas: past, present, future. Diabetes. 2011;60(11):2672-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zisser HC. Clinical hurdles and possible solutions in the implementation of closed-loop control in type 1 diabetes mellitus. J Diabetes Sci Technol. 2011;5(5):1283-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368(9):824-833. [DOI] [PubMed] [Google Scholar]
- 9. Doyle FJ, III, Huyett LM, Lee JB, Zisser HC, Dassau E. Closed loop artificial pancreas systems: engineering the algorithms. Diabetes Care. 2014;37(5):1191-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371(4):313-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kovatchev BP, Renard E, Cobelli C, et al. Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes Care. 2014;37(7):1789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hovorka R, Elleri D, Thabit H, et al. Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2014;37(5):1204-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Breton MD. Physical activity—the major unaccounted impediment to closed loop control. J Diabetes Sci Technol. 2008;2(1):169-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dalla Man C, Breton MD, Cobelli C. Physical activity into the meal glucose-insulin model of type 1 diabetes: in silico studies. J Diabetes Sci Technol. 2009;3(1):56-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jacobs PG, Resalat N, El Youssef J, et al. Incorporating an exercise detection, grading, and hormone dosign algorithm into the artificial pancreas using accelerometry and heart rate. J Diabetes Sci Technol. 2015;9(6):1175-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ben Brahim N, Place J, Renard E, Breton MD. Identification of main factors explaining glucose dynamics during and immediately after moderate exercise in patients with type 1 diabetes. J Diabetes Sci Technol. 2015;9(6):1185-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schiavon M, Dalla Man C, Kudva YC, Basu A, Cobelli C. In silico optimization of basal insulin infusion rate during exercise: implication for artificial pancreas. J Diabetes Sci Technol. 2013;7(6):1461-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turksoy K, Paulino TM, Zaharieva DP, et al. Classification of physical activity: information to artificial pancreas control systems in real time. J Diabetes Sci Technol. 2015;9(6):1200-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dasanayake IS, Bevier WC, Castorino K, et al. Early detection of physical activity for people with type 1 diabetes mellitus. J Diabetes Sci Technol. 2015;9(6):1236-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dasanayake IS, Seborg DE, Pinsker JE, Doyle FJ, III, Dassau E. Empirical dynamic model identification for blood-glucose dynamics in response to physical activity. In: Proceedings of the IEEE 54th Annual Conference on Decision and Control (CDC) Osaka, Japan; 2015:3834-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsalikian E, Kollman C, Tamborlane WB, et al. Prevention of hypoglycemia during exercise in children with type 1 diabetes by suspending basal insulin. Diabetes Care. 2006;29(10):2200-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Danne T, Tsioli C, Kordonouri O, et al. The PILGRIM study: in silico modeling of a predictive low glucose management system and feasibility in youth with type 1 diabetes during exercise. Diabetes Technol Ther. 2014;16(6):338-347. [DOI] [PubMed] [Google Scholar]
- 23. Breton MD, Brown SA, Karvetski CH, et al. Adding heart rate signal to a control-to-range artificial pancreas system improves the protection against hypoglycemia during exercise in type 1 diabetes. Diabetes Technol Ther. 2014;16(8):506-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Turksoy K, Bayrak ES, Quinn L, Littlejohn E, Cinar A. Multivariable adaptive closed-loop control of an artificial pancreas without meal and activity announcement. Diabetes Technol Ther. 2013;15(5):386-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Riddell MC, Zaharieva DP, Yavelberg L, Cinar A, Jamnik VK. Exercise and the development of the artificial pancreas: one of the more difficult series of hurdles. J Diabetes Sci Technol. 2015;9(6):1217-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Colmegna P, Sánchez-Peña RS, Gondhalekar R, Dassau E, Doyle FJ., III Reducing risks in type 1 diabetes using H∞ control. IEEE Trans Biomed Eng. 2014;61(12):2939-2947. [DOI] [PubMed] [Google Scholar]
- 27. Colmegna P, Sánchez-Peña RS. Linear parameter-varying control to minimize risks in type 1 diabetes. In: Proceedings of the 19th IFAC World Congress Cape Town, South Africa; 2014:9253-9257. [Google Scholar]
- 28. Colmegna P, Sánchez-Peña RS, Gondhalekar R, Dassau E, Doyle FJ., III Switched LPV glucose control in type 1 diabetes [published online ahead of print October 5, 2015]. IEEE Trans Biomed Eng. doi: 10.1109/TBME.2015.2487043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gondhalekar R, Dassau E, Doyle FJ., III MPC design for rapid pump-attenuation and expedited hyperglycemia response to treat T1DM with an artificial pancreas. In: Proceedings of the AACC American Control Conference Portland, OR; 2014:4224-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Bon AC, Verbitskiy E, von Basum G, Hoekstra JB, DeVries JH. Exercise in closed-loop control: a major hurdle. J Diabetes Sci Technol. 2011;5(6):1337-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schiavon M, Hinshaw L, Mallad A, et al. Postprandial glucose fluxes and insulin sensitivity during exercise: a study in healthy individuals. Am J Physiol Endocrinol Metab. 2013;305(4):E557-E566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Monnier L, Colette C. Target for glycemic control. Diabetes Care. 2009;32(suppl 2):S199-S204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nimri R, Atlas E, Ajzensztejn M, Miller S, Oron T, Phillip M. Feasibility study of automated overnight closed-loop glucose control under MD-logic artificial pancreas in patients with type 1 diabetes: the DREAM Project. Diabetes Technol Ther. 2012;14(8):728-735. [DOI] [PubMed] [Google Scholar]
- 34. Breton M, Farret A, Bruttomesso D, et al. Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes. 2012;61(9):2230-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Heusden K, Dassau E, Zisser HC, Seborg DE, Doyle FJ., III Control-relevant models for glucose control using a priori patient characteristics. IEEE Trans Biomed Eng. 2012;59(7):1839-1849. [DOI] [PubMed] [Google Scholar]
- 36. Dalla Man C, Raimondo DM, Rizza RA, Cobelli C. GIM, simulation software of meal glucose-insulin model. J Diabetes Sci Technol. 2007;1(3):323-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ghersin AS, Sánchez-Peña RS. Transient shaping of LPV systems. In: Proceedings of the European Control Conference Porto, Portugal; 2001:3080-3085. [Google Scholar]
- 38. Savitzky A, Golay MJ. Smoothing and differentiation of data by simplified least squares procedures. Anal Chem. 1964;36(8):1627-1639. [Google Scholar]
- 39. Keenan DB, Cartaya R, Mastrototaro JJ. Accuracy of a new real-time continuous glucose monitoring algorithm. J Diabetes Sci Technol. 2010;4(1):111-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dalla Man C, Micheletto F, Lv D, Breton M, Kovatchev B, Cobelli C. The UVA/PADOVA type 1 diabetes simulator: new features. J Diabetes Sci Technol. 2014;8(1):26-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Magni L, Raimondo DM, Dalla Man C, et al. Evaluating the efficacy of closed-loop glucose regulation via control-variability grid analysis. J Diabetes Sci Technol. 2008;2(4):630-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bakhtiani PA, El Youssef J, Duell AK, et al. Factors affecting the success of glucagon delivered during an automated closed-loop system in type 1 diabetes. J Diabetes Complications. 2015;29(1):93-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bakhtiani PA, Zhao LM, El Youssef J, Castle JR, Ward WK. A review of artificial pancreas technologies with an emphasis on bi-hormonal therapy. Diabetes Obes Metab. 2013;15(12):1065-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Bon AC, Jonker LD, Koebrugge R, Koops R, Hoekstra JB, DeVries JH. Feasibility of a bihormonal closed-loop system to control postexercise and postprandial glucose excursions. J Diabetes Sci Technol. 2012;6(5):1114-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haidar A, Legault L, Dallaire M, et al. Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. CMAJ. 2013;185(4):297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Bon AC, Luijf YM, Koebrugge R, Koops R, Hoekstra JB, DeVries JH. Feasibility of a portable bihormonal closed-loop system to control glucose excursions at home under free-living conditions for 48 hours. Diabetes Technol Ther. 2014;16(3):131-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Russell SJ, El-Khatib FH, Nathan DM, Magyar KL, Jiang J, Damiano ER. Blood glucose control in type 1 diabetes with a bihormonal bionic endocrine pancreas. Diabetes Care. 2012;35(11):2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. El-Khatib FH, Russell SJ, Magyar KL, et al. Autonomous and continuous adaptation of a bihormonal bionic pancreas in adults and adolescents with type 1 diabetes. J Clin Endocrinol Metab. 2014;99(5):1701-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]