Abstract
Background:
The majority of individuals with type 1 diabetes today have glucose levels exceeding guidelines. The primary aim of this study was to evaluate whether continuous glucose monitoring (CGM), using the Dexcom G4 stand-alone system, improves glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections (MDI).
Methods:
Individuals with type 1 diabetes and inadequate glycemic control (HbA1c ≥ 7.5% = 58 mmol/mol) treated with MDI were randomized in a cross-over design to the Dexcom G4 versus conventional care for 6 months followed by a 4-month wash-out period. Masked CGM was performed before randomization, during conventional treatment, and during the wash-out period to evaluate effects on hypoglycemia, hyperglycemia, and glycemic variability. Questionnaires were used to evaluate diabetes treatment satisfaction, fear of hypoglycemia, hypoglycemia confidence, diabetes-related distress, overall well-being, and physical activity during the different phases of the trial. The primary endpoint was the difference in HbA1c at the end of each treatment phase.
Results:
A total of 205 patients were screened, of whom 161 were randomized between February and December 2014. Study completion is anticipated in April 2016.
Conclusions:
It is expected that the results of this study will establish whether using the Dexcom G4 stand-alone system in individuals with type 1 diabetes treated with MDI improves glycemic control, reduces hypoglycemia, and influences quality-of-life indicators and glycemic variability.
Keywords: type 1 diabetes mellitus, CGM, randomized trial, glycemic control, quality of life
A keystone in preventing complications in patients with type 1 diabetes is good glycemic control.1 Today, intensive glycemic treatment is generally achieved through multiple daily insulin injections (MDI) or an insulin pump, also termed continuous subcutaneous insulin infusion (CSII).2 Regular capillary self-measured blood glucose values have been crucial in obtaining good glycemic control and guiding the patient on insulin dosing.3-5
During recent years continuous glucose monitoring (CGM) has become a treatment option for guiding the patient on insulin dosing and other activities.6 CGM has the advantage of informing the patient on estimated glucose values continuously, also illustrating trends on increases or decreases in glucose levels.
Data from several clinical trials of CGM have shown mixed results in regards to its impact on glycemic control.7 In some clinical trials, only patients on CSII were included or had initiated CGM and CSII simultaneously as an intervention. In other trials both patients with MDI and CSII were included, and post hoc analyses have also resulted in mixed findings, where the impact on glycemic control appears to differ when CGM is combined with MDI versus CSII.8-10 Although the absolute majority of adults with type 1 diabetes are treated with MDI, clinical trials evaluating the effect of CGM compared to conventional therapy in persons treated with MDI have not been carried out.
The aim of this study was to analyze the effect of CGM on glycemic control, hypoglycemia, quality of life, and glycemic variability in individuals with type 1 diabetes treated with MDI.
Method
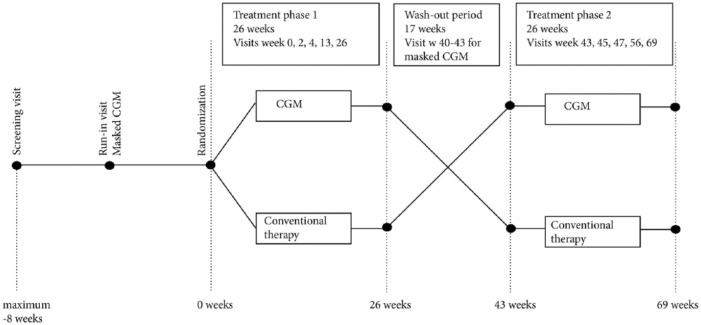
The present study was a randomized, open-label, controlled trial with a cross-over design conducted at 15 sites in Sweden with experience of treating adult patients with type 1 diabetes. After a run-in period of up to 8 weeks, patients were followed for 69 weeks where each treatment period consisted of 26 weeks with a between wash-out period of 17 weeks (Figure 1). The trial was investigator initiated and sponsored by the NU Hospital Group, Trollhättan and Uddevalla, Sweden. The primary endpoint was the effect on HbA1c.
Figure 1.
Flow chart of design.
Screening
Individuals with type 1 diabetes, HbA1c ≥ 7.5% (58 mmol/mol) treated with MDI were included. Patients were required to have a fasting C-peptide level < 0.3 nmol/l and diabetes duration > 1 year. Other inclusion and exclusion criteria are shown in Table 1. All laboratory tests for screening and throughout the study were analyzed at a central laboratory (Research Centre for Laboratory Medicine, Karolinska University Hospital, Stockholm, Sweden).
Table 1.
Inclusion and Exclusion Criteria in the CGM-MDI Trial.
| Inclusion criteria |
|---|
| 1. Type 1 diabetes. 2. Adults 18 years or older. 3. Written informed consent. 4. HbA1c greater than or equal to 7.5% (58 mmol/mol). |
| Exclusion criteria |
| 1. Pregnancy, planned pregnancy for the study duration, or pregnancy during the past 6 months. 2. Severe cognitive dysfunction or other disease, which is judged by the physician to be not suitable for inclusion. 3. Required continuous use of paracetamol. Paracetamol must not have been used the week before the study and shall not be used during CGM-use because it disturbs the interpretation of blood glucose levels estimated by the Dexcom G4. However, other pain killers can be used throughout the study duration. 4. Current CGM use (within the past 4 months). 5. History of allergic reaction to any of the CGMS materials or adhesives in contact with the skin. 6. History of allergic reaction to chlorhexidine or alcohol antiseptic solution. 7. Abnormal skin at the anticipated glucose sensor attachment sites (excessive hair, burn, inflammation, infection, rash, and/or tattoo). 8. Patient is uncomfortable by using the sensor during the blinded run-in period and believes it is unlikely that he or she will use the sensor more than 80% of the time during the trial. 9. The patient has on average performed 12 or fewer calibrations per week during the run-in period. 10. Insulin pump therapy = CSII. 11. Diabetes duration < 1 year. 12. Participation in another study. 13. Fasting C-peptide level of 0.3 mmol/l or higher. 14. Other investigator-determined criteria making patients unsuitable for participation. 15. eGFR < 30 ml/min (estimated from creatinine, age, and sex at the inclusion visit by the MDRD-formula). 16. Planned house move during the next 1.5 years, making it difficult to come to study visits. |
Run-In Period
One purpose of the run-in period was to perform masked CGM during 2 weeks before randomization. Before masked CGM, patients completed a series of self-report questionnaires to evaluate preintervention treatment satisfaction, fear of hypoglycemia, hypoglycemia confidence, diabetes-related distress, well-being, and physical activity:
Diabetes Treatment Satisfaction Questionnaire (DTSQ) is a widely used, 8-item scale that measures aspects of satisfaction scored on a 7-point Likert scale, including domains of current treatment satisfaction, convenience of treatment, understanding of diabetes, recommendation of treatment, and continuation of treatment. Two separate items measure perception of unacceptably high blood sugars (hyperglycemia) and unacceptably low blood sugars (hypoglycemia). Two versions were used: the DTSQs and DTSQc, where the DTSQs is used to record current treatment satisfaction throughout the trial and the DTSQc is used to retrospectively compare the treatment arms at week 69.11-13
Hypoglycemia Fear Survey, Swedish version (SWE-HFS) is a 23-item validated scale that consists of a behavior subscale (focusing on actions to prevent hypoglycemia) and a worry subscale (addressing fears about hypoglycemia).14-16
The hypoglycemia confidence questionnaire is a newly developed, 9-item scale that evaluates patient confidence regarding their ability to prevent and address hypoglycemic events. A manuscript on the validation of this questionnaire by Polonsky et al is in preparation.
Problem Areas in Diabetes Scale, Swedish version (SWE-PAID) is a 20-item scale which assesses worries and concerns specifically related to diabetes and its management. It has been shown to a be a good marker of diabetes-related distress.17,18
World Health Organization-5 (WHO-5) is a 5-item scale that assesses patient well-being.19
International Physical Activity Questionnaire (IPAQ) is a 7-item scale that assesses minutes spent in light, moderate and vigorous physical activity per week; scores are expressed as energy expenditure in estimated metabolic equivalent task (MET) minutes.20,21
An additional 19 questions, focusing on a variety of psychosocial, diabetes-related, and demographic factors, were included in the preintervention battery as potential predictors of CGM-related clinical outcomes (Supplement page 2).
All questionnaires were completed at the study site. It was emphasized to study sites that patients be allowed to complete questionnaires prior to clinical measurements and before other study activities, such as adjusting treatment, were discussed at the visit. Sites were instructed to allow patients to answer questionnaires in a reasonably quiet environment and on their own; however, the site personnel were instructed to help patients complete questionnaires, if necessary, without influencing patients’ responses. Site personnel were instructed to check questionnaires for completeness, and all questionnaires, aside from the final group of 19 predictor questions (which were administered only before randomization), were completed at set intervals throughout the study. In addition, at week 69 the DTSQc was completed.
After the masked CGM period patients who believed they would not wear the CGM sensor more than 80% of the study time during the period with CGM, and patients who did not perform adequate calibrations during the run-in period (on average at least 12 of 14 during a 7-day period), were excluded. Patients were shown an example picture of glucose curves (not their own curves) with trend arrows, explained by the physician/diabetes nurse to give them a better chance to judge how often they would use the sensor. Patients used their own blood glucose meter for self-monitoring of blood glucose (SMBG) during the run-in period and the rest of the study. In general, regions in Sweden only allow blood glucose meters with a CV of <10%, which ensures a high level of precision.
Randomization
After a maximum run-in period of 8 weeks, patients were randomized 1:1 to either the Dexcom G4 stand-alone system or conventional treatment in treatment period 1. A centralized web system was used, with block randomization performed on each site.
Treatment
The studied intervention was CGM (Dexcom G4 PLATINUM, Dexcom Inc, San Diego, CA) which was compared to conventional therapy using only SMBG for guiding the dosage of insulin. All patients in the trial were instructed regarding basic information on insulin dosing, such as bolus correction, types of foods elevating glucose levels, and the effect of physical activity on glucose control. The information was provided at the same level as in clinical practice for patients with type 1 diabetes, to guarantee that all patients have basic skills for dosing insulin. All patients were also educated on the proportion of rapid acting insulin analogues remaining at various time points after injection.22 Patients’ basic skills of SMBG-measurement were controlled and they were educated on using the Dexcom G4 system. During the study patients continued using the type of basal and meal-time insulin they had previously used in clinical practice.
At clinical visits the diabetes nurse or physician discussed glucose levels measured by SMBG and CGM data with the patient for possible improvements in their diabetes care. The discussions were performed to correspond with intensive therapy used in clinical practice. During the first week after CGM use commenced there were no alarm levels set on the CGM, other than a constantly active acute alarm at 55 mg/dl (3.1 mmol/l). This allowed the patient to become active in judging CGM trends instead of merely reacting to a variety of alarms. The clinical visit at screening was performed by a physician; this was also the case at the end of treatment phase 1 and start and end of treatment phase 2. Other visits could be carried out by either a diabetes nurse or physician, but preferentially by the same person in both treatment phases at the corresponding visits.
Alarm settings were introduced no later than 2 weeks after randomization. At each visit the patient was encouraged to use the CGM information at least every 1-2 hours during daytime. Correspondingly, patients were encouraged to measure blood glucose levels when randomized to conventional therapy in accordance with guidelines, that is, at least 4 times a day. At the first visit in each treatment period patients were evaluated for general skills adopted on dosing insulin, types of foods that elevate glucose levels, and the influence of physical activity on glucose levels. Blood glucose values were evaluated during visits of patients receiving conventional therapy for possible improvements in dosing insulin in relation to, for example, food intake and physical activity. In addition, CGM data were retrospectively analyzed and reviewed for patients treated with CGM. When randomized to CGM patients received 10 general guidelines that were considered during treatment and discussed with the diabetes nurse or physician (Supplement page 7). Insulin dosages were performed based on SMBG values and not CGM values.
Clinical Visits
In each treatment phase patients receiving Dexcom G4 and conventional therapy had the same number of visits. For treatment phase 1 the visit schedule was as follows: randomization, week 2, week 4, week 13, and week 26 (Figure 1). Corresponding visits for treatment phase 2 were week 43, week 45, week 47, week 56, and week 69 (Figure 1). HbA1c was recorded at the start of each treatment period and all subsequent study visits except weeks 2 and 45. Fasting blood samples for blood lipids, creatinine, sensitive CRP, apolipoproteins, and biobank samples were collected at randomization and weeks 26, 43, and 69. Urine albumin creatinine ratio was measured at the same visits as fasting blood samples.
Masked CGM was performed for all participants during 2 weeks before randomization and 2 weeks before week 43 (the starting point of the second treatment phase). During the treatment period with conventional therapy, patients also had masked CGM during 2 of the 4 last weeks (weeks 23-26 or 66-69). Masked CGM was used to evaluate time in hypoglycemia, time in euglycemia, time in hyperglycemia, and glycemic variability. At all visits the number of SMBG measurements were recorded by downloading data. CGM data were downloaded to evaluate glucose levels, proportion of time the CGM system was used, and potential improvements for optimizing glycemic control. A detailed description of procedures including various blood samples and questionnaires is provided in Table 2.
Table 2.
Detailed Description of Procedures.
| Information | Informed consent | Check criteria | Blinded CGM | Demographics, medical history | DTSQs, WHO-5, SWE-HFS, hypoglycemia confidence, SWE-PAID-20, IPAQ | DTSQc | HbA1c | Creatinine, sensitive CRP, blood lipids, apolipoproteins, biobank samples | BMI, waist-hip ratio | Blood pressure | A/C ratio | Insulin dose | Download SMBG | Download CGM, evaluate use | Optimization by CGM/SMBG only |
Concomitant medication | AE, SAE | Wash-out period | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit 1 | X | ||||||||||||||||||
| Visit 2 | x | x | |||||||||||||||||
| Run-in | x | x | x | x | x | ||||||||||||||
| Randomization | x | x | x | x | x | x | x | x | x | x | x | ||||||||
| Week 2 | x | x | x | x | x | ||||||||||||||
| Week 4 | x | x | x | x | x | x | |||||||||||||
| Week 13 | x | x | x | x | x | x | |||||||||||||
| Week 23-26 | x | ||||||||||||||||||
| Week 26 | x | x | x | x | x | x | x | x | x | x | x | ||||||||
| Week 27-43 | x | ||||||||||||||||||
| Week 40-43 | x | x | x | x | x | ||||||||||||||
| Week 43 | x | x | x | x | x | x | x | x | x | x | x | ||||||||
| Week 45 | x | x | x | x | x | ||||||||||||||
| Week 47 | x | x | x | x | x | x | |||||||||||||
| Week 56 | x | x | x | x | x | x | |||||||||||||
| Week 66-69 | x | ||||||||||||||||||
| Week 69 | x | x | x | x | x | x | x | x | x | x | x | x |
Physical examination is to be performed at visit 2 (inclusion), visit 9 (week 26), visit 11 (week 43), and visit 16 (week 69). Education on Dexcom G4 system will be performed at start visit, week 2, and week 4 in each period. Knowledge on how to perform SMBG will be checked and education will be performed if judged clinically indicated. At randomization and week 43, basic knowledge of insulin dosing will be checked and, if necessary, patients will be educated about basic insulin dosing. At visit week 13 and week 26 (and corresponding to week 56 and week 69) participants using CGM will be asked how frequently they look at the receiver screen.
Endpoints
The primary endpoint was the difference in HbA1c between the last visit in each treatment phase, weeks 26 and 69. HbA1c was analyzed according to IFCC standard using the instrument Variant II Turbo (Bio-Rad Laboratories).23 All predefined endpoints are shown in Table 3.
Table 3.
Predefined Endpoints.
| Primary endpoint |
|---|
| • The primary endpoint is the difference in HbA1c between week 26 and week 69. |
| Secondary endpoints |
| • The difference in mean glucose level (measured by CGM during 2 weeks) between weeks 23-26 and 66-69. • The difference in MAGE (measured by CGM during 2 weeks) between weeks 23-26 and 66-69.24 • The difference in standard deviation of glucose levels measured by CGM during 2 weeks between weeks 23-26 and weeks 66-69, measured by CGM. • The difference in DTSQ scores between weeks 26 and 69. • DTSQc score at week 69 • The difference in WHO-5 scores between weeks 26 and 69. • The difference in SWE-HFS scores between weeks 26 and 69. • The difference in SWE-PAID-20 scores between weeks 26 and 69. • The difference in the proportion of time with low glucose levels measured by CGM during 2 weeks between week 23-26 and week 66-69 measured by CGM (below 54 mg/dl [3.0 mmol/l] and below 72 mg/dl [4.0 mmol/l] respectively). • The difference in the proportion of time with high glucose levels measured by CGM during 2 weeks between week 23-26 and week 66-69 measured by CGM (above 180 mg/dl [10.0 mmol/l] and above 250 mg/dl [13.9 mmol/l] respectively). • The difference in the proportion of time with euglycemic levels measured by CGM during 2 weeks between weeks 23-26 and weeks 66-69 (99-180 mg/dl [5.5-10.0 mmol/l] and 70-180 mg/dl [3.9-10.0 mmol/l] respectively). • The difference in the proportion of patients reducing their HbA1c by 0.5% (5 mmol/mol) or more. • The difference in the proportion of patients lowering their HbA1c 1% (10 mmol/mol) or more. • The difference in the mean number of severe hypoglycemic events between weeks 1-26 and weeks 44-69 defined as unconsciousness due to hypoglycemia or need of assistance from another person to resolve the hypoglycemia. • The difference in mean number of capillary glucose measurements per day between weeks 1-26 and weeks 43-69, from time periods when values are available in glucometers. |
Monitoring and Laboratory Analyses
Gothia Forum, Gothenburg, Sweden, monitored the trial and the Research Centre for Laboratory Medicine at Karolinska University Hospital, Stockholm, Sweden, was the central laboratory analyzing all blood samples. Urine albumin/creatinine ratio was the only sample analyzed at the local laboratory. The study was approved by the Ethics Committee at the University of Gothenburg, Gothenburg, Sweden as a multicentre, randomized trial (December 12, 2013, diary number 857-13). The trial was registered on ClinicalTrials.gov, number NCT02092051.
Statistics
The full analysis set consists of all randomized patients who received at least 1 follow-up measurement in each treatment phase.
The per-protocol population (PP-population) consists of all patients in the full analysis set without any significant protocol deviations. The PP-population is defined at the clean-file meeting before the database is locked.
The safety population consists of all randomized patients who received treatment (conventional or CGM) at any time. In the safety analysis patients will be assigned to treatment given, not the randomized treatment.
The primary efficacy analysis was the difference in HbA1c at weeks 26 and 69 between CGM and conventional therapy for the full analysis set using a general linear model adjusted for both period’s effect and subject effect. This implies that treatment effect will be analyzed within subjects and period’s effect will be handled correctly. If efficacy measurements from 26 and 69 weeks follow-up, respectively, are missing, the last observation carried forward (LOCF) principle will be applied. LOCF will not be applied to measurements at the first visit in each treatment period (randomization and week 43).
Secondary efficacy analyses, the differences in the secondary endpoints (Table 3) between CGM and conventional therapy, will also be analyzed adjusted for period’s effect and subject effect on the full analysis set. The theory of sequential multiple test procedures will be applied for the primary analysis and for secondary analyses. If a test gives a significant result at the 5% significance level, the total test mass will be transferred to the following number in the test sequence until a nonsignificant result is achieved. The above efficacy analyses will also be performed on the PP-population. All significance tests will be performed 2-sided at significance level of .05.
The study was powered to detect a difference of 0.3% (3 mmol/mol) in HbA1c between weeks 26 and 69, at 90% power, assuming a standard deviation of 1.1%, which requires 144 participants. Assuming a drop-out rate of 10% 160 individuals were required to be enrolled.
Results
A total of 205 patients were screened, of whom 161 patients were randomized. Of excluded patients, 22 declined participation, 10 had HbA1c below 7.5% (58 mmol/mol), 5 experienced problems with CGM during run-in, 3 had C-peptide of 0.3 nmol/l or higher, 1 had insulin pump therapy, 1 was planning pregnancy, 1 was evaluated for kidney transplantation and 1 fulfilled more than 1 of the mentioned criteria. Patients were randomized between February 2014 and December 2014. Study completion is anticipated in April 2016.
Discussion
This trial is believed to be the first randomized trial evaluating the effect of CGM in a pure MDI treated population with type 1 diabetes. In total, 161 individuals were randomized in a cross-over design to CGM or conventional therapy consisting of SMBG for monitoring glucose levels. Besides showing whether CGM has an effect on improving glycemic control in this patient group, where few treatment options currently exist, the trial will evaluate effects of CGM on hypoglycemia, glycemic variability, and patient experience with respect to treatment satisfaction, hypoglycemia fear, hypoglycemia confidence, diabetes-related distress, well-being, and physical activity.
Recently it has been shown that there is still a high excess risk of mortality in individuals with type 1 diabetes.25-27 In individuals younger than 40-50 years of age acute complications in the form of ketoacidosis and hypoglycemia are major drivers for this excess risk, while cardiovascular disease is the major cause in older individuals.25,27 Improved glycemic control is associated with both lower risk of cardiovascular disease and all-cause mortality.25,28,29 Since most adult individuals with type 1 diabetes are treated with MDI,30 there is a great need for treatments improving glycemic control and reducing the risk of hypoglycemia in this population.
Since the current trial is believed to be the first in a pure MDI population of type 1 diabetes, any beneficial effects on HbA1c, hypoglycemia, or other parameters are of interest. Moreover, the Dexcom sensor per se, has been sparsely evaluated in long-term and large randomized trials, both in patients treated with MDI and CSII. The Dexcom G4 sensor used in the current trial has shown higher accuracy in estimating blood glucose levels than other CGM sensors.31-33 High accuracy is likely essential not only for making correct treatment decisions, but also for gaining patients’ confidence in using the CGM system.34 It may also influence the possibility of detecting hypoglycemia, and the Dexcom G4 has shown high accuracy in this glycemic range.31 Furthermore, the Dexcom G4 stand-alone system has been associated with high treatment satisfaction.31
In earlier studies the effect of CGM on HbA1c has been strongly associated with the time of sensor use.35 A greater proportion of time the sensor is used has been associated with a greater reduction in HbA1c. Long-term as opposed to short-term sensor use has also been associated with reductions in HbA1c in clinical practice.36 High accuracy and treatment satisfaction with the CGM system may therefore be essential regarding effects on HbA1c and other parameters. Another novelty in the current trial, in contrast to many earlier randomized CGM trials, is that no upper limit of HbA1c was set for inclusion. Recently it was shown, that those patients with very poor glycemic control have 8-10 times excess risk of all-cause and cardiovascular mortality.25 Therefore it will also be essential to evaluate effects in this subgroup of patients.
In the current trial extensive questionnaires of treatment satisfaction, hypoglycemia fear, hypoglycemia confidence, diabetes-related distress, well-being, and physical activity were included. Such evaluations may also lead to further understanding of CGM effects besides those of glycemic parameters. For evaluating how glycemic variability or other glucose patterns affect different metabolic parameters in the future, biobank samples were collected.
Regarding the current study design, it is noteworthy that a potential draw-back with a cross-over design can be possible carryover effects. It could be speculated that patients will learn from CGM regarding their treatment, for example insulin dosing, eating habits, and effects of exercise. However, if a carryover effect of CGM on conventional therapy exists it would rather underestimate the effect of CGM than overestimate any beneficial effect. In an earlier cross-over study of patients with CSII, those randomized to CGM had no clear carryover effects.9
Conclusion
In conclusion, the CGM-MDI trial intends to improve knowledge of CGM in adult individuals with type 1 diabetes treated with MDI. It will extend knowledge of effects on HbA1c, hypoglycemia, quality-of-life indicators, and glycemic variability in this population. In the CGM-MDI trial there was no upper limit of HbA1c for inclusion, which will hopefully improve knowledge also for patients with very poor glycemic control, who generally have the highest risk for excess complications and mortality.
Acknowledgments
The steering committee of the CGM-MDI trial consists of ML (PI), WP, IBH, TH, JB, and SD. We want to thank all participating sites where costs for salaries are mainly locally covered and not by financial support from Dexcom.
Footnotes
Abbreviations: A/C, albumin/creatinine; AE, adverse event; BMI, body mass index; CGM, continuous glucose monitoring; CRP, C-reactive protein; CSII, continuous subcutaneous insulin infusion; CV, coefficient of variation; DTSQ, Diabetes Treatment Satisfaction Questionnaire; GOLD, Glycaemic control & Optimisation of Life quality in type 1 Diabetes; IPAQ, International Physical Activity Questionnaire; LOCF, last observation carried forward; MAGE, mean amplitude of glycaemic excursions; MDI, multiple daily insulin injections; MET, metabolic equivalent task; PP-population, per-protocol population; SAE, serious adverse event; SMBG, self-monitoring of blood glucose; SWE-HFS, Hypoglycemia Fear Survey, Swedish version; SWE-PAID, Problem Areas in Diabetes Scale, Swedish version; WHO-5, World Health Organization–5.
Declaration of Conflicting Interests: ML has received research grants from AstraZeneca, Dexcom, and Novo Nordisk, been a consultant or received honoraria from Medtronic, Eli Lilly, Pfizer, Abbot Scandinavia, Bayer, Novo Nordisk, and Rubin Medical. ML has participated in advisory boards for Novo Nordisk. WP has served as a consultant for Dexcom and Abbott Diabetes Care. IBH has received research grants from Novo Nordisk, and been a consultant for Abbott Diabetes Care, Roche, and Becton Dickinson. TH has received research funds from Adocia, AstraZeneca, BD, Biocon, Boehringer Ingelheim, Dance Pharmaceuticals, Grünenthal, Eli Lilly, Medtronic, Novo Nordisk, Novartis, Sanofi, and Senseonics. TH has participated in advisory panels for Novo Nordisk and received speaker honoraria and travel grants from Eli Lilly, Mylan, and Novo Nordisk. JB has received honoraria for consulting and/or lecture fees from Abbott Diabetes Care, AstraZeneca, Integrity Applications, Eli Lilly, and Sanofi. SD, NGP, and PM have no conflicts of interest to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The NU Hospital Group has received financial support for the current trial and CGM systems and sensors from Dexcom Inc. The trial was investigator initiated and Dexcom was not involved in carrying out the trial. Dexcom will not be involved in the statistical analysis, data management, data analysis, and publication.
Supplemental Material: The supplementary material is available at http://dst.sagepub.com/supplemental
References
- 1. DCCT Study Group. The Effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [DOI] [PubMed] [Google Scholar]
- 2. Misso ML, Egberts KJ, Page M, O’Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010;(1):CD005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hansen MV, Pedersen-Bjergaard U, Heller SR, et al. Frequency and motives of blood glucose self-monitoring in type 1 diabetes. Diabetes Res Clin Pract. 2009;85(2):183-188. [DOI] [PubMed] [Google Scholar]
- 4. Miller KM, Beck RW, Bergenstal RM, et al. Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care. 2013;36(7):2009-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Evans JM, Newton RW, Ruta DA, MacDonald TM, Stevenson RJ, Morris AD. Frequency of blood glucose monitoring in relation to glycaemic control: observational study with diabetes database. BMJ. 1999;319(7202):83-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hirsch I. Clinical review: realistic expectations and practical use of continuous glucose monitoring for the endocrinologist. J Clin Endocrinol Metab. 2009;94(7):2232-2238. [DOI] [PubMed] [Google Scholar]
- 7. Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464-1476. [DOI] [PubMed] [Google Scholar]
- 9. Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55(12):3155-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Riveline JP, Schaepelynck P, Chaillous L, et al. Assessment of patient-led or physician-driven continuous glucose monitoring in patients with poorly controlled type 1 diabetes using basal-bolus insulin regimens: a 1-year multicenter study. Diabetes Care. 2012;35(5):965-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bradley C, Gilbride CJ. Improving treatment satisfaction and other patient-reported outcomes in people with type 2 diabetes: the role of once-daily insulin glargine. Diabetes Obes Metab. 2008;10(suppl 2):50-65. [DOI] [PubMed] [Google Scholar]
- 12. Bradley C. The Diabetes Treatment Satisfaction Questionnaire: DTSQ. In: Bradley C, ed. Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Practice. Chur, Switzerland: Harwood; 1994. [Google Scholar]
- 13. Bradley C. Diabetes treatment satisfaction questionnaire. Change version for use alongside status version provides appropriate solution where ceiling effects occur. Diabetes Care. 1999;22:530-532. [DOI] [PubMed] [Google Scholar]
- 14. Anderbro T, Amsberg S, Wredling R, et al. Psychometric evaluation of the Swedish version of the Hypoglycaemia Fear Survey. Patient Educ Couns. 2008;73(1):127-131. [DOI] [PubMed] [Google Scholar]
- 15. Gonder-Frederick LA, Schmidt KM, Vajda KA, et al. Psychometric properties of the hypoglycemia fear survey-ii for adults with type 1 diabetes. Diabetes Care. 2011;34(4):801-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Irvine A, Cox D, Gonder-Frederick L. The Fear of Hypoglycaemia Scale. In: Bradley C, ed. Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Practice. Chur, Switzerland: Harwood; 1994. [Google Scholar]
- 17. Polonsky WH, Anderson BJ, Lohrer PA, Welch G, Jacobson AM, Schwartz C. Assessment of diabetes-specific distress. Diabetes Care. 1995;18:754-760. [DOI] [PubMed] [Google Scholar]
- 18. Amsberg S, Wredling R, Lins PE, Adamson U, Johansson UB. The psychometric properties of the Swedish version of the Problem Areas in Diabetes Scale (Swe-PAID-20): scale development. Int J Nurs Stud. 2008;45:1319-1328. [DOI] [PubMed] [Google Scholar]
- 19. Hajos TR, Pouwer F, Skovlund SE, et al. Psychometric and screening properties of the WHO-5 well-being index in adult outpatients with type 1 or type 2 diabetes mellitus. Diabet Med. 2013;30(2):e63-e69. [DOI] [PubMed] [Google Scholar]
- 20. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395. [DOI] [PubMed] [Google Scholar]
- 21. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 suppl):S498-S504. [DOI] [PubMed] [Google Scholar]
- 22. Hirsch IB. Insulin analogues. N Engl J Med. 2005;352(2):174-183. [DOI] [PubMed] [Google Scholar]
- 23. Jeppsson JO, Kobold U, Barr J, et al. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin Chem Lab Med. 2002;40(1):78-89. [DOI] [PubMed] [Google Scholar]
- 24. Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19(9):644-655. [DOI] [PubMed] [Google Scholar]
- 25. Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371(21):1972-1982. [DOI] [PubMed] [Google Scholar]
- 26. Livingstone SJ, Looker HC, Hothersall EJ, et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLOS MED. 2012;9(10):e1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Livingstone SJ, Levin D, Looker HC, et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. JAMA. 2015;313(1):37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Writing Group for the DCCT/EDIC Research Group. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA. 2015;313(1):45-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Swedish National Diabetes Register. Annual report 2013. Available at: https://www.ndr.nu/pdfs/Annual_Report_NDR_2013.pdf. Accessed January 18, 2016.
- 31. Matuleviciene V, Joseph JI, Andelin M, et al. A clinical trial of the accuracy and treatment experience of the Dexcom G4 sensor (Dexcom G4 system) and Enlite sensor (guardian REAL-time system) tested simultaneously in ambulatory patients with type 1 diabetes. Diabetes Technol Ther. 2014;16(11):759-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Damiano ER, McKeon K, El-Khatib FH, Zheng H, Nathan DM, Russell SJ. A comparative effectiveness analysis of three continuous glucose monitors: the Navigator, G4 Platinum and Enlite. J Diabetes Sci Technol. 2014;8:699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kropff J, Bruttomesso D, Doll W, et al. Accuracy of two continuous glucose monitoring systems: a head-to-head comparison under clinical research centre and daily life conditions. Diabetes Obes Metab. 2015;17(4):343-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Polonsky WH, Hessler D. Perceived accuracy in continuous glucose monitoring: understanding the impact on patients. J Diabetes Sci Technol. 2015;9(2):339-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. JDRF study group. Factors predictive of use and benefit from CGM in type 1 diabetes. Diabetes Care. 2009;32(11):1947-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anderson J, Attvall S, Sternemalm L, et al. Effect on glycemic control by short- and long-term use of continuous glucose monitoring in clinical practice. J Diabetes Sci Technol. 2011;5(6):1472-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]