Abstract
Mortality associated with infections due to carbapenem-resistant Klebsiella pneumoniae (CR-KP) is high and the infections need to be predicted early. The risk factors for CR-KP infection are heterogeneous. The aim of the present study was to construct a model allowing for the early prediction of CR-KP infection. Nosocomial infections due to K. pneumoniae were evaluated retrospectively over a 2-year period. The case cohort consisted of 370 inpatients with CR-KP infection. For each case enrolled, two matched controls with no CR-KP infection during their hospitalization were randomly selected. Matching involved month of admission, ward, as well as interval days. The Vitek 2 system was used for identification of isolates and antimicrobial susceptibility testing. General linear model with logistic regression was used to identify possible risk factors. The predicted power of the model was expressed as the area under the receiver-operating characteristic curve. Age, male gender, with cardiovascular disease, hospital stay, recent admission to intensive care unit, indwelling urinary catheter, mechanical ventilation, recent β-lactam-β-lactamase inhibitors, fourth-generation cephalosporins and/or carbapenems therapy were independent risk factors for CR-KP infection. Models predicting CR-KP infection developed by cumulative risk factors exhibited good power, with areas under the receiver-operating characteristic curves of 0.902 [95% confidence interval (CI), 0.883–0.920; P<0.001] and 0.899 (95% CI, 0.877–0.921; P<0.001) after filtering by age (≥70 years). The Yonden index was at the maximum when the cumulative risk factors were ≥3 in the two prediction models. The results show that the prediction model developed in the present study might be useful for controlling infections caused by CR-KP strains.
Keywords: nosocomial infection, Klebsiella pneumoniae, carbapenem resistance, risk factors
Introduction
Infections caused by carbapenem-resistant Klebsiella pneumoniae (CR-KP) isolates are worldwide. The prevalence of CR-KP infection in areas of endemicity may vary between 20 and 40%. In addition, these infections often occur in debilitated and immunocompromised patients, in association with prolonged hospital stays (1). The isolates are often resistant to multiple antibiotics, and the mortality associated with infections due to CR-KP is extremely high (2–4). Early identification of possible CR-KP-infected patients and implementation of proper precaution are core measures for controlling CR-KP infections.
Risk factors involved in CR-KP infections have been previously investigated (5–8). These factors were heterogeneous. A retrospective study was conducted in a Chinese tertiary care hospital to identify the main factors associated with nosocomial CR-KP infections, and a model was established for the early prediction of patients with such infection. The results show that the prediction model developed in the present study might be useful for controlling infections caused by CR-KP strains.
Materials and methods
Setting and participants
The Beijing Shijitan Hospital of the Capital Medical University is a 1,100-bed tertiary care hospital in Beijing, China, with a 26-bed general intensive care unit (ICU), an 8-bed cardiology ICU, an 8-bed respiratory ICU, and a 6-bed emergency medicine ICU.
This retrospective study was conducted based on the hospital electronic database. During the 2-year study period (from January 1, 2012 to December 31, 2013), patients with K. pneumoniae nosocomial infection were evaluated. During the study period, rectal swab screening was not a routine admission procedure and patients were clustered within at least 48 h once CR-KP infection was confirmed.
In one hospitalization period, each patient was evaluated only once at the time of the index culture (nosocomial infections were validated by trained infection management doctors according to the criteria based on a previous study) (9), and the index culture was defined with the K. pneumoniae strain first isolated from a clinical specimen and then the corresponding nosocomial infection was confirmed. Patients with CR-KP infections were defined as cases. For each case enrolled, two matched controls with no CR-KP infection during their hospitalization were randomly selected. Matching involved month of admission, ward, as well as interval days (interval from admission to confirmation of the index culture). The length of the entire hospital stay of the controls was equal or more to the interval days of the matched cases.
The following patient data were extracted: Age; gender; transfer from another hospital; comorbidity (at the time of index culture); recent admission to ICU (defined as patients admitted to ICU for >24 h before the index culture in one hospitalization period); with CR-KP-positive patients in nearby beds (defined as patients with CR-KP-positive patients in the same ward for >24 h before the index culture within the hospitalization period); adopted invasive procedures including central venous catheterization, urinary catheter, nasogastric tube, surgical drain, and invasive mechanical ventilation (at the time of the index culture); and on prior antibiotic therapy (defined as the use of a systemic antimicrobial agent for ≥48 h within the preceding 10 days of index culture in one hospitalization period).
Approval for the study was obtained from the ethics committee of the Beijing Shijitan Hospital of Capital Medical University.
Microbiological procedures
The Vitek 2 system (bioMérieux, Marcy l'Étoile, France) was used for identification of isolates and antimicrobial susceptibility testing. The production of extended-spectrum β-lactamases (ESBLs) was suggested following use of the Advanced Expert System of the Vitek 2 system. Susceptibility results to carbapenems (imipenem, meropenem, and ertapenem) were interpreted according to Clinical Laboratory Standards Institute guidelines coevally (i.e., susceptibility results of year 2012 were interpreted according to 2012 guideline and results of year 2013 were interpreted according to 2013 standards) (10). Isolates were considered CR-KP if any of the carbapenems (ertapenem, meropenem, and imipenem) was resistant. If the carbapenem MIC of one isolate was intermediary, the isolate was excluded from the analysis.
Statistical analysis
The SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA) software was used for data analysis. Non-normally distributed continuous variables were reported as medians (interquartile range); and numbers and percentages were reported for categorical variables. The Mann-Whitney U test was used to compare non-normally distributed continuous variables (i.e., ages). Categorical variables were evaluated with the Chi-square test.
The multivariate logistic regression model was used to adjust for potential confounding. Variables associated with CR-KP infection in the univariate analysis (P≤0.05) were included in a logistic regression analysis to identify those independently associated with the development of CR-KP nosocomial infection. Statistical tests were two-tailed. P≤0.05 was considered statistically significant.
The power of age and cumulative risk factors to discriminate cases and controls were expressed as the area under the receiver-operating characteristic curve.
Results
Characteristics of isolates
During the 2-year study period, 370 non-repetitive episodes of nosocomial infections due to CR-KP were found. The characteristics of the cases and controls are shown in Table I. The median (IQR) time of interval days in the case cohort was 17 days (13–21 days). No active outbreak of CR-KP during the 2-year study period was recorded.
Table I.
The characteristics of cases and controls.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variables | Case (n=370) | Control (n=740) | P-value | OR | 95% CI | P-value |
| Demographics | ||||||
| Age (year) | 85 (80–87) | 74 (59–84) | <0.001 | 1.08 | 1.06–1.10 | <0.001 |
| Male | 321 (86.8) | 434 (58.6) | <0.001 | 3.839 | 2.36–6.26 | <0.001 |
| Transfer from another hospital | 23 (6.2) | 16 (2.2) | 0.001 | 0.750 | 0.29–1.94 | 0.553 |
| Comorbiditiesa | ||||||
| Cardiovascular disease | 80 (21.6) | 32 (15.0) | <0.001 | 10.00 | 4.52–22.15 | <0.001 |
| Chronic pulmonary disease | 162 (43.8) | 303 (40.9) | 0.366 | |||
| Cerebrovascular disease | 88 (23.8) | 192 (25.9) | 0.434 | |||
| Liver disease | 22 (5.9) | 50 (6.8) | 0.605 | |||
| Chronic renal failure | 27 (7.3) | 51 (6.9) | 0.803 | |||
| Diabetes mellitus | 23 (6.2) | 43 (5.8) | 0.788 | |||
| Peptic ulcer disease | 8 (3.0) | 32 (4.3) | 0.068 | |||
| Malignancy | 26 (7.0) | 56 (7.6) | 0.746 | |||
| Recent surgeryb | 36 (9.7) | 101 (13.6) | 0.061 | |||
| Hospitalization data | ||||||
| Admission to ICUc | 225 (60.8) | 75 (10.1) | <0.001 | 2.06 | 1.03–4.10 | 0.040 |
| CR-KP-positive patients in nearby bedsd | 251 (67.8) | 111 (15.0) | <0.001 | 1.18 | 0.60–2.29 | 0.636 |
| Invasive proceduresa | ||||||
| Central venous catheter | 198 (53.5) | 109 (14.7) | <0.001 | 1.64 | 0.99–2.71 | 0.052 |
| Urinary catheter | 297 (80.2) | 240 (32.4) | <0.001 | 2.21 | 1.36–3.59 | 0.001 |
| Nasogastric tube | 207 (55.9) | 65 (8.8) | <0.001 | 0.95 | 0.41–2.23 | 0.904 |
| Surgical drain | 10 (2.7) | 22 (3.0) | 0.800 | |||
| Mechanical ventilation | 193 (52.2) | 56 (7.6) | <0.001 | 2.80 | 1.15–6.86 | 0.024 |
| Prior antibiotic therapye | ||||||
| Second-generation cephalosporins | 40 (10.8) | 335 (45.2) | <0.001 | 0.787 | 0.43–1.43 | 0.433 |
| Third-generation cephalosporins | 65 (17.6) | 102 (13.8) | 0.096 | |||
| Fourth-generation cephalosporins | 27 (7.3) | 18 (2.4) | <0.001 | 3.79 | 1.44–10.03 | 0.007 |
| β-lactam-β-lactamase inhibitors | 156 (42.2) | 111 (15.0) | <0.001 | 10.65 | 5.93–19.10 | <0.001 |
| Carbapenems | 194 (52.4) | 123 (16.6) | <0.001 | 7.75 | 4.21–14.27 | <0.001 |
| Fluoroquinolones | 47 (12.7) | 106 (14.3) | 0.460 | |||
| Aminoglycosides | 25 (6.8) | 38 (5.1) | 0.271 | |||
| Othersf | 55 (14.9) | 137 (18.5) | 0.130 | |||
Results are shown as number (%) or median (range). CR-KP, carbapenem-resistant Klebsiella pneumoniae; OR, odds ratio; CI, confidence interval; ICU, intensive care unit.
At the time of the index culture.
Surgical procedure performed within 14 days before the index culture.
Admitted to ICU for more than 24 h before the index culture within the hospitalization period.
A CR-KP-positive patient in the nearby bed for more than 24 h before the index culture within the hospitalization period.
Zero to 30 days before the index culture within the hospitalization period.
Including semisynthetic penicillins, oxyimino-cephalosporins, glycopeptides, linezolid, rifampin, erythromycins, and antifungal drugs.
CR-KP isolates were recovered from the sputum/bronchoalveolar lavage fluid (n=268), urine (n=48), blood (n=35), skin/mucosa (n=10), surgical site (n=7), and cerebrospinal fluid (n=2). ESBLs were produced by 61% of CR-KP strains (n=225).
Risk factors associated with CR-KP infection
The main findings of the univariate and multivariate analysis associated with CR-KP infection are shown in Table I. The multivariate analysis revealed that age, male gender, with cardiovascular disease, recent admission to ICU, indwelling urinary catheter and/or surgical drain, mechanical ventilation, and prior administration of β-lactam-β-lactamase inhibitors, fourth-generation cephalosporins, and/or carbapenems were significantly different between CR-KP infections and controls.
Cumulative risk factors and their predicting power of CR-KP infection
Male gender, with cardiovascular disease, recent admission to ICU, indwelling urinary catheter, mechanical ventilation, and prior use of antibiotics of β-lactam-β-lactamase inhibitors, fourth-generation cephalosporins and/or carbapenems were included in the calculation of cumulative risk factors (Table II).
Table II.
Distribution of cumulative risk factors for CR-KP infection.
| No. of risk factors | Casea | Controla | Totala | Caseb | Controlb | Totalb |
|---|---|---|---|---|---|---|
| 0 | 4 | 154 | 158 | 4 | 93 | 97 |
| 1 | 13 | 279 | 292 | 11 | 160 | 171 |
| 2 | 39 | 171 | 210 | 35 | 102 | 137 |
| 3 | 76 | 94 | 170 | 71 | 54 | 125 |
| 4 | 86 | 29 | 115 | 86 | 19 | 105 |
| 5 | 86 | 13 | 99 | 71 | 9 | 80 |
| 6 | 66 | 0 | 66 | 60 | 0 | 60 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 370 | 740 | 1,110 | 338 | 437 | 775 |
Risk factors included the following: Male gender, cardiovascular disease, recent admission to intensive care unit, urinary catheter, mechanical ventilation, fourth-generation cephalosporins therapy, β-lactam-β-lactamase inhibitors therapy, and carbapenems therapy.
Results are shown as numbers and tested in total 1,110 (370 cases and 740 matched controls) patients.
Results are shown as numbers and tested in 775 patients [338 CR-KP infection positive cases and 437 matched controls after filtering by age (≥70 years)]. CR-KP, carbapenem-resistant Klebsiella pneumoniae.
The area under the curve (AUC) was 0.739 (95% CI, 0.709–0.768; P≤0.001) for age prediction CR-KP infection. The Yonden index was at its maximum when the patient age was 70 years. After filtering by age (≥70 years), 338 CR-KP infection cases and 437 controls remained.
The sensitivity, specificity, and total consistency rate for predicting CR-KP infection at different cut-off levels using cumulative risk factors are shown in Table III. The AUC was 0.902 (95% CI, 0.883–0.920; P≤0.001) for cumulative risk factors predicting CR-KP infection tested in the total 1,110 patients and 0.899 (95% CI, 0.877–0.921; P≤0.001) tested in the 775 patients filtered by age (Fig. 1), respectively. The Yonden index was at its maximum when the cumulative risk factors were ≥3 in the two prediction models. However, the sensitivity of prediction increased from 84.8 to 85.2% and the specificity increased from 68.1 to 81.2% with age (≥70 years), respectively.
Table III.
Sensitivity, specificity, and total consistency rate for predicting CR-KP infection at different cut-off levels.
| No. of risk factors | SEa | SPa | TCRa | SEb | SPb | TCRb |
|---|---|---|---|---|---|---|
| ≥1 | 98.9 | 20.8 | 46.8 | 98.8 | 21.3 | 55.1 |
| ≥2 | 95.4 | 58.5 | 70.8 | 95.6 | 57.9 | 774.3 |
| ≥3 | 84.8 | 68.1 | 82.7 | 85.2 | 81.2 | 83.0 |
| ≥4 | 64.3 | 94.3 | 84.3 | 64.2 | 93.6 | 80.7 |
| ≥5 | 41.1 | 98.2 | 79.2 | 38.8 | 97.9 | 72.1 |
| ≥6 | 17.8 | 100.0 | 72.6 | 17.8 | 100.0 | 64.1 |
Risk factors included the following: Male gender, cardiovascular disease, recent admission to intensive care unit, urinary catheter, mechanical ventilation, fourth-generation cephalosporins therapy, β-lactam-β-lactamase inhibitors therapy, and carbapenems therapy. SE, sensitivity; SP, specificity; TCR, total consistency rate.
Results are shown as percentage and tested in total 1,110 (370 cases and 740 matched controls) patients.
Results are shown as percentage and tested in 775 patients [338 CR-KP infection positive cases and 437 matched controls after filtered by age (≥70 years)]. CR-KP, carbapenem-resistant Klebsiella pneumoniae.
Figure 1.
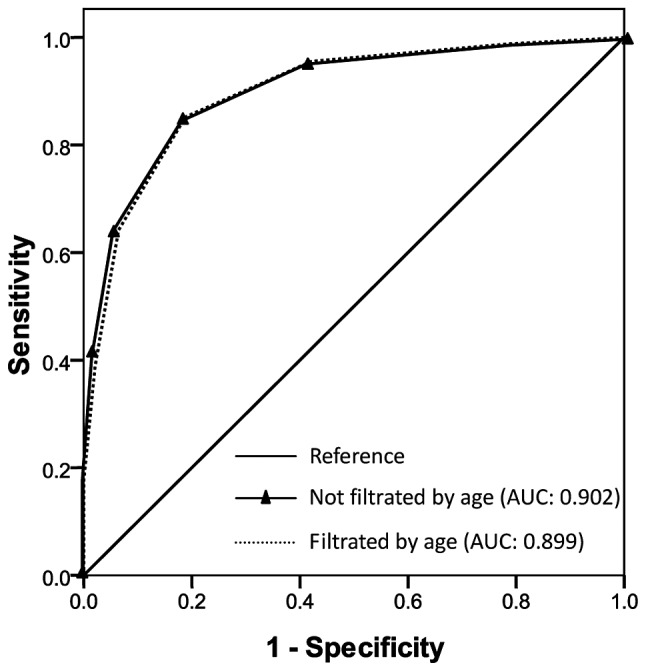
Receiver-operating characteristic curves for cumulative risk factors predicting carbapenem-resistant Klebsiella pneumoniae infection. AUC, area under the curve.
Discussion
The results of the present study showed that age, male gender, with cardiovascular disease, recent admission to ICU for more than 24 h, indwelling urinary catheter, mechanical ventilation, exposure to β-lactam-β-lactamase inhibitors, fourth-generation cephalosporins and carbapenems were associated with CR-KP infection.
Compared with young patients, elderly patients were more likely to have comorbidities and susceptibility to infections (11). In the present study, patients with CR-KP infection were elderly compared to those with no CR-KP infection.
Male gender was associated with CR-KP infection. Daikos et al found that the proportion of male patients surviving was significantly higher than those that succumbed due to carbapenemase-producing K. pneumoniae bloodstream infections (2). However, to the best of our knowledge, this is the first time that the male gender and cardiovascular disease constituted risk factors for CR-KP infection. Regarding the latter, it may be explained by the fact that cardiovascular disease is diagnosed more frequently in men (12).
As expected, ICU stay was an independent risk factor for CR-KP infections. ICUs are the principal hospital reservoirs of MDR bacteria. Patients with ICU admission represent a population subjected to many invasive procedures and medical devices, which facilitate cross-transmission of resistance mechanisms (7,13,14).
In the current study, CR-KP infection was also associated with indwelling urinary catheter and mechanical ventilation. These invasive procedures are well-known risk factors for MDR bacteria infection/colonization (7,13,15).
Prior administration of β-lactam-β-lactamase inhibitors and/or carbapenems was associated with CR-KP infection, as reported in other studies (7,8,16). This observation may be due to the fact that treatment with broad-spectrum antibiotics potentially promotes the selection of CR-KP strains by eliminating susceptible competing clones. In a previous study, it was reported that a combination therapy containing carbapenem was strongly associated with survival in CR-KP bloodstream infections (2). Thus, administration of carbapenem should be used prudently in empirical therapy, and once the CR-KP infection is highly suspected or verified, a regimen including carbapenem is likely to benefit the outcome.
Previous findings have shown that the presence of CR-KP-colonized patients in nearby beds was associated with CR-KP infection/colonization (17,18). In the present study, although the proportion of patients who had CR-KP-positive patients in nearby beds was higher in the case group than in the control group, CR-KP-positive patients in nearby beds was not an independent risk factor of CR-KP infection. This discrepancy may partly be because of the different case-control design, and remains to be confirmed.
The model for predicting CR-KP infection developed in this study displayed good discriminatory power (AUC=0.902), which is similar to the model developed by Tumbarello et al (7). The sensibility was 84.8% and specificity was 68.1% when there were three or more risk factors for predicting CR-KP infection. The age of patients (≥70 years) increased the predicting specificity to 81.2%. The predicting model is potentially useful in hospitals where CR-KP infection is epidemic. Empirical application of infection control measures, such as rectal swabs, may be limited to individuals with a higher possibility of CR-KP infection, thereby reducing workloads and costs. This prediction model may also be useful for empirical antimicrobial therapy and potentially reduce the risk of ineffective therapy during the initial phase of treatment. Notably, the prevention of nosocomial acquisition of resistant bacteria is dependent on implementation of and adherence to infection control standards.
In conclusion, the present findings provide information that may be useful for identifying patients at high risk for CR-KP infection and may assist physicians to use more effective treatments for CR-KP infections.
Glossary
Abbreviations
- CR-KP
Carbapenem-resistant Klebsiella pneumonia
- CS-KP
Carbapenem-sensitive Klebsiella pneumonia
- ICU
intensive care unit
- ESBLs
extended-spectrum β-lactamases
- MIC
minimal inhibitory concentration
- AUC
area under the curve
- MDR
multidrug resistance
References
- 1.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: An evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25:682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: Lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother. 2014;58:2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraenkel-Wandel Y, Raveh-Brawer D, Wiener-Well Y, Yinnon AM, Assous MV. Mortality due to blaKPC Klebsiella peumoniae bacteraemia. J Antimicrob Chemother. 2016;71:1083–1087. doi: 10.1093/jac/dkv414. [DOI] [PubMed] [Google Scholar]
- 4.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: Superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108–2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falagas ME, Rafailidis PI, Kofteridis D, Virtzili S, Chelvatzoglou FC, Papaioannou V, Maraki S, Samonis G, Michalopoulos A. Risk factors of carbapenem-resistant Klebsiella pneumoniae infections: A matched case control study. J Antimicrob Chemother. 2007;60:1124–1130. doi: 10.1093/jac/dkm356. [DOI] [PubMed] [Google Scholar]
- 6.Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother. 2008;52:1028–1033. doi: 10.1128/AAC.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tumbarello M, Trecarichi EM, Tumietto F, Del Bono V, De Rosa FG, Bassetti M, Losito AR, Tedeschi S, Saffioti C, Corcione S, et al. Predictive models for identification of hospitalized patients harboring KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2014;58:3514–3520. doi: 10.1128/AAC.02373-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarkotou O, Pournaras S, Voulgari E, Chrysos G, Prekates A, Voutsinas D, Themeli-Digalaki K, Tsakris A. Risk factors and outcomes associated with acquisition of colistin-resistant KPC-producing Klebsiella pneumoniae: A matched case-control study. J Clin Microbiol. 2010;48:2271–2274. doi: 10.1128/JCM.02301-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute (CLSI) CLSI; Wayne, PA: 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved standard M100. [Google Scholar]
- 11.Rodríguez-Baño J, Alcalá JC, Cisneros JM, Grill F, Oliver A, Horcajada JP, Tórtola T, Mirelis B, Navarro G, Cuenca M, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008;168:1897–1902. doi: 10.1001/archinte.168.17.1897. [DOI] [PubMed] [Google Scholar]
- 12.Pilote L, Dasgupta K, Guru V, Humphries KH, McGrath J, Norris C, Rabi D, Tremblay J, Alamian A, Barnett T, et al. A comprehensive view of sex-specific issues related to cardiovascular disease. CMAJ. 2007;176:S1–S44. doi: 10.1503/cmaj.051455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trecarichi EM, Cauda R, Tumbarello M. Detecting risk and predicting patient mortality in patients with extended-spectrum β-lactamase-producing Enterobacteriaceae bloodstream infections. Future Microbiol. 2012;7:1173–1189. doi: 10.2217/fmb.12.100. [DOI] [PubMed] [Google Scholar]
- 14.Lucena A, Dalla Costa LM, Nogueira KS, Matos AP, Gales AC, Paganini MC, Castro ME, Raboni SM. Nosocomial infections with metallo-beta-lactamase-producing Pseudomonas aeruginosa: Molecular epidemiology, risk factors, clinical features and outcomes. J Hosp Infect. 2014;87:234–240. doi: 10.1016/j.jhin.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Lin MY, Lyles-Banks RD, Lolans K, Hines DW, Spear JB, Petrak R, Trick WE, Weinstein RA, Hayden MK. Centers for Disease Control and Prevention Epicenters Program: The importance of long-term acute care hospitals in the regional epidemiology of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis. 2013;57:1246–1252. doi: 10.1093/cid/cit500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papadimitriou-Olivgeris M, Marangos M, Fligou F, Christofidou M, Bartzavali C, Anastassiou ED, Filos KS. Risk factors for KPC-producing Klebsiella pneumoniae enteric colonization upon ICU admission. J Antimicrob Chemother. 2012;67:2976–2981. doi: 10.1093/jac/dks316. [DOI] [PubMed] [Google Scholar]
- 17.Papadimitriou-Olivgeris M, Christofidou M, Fligou F, Bartzavali C, Vrettos T, Filos KS, Marangos M, Anastassiou ED. The role of colonization pressure in the dissemination of colistin or tigecycline resistant KPC-producing Klebsiella pneumoniae in critically ill patients. Infection. 2014;42:883–890. doi: 10.1007/s15010-014-0653-x. [DOI] [PubMed] [Google Scholar]
- 18.Bonten MJ. Colonization pressure: A critical parameter in the epidemiology of antibiotic-resistant bacteria. Crit Care. 2012;16:142. doi: 10.1186/cc11417. [DOI] [PMC free article] [PubMed] [Google Scholar]


