Abstract
A 71-year-old woman with arthralgia and lung fibrosis was referred to Mito Kyodo General Hospital (Mito, Japan) for a mass, which was incidentally observed on a chest radiograph. The chest computed tomography scan demonstrated fibrotic lesions in the lower lobes of the lung and a nodule in the left upper lobe. The serum levels of myeloperoxidase (MPO)-anti-neutrophil cytoplasmic antibody (ANCA) and proteinase 3 (PR3)-ANCA were 60.3 and 7.5 U/ml, respectively. A transbronchial biopsy obtained from the nodule in the left upper lobe of the lung revealed a lung adenocarcinoma and the patient underwent standard upper lobectomy of the left lung. Subsequent to the resection, the serum levels of PR3-ANCA and MPO-ANCA returned to 10.0 and <1.0 U/ml, respectively. Notably, titers of antinuclear antibodies were also decreased during the postoperative course. Although elevated serum ANCA levels are rarely seen in lung cancer, they may be associated with the occurrence of lung cancer in certain patients, as observed in the present case.
Keywords: ANCA, lung cancer, proteinase 3-ANCA, myeloperoxidase-ANCA
Introduction
Anti-neutrophil cytoplasmic antibodies (ANCAs) are autoantibodies produced by the immune system, which mistakenly target and attack proteins within an individual's neutrophils (1). Using indirect fluorescence antibody testing, ANCAs are typically classified as either myeloperoxidase (MPO)-ANCA, proteinase 3 (PR3)-ANCA and atypical-ANCA (1). The clinical significance of the two common types or ANCA subsets, MPO-ANCA and PR3-ANCA, differs; MPO-ANCA is often associated with microscopic polyarteritis, necrotizing glomerulonephritis and Churg-Strauss syndrome, whereas PR3-ANCA is found primarily in patients exhibiting granulomatosis with polyangiitis (previously Wegener's granulomatosis) (1). The association between MPO-ANCA and lung fibrosis has also been demonstrated (2,3). The present case report describes a patient with elevated serum levels of MPO-ANCA and PR3-ANCA, which decreased during the postoperative course. To the best of our knowledge, this is the first case to show a decrease in elevated serum levels of the two types of ANCA following treatment for lung cancer.
Case report
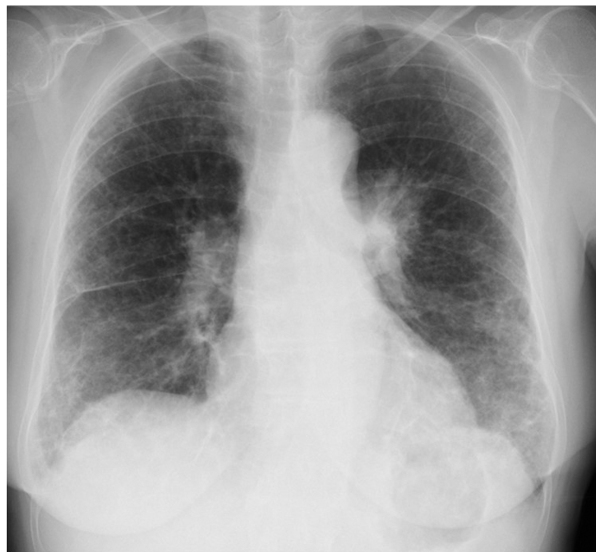
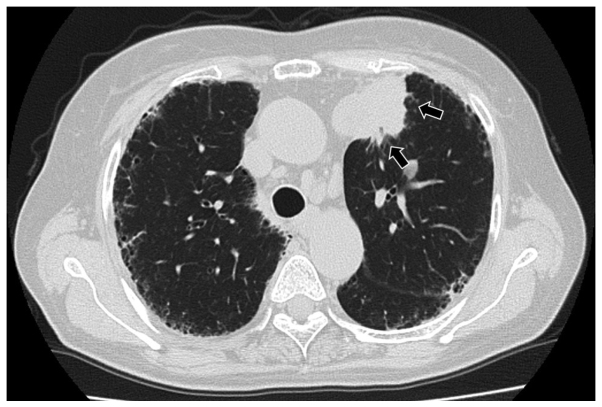
A 71-year-old woman with arthralgia and lung fibrosis was referred to Mito Kyodo General Hospital (Mito, Japan) for a mass that was incidentally observed on a chest radiograph. The chest radiograph and a computed tomography scan showed ground-glass opacity and honeycombing in the lower lobes of the lungs, and a nodule in the left upper lobe of the lung (Figs. 1 and 2). The differential diagnosis was primary lung cancer and a nodule with granulomatosis with polyangiitis. Laboratory analysis demonstrated antinuclear antibodies were positive with a titer of 1:160 homogenous pattern, and a titer of 1:160 speckled pattern. Serum levels of MPO-ANCA and PR3-ANCA were elevated to 60.3 and 7.5 U/ml, respectively (normal range, ≤3.5 IU/ml). A transbronchial biopsy obtained from the lesion revealed a lung adenocarcinoma and the patient underwent standard upper lobectomy of the left lung. Written informed consent was obtained from the patient. The postoperative period was not eventful and the postoperative diagnosis was pT3N1M0, stage IIIA (according to the seventh edition of the International Union Against Cancer TNM Staging System for Lung Cancer), epidermal growth factor receptor mutation-negative and anaplastic lymphoma receptor tyrosine kinase-negative fusion gene. One month subsequent to the resection, MPO-ANCA and PR3-ANCA serum levels had returned to 10.0 and <1.0 U/ml, respectively. Antinuclear antibodies were positive, with a titer of 1:80 homogenous pattern and a titer of 1:80 speckled pattern. The patient experienced an uneventful postoperative course for 6 months; however, developed lung and brain metastases, and succumbed due to lung cancer 15 months after surgery.
Figure 1.
Chest radiograph demonstrating ground-glass opacitiy and honeycombing in the lower lobes of the lung.
Figure 2.
Chest computed tomography scan demonstrating a nodule in the left upper lobe of the lung (arrows).
Discussion
The presence of antibodies that react with neutrophil cytoplasm in patients who had necrotizing glomerulonephritis and small-vessel vasculitis was first reported by Davies et al in 1982 (4). A wide variety of pulmonary abnormalities, including alveolar hemorrhage and pulmonary fibrosis, have been reported to be associated with MPO-ANCA (5–7). Whereas the majority of patients with granulomatosis with polyangiitis have elevated serum levels of PR3-ANCA (8,9). It currently remains unknowns as to whether there is a definite association between solid tumors and elevated serum levels of ANCAs. Edgar et al (10) were the first to report an association between solid tumors and ANCA-associated vasculitis. Thereafter, Tatsis et al (11) evaluated the frequencies and types of malignant diseases occurring before or simultaneously with the diagnosis of patients positive for PR3-ANCA. The authors reported that 23 of 477 patients that were PR3-ANCA-positive had solid tumors; the most common malignant disease was renal cell carcinoma and one patient had lung cancer (11). Including this patient, to the best of knowledge, there have been four ANCA-positive lung cancer patients (Table I) (10–13). The patients were the fifth and sixth decades, and were all male. Three of the patients were positive for PR3-ANCA, and the others were positive for MPO-ANCA. Two patients presented with squamous cell carcinoma, and one patient had adenocarcinoma (and there was no description for one patient). Two patients had renal impairment, but none of the patients had pulmonary fibrosis. The patient in the current study complained of arthralgia for 5 years and lung fibrosis was detected on a chest radiograph taken 2 years before referral to our department. Elevated serum levels of MPO-ANCA may be associated with lung fibrosis in this patient. As the patient presented with a mass on the chest radiograph, measurement of serum PR3-ANCA levels was performed to rule out the possibility of developing granulomatosis with polyangiitis. Notably, in the present case, elevated PR3-ANCA and MPO-ANCA serum levels were noted pretreatment, and decreases in the levels of the two types of ANCA were observed postoperatively. Furthermore, titers of antinuclear antibodies were observed to decrease during the postoperative course. As shown in Table I, decreases in the elevated serum levels of either MPO-ANCA or PR3-ANCA following therapy for lung cancer were reported in two patients (12,13). Navarro et al (12) reported a case of lung adenocarcinoma with elevated MPO-ANCA serum levels and its decrease as a result of therapy for lung cancer with prednisolone and cyclophosphamide. Morisako et al (13) reported a case with elevated levels of PR3-ANCA in a patient with advanced squamous cell lung carcinoma, and the ANCA level was decreased subsequent to successful chemotherapy. However, to the best of our knowledge, the present case is the first to exhibit decreases in elevated serum levels of the two types of ANCA due to therapy for lung cancer. In certain cases of ANCA-associated vasculitis, malignant disease may be a trigger for either the generation of ANCAs or the development of vasculitis (10). In conclusion, together with the results of previously reported cases (12,13), elevated serum ANCA levels may be associated with the existence of malignant disease, although the occurrence of the two types of ANCA being elevated is rare. The present case provides information, which may facilitate the diagnosis and treatment of similar cases of patients presenting with these diseases.
Table I.
ANCA-positive lung cancer patients.
| Author, year (Refs.) | Age (years) | Gender | ANCA-positive | Pathology | Renal impairment | IPF | Lung cancer therapy | ANCA improvement post-treatment |
|---|---|---|---|---|---|---|---|---|
| Edgar et al, 1993 (10) | 62 | M | PR3-ANCA | SQ | Present | ND | BSC | ND |
| Tatsis et al, 1999 (11) | 63 | M | PR3-ANCA | ND | ND | ND | ND | ND |
| Navarro et al, 1994 (12) | 68 | M | MPO-ANCA | AD | Present | ND | Irradiation | Present |
| Morisako et al, 2006 (13) | 57 | M | PR3-ANCA | SQ | Absent | Absent | Chemotherapy | Present |
| Present case | 70 | F | PR3-ANCA | AD | Absent | Present | Surgery | Present |
| MPO-ANCA |
ANCA, anti-neutrophil cytoplasmic antibodies; M, male; F, female; PR3, proteinase 3; MPO, myeloperoxidase; SQ, squamous cell lung cancer; AD, adenocarcinoma of the lung; ND, not described; IPF, idiopathic pulmonary fibrosis; BSC, best supportive care.
References
- 1.Beauvillain C, Delneste Y, Renier G, Jeannin P, Subra JF, Chevailler A. Antineutrophil cytoplasmic autoantibodies: how should the biologist manage them? Clin Rev Allergy Immunol. 2008;35:47–58. doi: 10.1007/s12016-007-8071-9. [DOI] [PubMed] [Google Scholar]
- 2.Hervier B, Pagnoux C, Agard C, Haroche J, Amoura Z, Guillevin L, Hamidou MA. French Vasculitis Study Group: Pulmonary fibrosis associated with ANCA-positive vasculitides. Retrospective study of 12 cases and review of the literature. Ann Rheum Dis. 2009;68:404–407. doi: 10.1136/ard.2008.096131. [DOI] [PubMed] [Google Scholar]
- 3.Yamada H. ANCA: Associated lung fibrosis. Semin Respir Crit Care Med. 2011;32:322–327. doi: 10.1055/s-0031-1279828. [DOI] [PubMed] [Google Scholar]
- 4.Davies DJ, Moran JE, Niall JF, Ryan GB. Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? Br Med J (Clin Res Ed) 1982;285:606. doi: 10.1136/bmj.285.6342.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arimura Y, Minoshima S, Tanaka U, Fujii A, Kobayashi M, Nakabayashi K, Kitamoto K, Nagasawa T. Pulmonary involvement in patients with myeloperoxidase specific-antineutrophil cytoplasmic antibody. Ryumachi. 1995;35:46–55. (In Japanese) [PubMed] [Google Scholar]
- 6.Homma S, Matsushita H, Nakata K. Pulmonary fibrosis in myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitides. Respirology. 2004;9:190–196. doi: 10.1111/j.1440-1843.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- 7.Gaudin PB, Askin FB, Falk RJ, Jennette JC. The pathologic spectrum of pulmonary lesions in patients with anti-neutrophil cytoplasmic autoantibodies specific for anti-proteinase 3 and anti-myeloperoxidase. Am J Clin Pathol. 1995;104:7–16. doi: 10.1093/ajcp/104.1.7. [DOI] [PubMed] [Google Scholar]
- 8.Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337:1512–1523. doi: 10.1056/NEJM199711203372106. [DOI] [PubMed] [Google Scholar]
- 9.Jennette JC. Antineutrophil cytoplasmic autoantibody-associated diseases: a pathologist's perspective. Am J Kidney Dis. 1991;18:164–170. doi: 10.1016/S0272-6386(12)80874-2. [DOI] [PubMed] [Google Scholar]
- 10.Edgar JD, Rooney DP, McNamee P, McNeill TA. An association between ANCA positive renal disease and malignancy. Clin Nephrol. 1993;40:22–25. [PubMed] [Google Scholar]
- 11.Tatsis E, Reinhold-Keller E, Steindorf K, Feller AC, Gross WL. Wegener's granulomatosis associated with renal cell carcinoma. Arthritis Rheum. 1999;42:751–756. doi: 10.1002/1529-0131(199904)42:4<751::AID-ANR19>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 12.Navarro JF, Quereda C, Rivera M, Navarro FJ, Ortuño J. Anti-neutrophil cytoplasmic antibody-associated paraneoplastic vasculitis. Postgrad Med J. 1994;70:373–375. doi: 10.1136/pgmj.70.823.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morisako T, Tsuchida F, Nakamura H, Ohishi S, Matsuoka T. A case of squamous cell carcinoma of the lung with a high titer of proteinase 3 antineutrophil cytoplasmic antibody. Nihon Kokyuki Gakkai Zasshi. 2006;44:139–143. (In Japanese) [PubMed] [Google Scholar]