Abstract
Background
Larger gastrojejunal (GJ) anastomosis aperture is one independent predictor for weight regain after Roux-en-Y gastric bypass. Transoral outlet reduction (TORe) has proven safe and effective for treatment of weight regain by using a superficial-thickness (ST) suturing device. Full-thickness (FT) suturing devices are now available, potentially providing more effective results.
Objective
To compare effectiveness of superficial-thickness with full-thickness TORe.
Design
Matched cohort study: patients were matched sequentially by GJ anastomosis aperture, body mass index, and age.
Setting
Tertiary-care center.
Patients
A total of 59 consecutive patients undergoing full-thickness TORe were matched with 59 patients undergoing superficial-thickness TORe. All had GJ anastomosis apertures >20 mm.
Intervention
Transoral outlet reduction.
Main Outcome Measurements
Weight loss and rate of adverse events.
Results
Post-TORe GJ anastomosis apertures were similar between groups (ST 6.9 ± 0.2 mm vs FT 7.1 ± 0.3 mm). Weight loss was greater at 6 months in the FT group (10.6 ± 1.8 kg in FT vs 4.4 ± 0.8 kg in ST; P < .01) and at 1 year (8.6 ± 2.5 kg in FT vs 2.9 ± 1.0 kg in ST; P < .01). Excess weight loss was greater in the FT group at 6 months (20.4 ± 3.3% in FT vs 8.1 ± 2.5% in ST; P < .01) and at 1 year (18.9 ± 5.4% in FT vs 9.1 ± 2.3% in ST; P = .03).
Limitations
This was a single-center, retrospective, cohort study.
Conclusion
There is level 1b evidence for effectiveness of TORe by using a superficial mucosal suturing device. This matched cohort study compared TORe by using the same ST suturing device with TORe by using a newer, FT suturing device and the same operative methods. FT TORe resulted in significantly more weight loss than ST TORe at 6 months and at 1 year. Full-thickness TORe is a significant improvement over ST TORe for endoscopic therapy of weight regain in patients with dilated GJ anastomosis.
As the population of bariatric surgery patients increases, postoperative weight regain is a growing concern. Reversal of weight loss reintroduces comorbidities and decreases quality of life.1 After dramatic initial postoperative weight loss, many bariatric surgery patients reach a weight plateau in 1 to 2 years.2 Approximately 20% of patients do not reach 1-year weight loss goals, and 30% of patients begin to regain weight within 2 years. Nearly two-thirds of patients regain substantial weight within 4 years.3-5
Larger gastrojejunal (GJ) anastomosis aperture is one independent predictor for postoperative weight regain.6-7 Surgical revision is problematic. Adverse event rates are prohibitively high, reaching over 15%, and procedures entail longer intraoperative time and greater blood loss.8-10
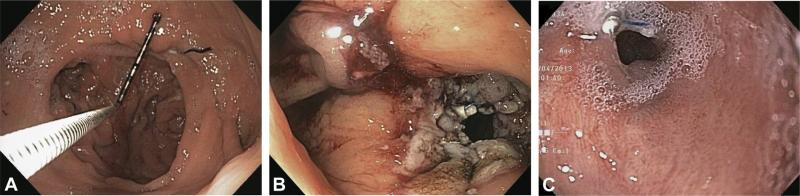
Transoral outlet reduction (TORe) offers a less-invasive alternative (Fig. 1). Revision of bariatric surgery by using endoluminal suturing was first reported in 2004.11-12 Since then, a number of devices have been studied for revision, including the Incisionless Operating Platform (USGI Medical, San Clemente, CA) and StomaphyX (EndoGastric Solutions, Redmond, WA).13-16 Recently, a randomized, double-blinded, sham-controlled trial that used the Endo-Cinch (CR Bard, Murray Hill, NJ), a suction-based superficial suturing device, provided level 1b evidence for effectiveness of TORe.17 More recently, the OverStitch (Apollo Endosurgery, Austin, Tex) has demonstrated safety and effectiveness for TORe.18 Unlike the prior EndoCinch device, the OverStitch uses a curved needle for tissue purchase and allows placement of full-thickness (FT) sutures.
Figure 1.
A, Dilated gastrojejunal anastomosis (GJA). B, GJA after transoral outlet reduction (TORe) with a full-thickness suturing device. C, GJA 6 months after full-thickness TORe.
The aim of this study was to compare the relative effectiveness of a suction-based, superficial-thickness (ST) suturing device versus an FT endoscopic suturing device for TORe in patients who are status post Roux-en-Y gastric bypass (RYGB) with weight regain.
METHODS
Patients
All consecutive patients undergoing full-thickness TORe between 2010 and 2012 were included. To be eligible for TORe, patients had GJ anastomosis dilated to apertures >20 mm. Procedures in the ST cohort were performed between 2004 and 2008, after which the device was withdrawn. The device for FT TORe became available in 2010; hence, there was no temporal overlap regarding patient allocation to a particular procedure. All consecutive patients undergoing FT TORe were matched; each patient undergoing FT TORe was matched 1:1 with a patient from the ST group. Matching factors were applied sequentially: GJ anastomosis aperture at time of TORe, body mass index (BMI) at time of TORe, and age.
Procedure
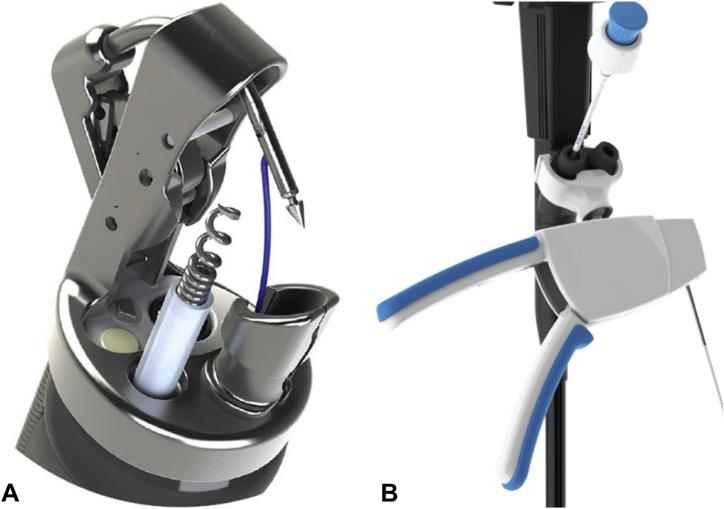
In the FT group, TORe was performed by using the Overstitch Endoscopic Suturing System (Fig. 2). The Overstitch attaches to a double-channel endoscope (GIF-2T160; Olympus America, Center Valley, Pa). It comprises a curved suture arm attached to the endoscope tip and an anchor exchange deployed through one channel. When the handle is activated, the suture arm pushes the needle through the tissue and passes the needle to the anchor exchange. The handle is then opened, releasing the tissue. The anchor then can be passed back to the suture arm for additional stitch placement (Video 1, available online at www.giejournal.org).
Figure 2.
A, Apollo OverStitch tip with suturing arm. B, Apollo OverStitch handle (Apollo Endosurgery, Austin, Tex).
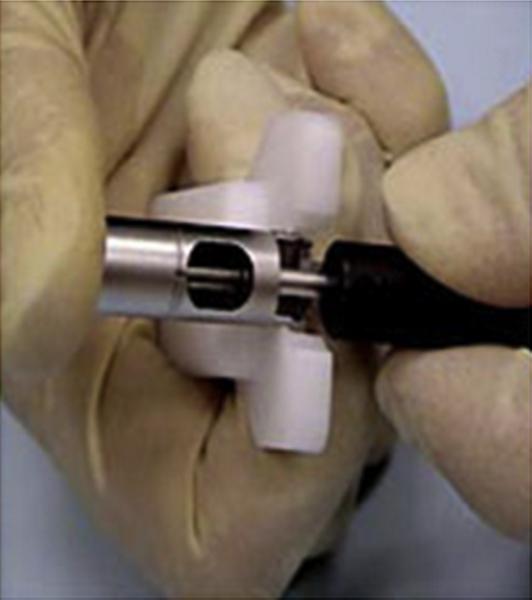
In the ST group, TORe was performed by using the most recent iteration of EndoCinch (Fig. 3). Patients included in other trials were not included in the present study, because they underwent specific and varied trial protocols. Furthermore, procedures performed with previous iterations of EndoCinch and other devices were not included in the present study. Tissue at the rim of the GJ anastomosis is suctioned into the device, and the handle is activated to place a stitch. The device is then removed and reloaded. The device must be reinserted to place the next stitch. The sutures are tightened and secured to form a tissue plication or left to be secured at the end of the procedure (Video 2, available online at www.giejournal.org).
Figure 3.
Bard EndoCinch tip, with cap used to suction mucosa for apposition.
All procedures in both arms were performed by using the same method. General anesthesia with endotracheal intubation was used. Upper endoscopy was performed to record GJ anastomosis aperture and pouch length, and an overtube was inserted. A 5 to 10–mm area around the margin of the GJ anastomosis was ablated by using end-firing forced argon plasma coagulation at 30 W. Interrupted stitches were placed sequentially from the lower left to the upper right of the GJ anastomosis until the aperture was reduced to 12 mm or less. If the gastric pouch was dilated, interrupted stitches were placed in the distal pouch to reduce pouch volume.
Patients were given nothing by mouth on the evening after the procedure. They proceeded to a clear liquid diet for 1 day, followed by full liquids for 6 weeks and soft solids for 2 weeks. Follow-up clinical examinations were scheduled at 3, 6, and 12 months and entailed weight recording and interview regarding postprocedure symptoms, dietary and exercise habits, and satiety.
RESULTS
All 59 patients who underwent FT TORe and 59 of 129 patients who underwent ST TORe were included. Baseline characteristics are reported in Table 1 for the matched cohort and in Table 2 for all consecutive patients undergoing ST TORe. The FT TORe group had a higher proportion of men, but there was no other significant difference in baseline demographics, prevalence of diabetes, preoperative weight regain, preoperative BMI, or preoperative anatomy.
TABLE 1.
Baseline characteristics (matched cohort)
| Superficial (n = 59) | Full-thickness (n = 59) | P value | |
|---|---|---|---|
| Sex,* no. | 3 M/56 F | 15 M/44 F | < .01 |
| Age, y | 48.8 ± 1.1† | 49.9 ± 1.3 | .52 |
| Diabetes mellitus, % | 17.2 | 23.7 | .49 |
| Lost weight regained, % | 32.5 ± 3.0 | 40.9 ± 3.2 | .06 |
| Weight regained, kg | 18.7 ± 1.8 | 18.6 ± 1.5 | .97 |
| Before TORe BMI | 40.4 ± 1.0 | 41.1 ± 1.3 | .67 |
| Before TORe GJA, mm | 24.3 ± 0.8 | 24.8 ± 0.9 | .68 |
| Before TORe pouch, mm | 51.8 ± 1.5 | 49.7 ± 2.4 | .46 |
M, Male; F, female; TORe, transoral outlet reduction; BMI, body mass index; GJA, gastrojejunal anastomosis.
Statistical significance.
(Mean ± SEM)
TABLE 2.
Baseline characteristics (all consecutive patients)
| Superficial (n = 129) | Full-thickness (n = 59) | P value | |
|---|---|---|---|
| Sex, no. | 19 M/110 F | 15 M/44 F | .085 |
| Age, y | 47.0 ± 0.9* | 49.9 ± 1.3 | .061 |
| Diabetes mellitus, % | 21.7 | 23.7 | .85 |
| Lost weight regained, % | 33.4 ± 3.0 | 40.9 ± 3.2 | .13 |
| Weight regained, kg | 19.2 ± 1.6 | 18.6 ± 1.5 | .82 |
| Before TORe BMI | 39.7 ± 0.8 | 41.1 ± 1.3 | .34 |
| Before TORe GJA, mm | 23.6 ± 0.6 | 24.8 ± 0.9 | .27 |
| Before TORe pouch, mm | 52.8 ± 1.9 | 49.7 ± 2.4 | .34 |
M, Male; F, female; TORe, transoral outlet reduction; BMI, body mass index; GJA, gastrojejunal anastomosis.
(Mean ± SEM)
The overall number of stitches was similar between groups (Table 3). Significantly fewer stitches were required to achieve smaller GJ anastomosis diameters in the FT TORe group. Patients who underwent FT TORe had significantly more stitches placed for pouch size reduction, but post-TORe GJ anastomosis aperture and pouch diameter were similar between groups.
TABLE 3.
Procedure characteristics
| Superficial (n = 59) | Full-thickness (n = 59) | P value | |
|---|---|---|---|
| Total stitches | 3.5 ± 0.1* | 3.8 ± 0.2 | .18 |
| Stitches, GJA† | 3.3 ± 0.1 | 2.5 ± 0.2 | < .01 |
| Stitches, pouch† | 0.3 ± 0.1 | 1.2 ± 0.2 | < .01 |
| After-TORe GJA, mm | 6.9 ± 0.2 | 7.1 ± 0.3 | .58 |
| After-TORe pouch, mm | 48.6 ± 2.0 | 46.4 ± 2.1 | .45 |
GJA, Gastrojejunal anastomosis; TORe, transoral outlet reduction.
(Mean ± SEM)
Statistical significance.
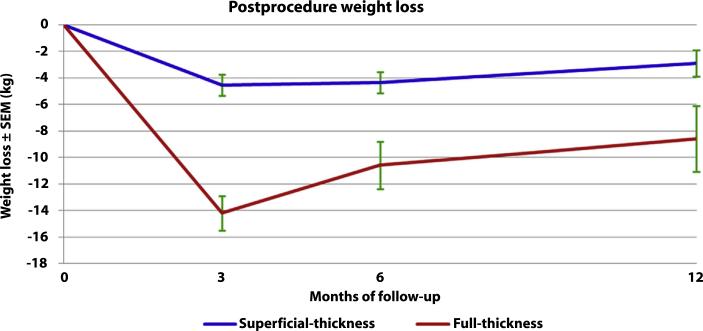
One transfusion-requiring bleeding event occurred in each group (P = not significant). Weight loss at all time points was significantly greater in the FT TORe group. Weight loss at 3 months was 14.2 ± 1.3 kg after FT versus 2.1 ± 0.8 kg after ST; P < .01. Weight loss at 6 months was 10.6 ± 1.8 kg after FT versus 4.4 ± 0.8 kg after ST; P < .01. Weight loss at 1 year was 8.6 ± 2.5 kg after FT versus 2.9 ± 1.0 kg after ST; P < .01. Excess weight loss (EWL) was significantly greater in the FT TORe group. EWL at 6 months was 20.4 ± 3.3% after FT versus 8.1 ± 2.5% after ST; P < .01. EWL at 1 year was 18.9 ± 5.4% after FT versus 9.1 ± 2.3% after ST; P = .03. BMI loss was significantly greater after FT TORe. Six-month BMI loss was 8.0 ± 0.8% after FT TORe versus 3.1 ± 1.0% after ST TORe; P < .01. One-year BMI loss was 7.4 ± 1.6% after FT TORe versus 3.4 ± 0.6% after ST TORe; P = .02. Weight trends are shown in Figure 4. Endoscopic follow-up has been limited because of lack of indication for repeat endoscopy, although endoscopy 6 to 12 months after FT TORe in two cases demonstrated stable GJ anastomosis size in both (Fig. 1C).
Figure 4.
Weight trend. SEM, standard error of the mean.
The 59 patients who underwent ST TORe and were chosen for the matched cohort experienced similar weight loss compared with the entire ST TORe group. Six-month weight loss in the cohort was 4.4 ± 0.8 kg in the cohort versus 4.4 ± 0.6 kg in the entire group. One-year weight loss in the cohort was 2.9 ± 1.0 kg in the cohort versus 2.6 ± 0.7 kg in the entire group.
DISCUSSION
This study is a matched cohort comparison of TORe with an ST suturing device and TORe with a newer, FT suturing device, otherwise using the same methods. Both suturing devices demonstrated safety for transoral outlet reduction. Patients who underwent TORe with an FT suturing device demonstrated significantly higher weight loss at 6 months and at 1 year than did those who had TORe with a superficial, suction-based suturing device. There are several possible reasons for better weight loss results after use of the FT platform. It is possible that the FT plications are more durable than superficial mucosal apposition. Additionally, FT plications may result in less tissue compliance across the plication. Consequently, FT plications may result in less GJ anastomosis dynamic compliance and smaller effective GJ anastomotic apertures than with mucosal apposition. Furthermore, because the FT device can target suture placement precisely by using a curved needle rather than tissue acquisition that uses suction, suture placement may be more accurate. Finally, the suction-based tissue acquisition used by ST devices may result in more collateral tissue trauma and inflammation, resulting in a higher rate of suture loss.
Level 1b evidence for the effectiveness of TORe for weight regain after RYGB recently has been published from the RESTORe trial.17 That study used a superficial mucosal suturing device and compared TORe with a sham procedure in a blinded and randomized fashion. A total of 77 patients with GJ anastomosis diameters >20 mm were included. GJ anastomosis diameters were reduced to <10 mm in 89%. There were no perforations, and the adverse event rate was similar to that of the sham group. A total of 96% of patients who underwent revision had weight loss or stabilization in the following 6 months. Mean weight loss in the revised group was 3.8% of body weight versus 0.3% in the sham group (P = .02) in intent-to-treat analysis.
The current study is limited by its retrospective design. However, patients were matched by criteria known to affect weight loss after the procedure, and baseline characteristics were similar between groups. More stitches were placed for pouch reduction in the FT group, given that the device is reloadable without removal and more maneuverable within the pouch. The effect of this difference on weight loss will require further study. Additionally, the study is limited by single-center performance. However, this resulted in a similar patient population between groups and allowed standardization of many procedure parameters and clinical follow-up.
The results of this study should be considered in the context of recently published level 1b evidence from the RESTORe trial, which exclusively used the superficial suturing device compared in this study. Although that device proved safe and effective, this study demonstrates that FT TORe is even more effective in the same population, with greater weight loss at 6 months and 1 year. Full-thickness is a significant improvement over ST TORe for endoscopic therapy of weight regain in patients with dilated GJ anastomoses.
Supplementary Material
Take-home Message.
Full-thickness transoral outlet reduction (TORe) is a significant improvement over superficial-thickness TORe for endoscopic therapy of weight regain in patients with dilated gastrojejunal anastomoses.
Abbreviations
- BMI
body mass index
- EWL
excess weight loss
- FT
full thickness
- GJ
gastrojejunal
- RYGB
Roux-en-Y gastric bypass
- ST
superficial thickness
- TORe
transoral outlet reduction
Footnotes
DISCLOSURE: C. Thompson is a consultant for Apollo Endosurgery and CR Bard. All other authors disclosed no financial relationships relevant to this publication.
This video can be viewed directly from the GIE website or by using the QR code and your mobile device. Download a free QR code scanner by searching “QR Scanner” in your mobile device's app store.
REFERENCES
- 1.Christou NV, Look D, MacLean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244:734–40. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell JE, Lancaster KL, Burgard MA, et al. Long-term follow-up of patients’ status after gastric bypass. Obes Surg. 2001;11:464–8. doi: 10.1381/096089201321209341. [DOI] [PubMed] [Google Scholar]
- 3.Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793–6. doi: 10.1001/jama.288.22.2793. [DOI] [PubMed] [Google Scholar]
- 4.Hsu LK, Benotti PN, Dwyer J, et al. Nonsurgical factors that influence the outcome of bariatric surgery: a review. Psychosom Med. 1998;60:338–46. doi: 10.1097/00006842-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Magro DO, Gelonese B, Delfini R, et al. Long-term weight regain after gastric bypass: a 5-year prospective study. Obes Surg. 2008;18:648–65. [Google Scholar]
- 6.Dayyeh BK, Lautz DB, Thompson CC. Gastrojejunal stoma diameter predicts weight regain after Roux-en-Y gastric bypass. Clin Gastroenterol Hepatol. 2011;9:228–33. doi: 10.1016/j.cgh.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller MK, Wildi S, Scholz T, et al. Laparoscopic pouch resizing and redo of gastro-jejunal anastomosis for pouch dilatation following gastric bypass. Obes Surg. 2005;15:1089–95. doi: 10.1381/0960892055002257. [DOI] [PubMed] [Google Scholar]
- 8.Coakley BA, Deveney CW, Spight DH, et al. Revisional bariatric surgery for failed restrictive procedures. Surg Obes Relat Dis. 2008;4:581–6. doi: 10.1016/j.soard.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Dapri G, Cadiere GB, Himpens J. Laparoscopic conversion of adjustable gastric banding and vertical banded gastroplasty to duodenal switch. Surg Obes Relat Dis. 2009;5:678–83. doi: 10.1016/j.soard.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Ryou M, Ryan MB, Thompson CC. Current status of endoluminal bariatric procedures for primary and revision indications. Gastrointest Endosc Clin N Am. 2011;21:315–33. doi: 10.1016/j.giec.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson CC, Carr-Locke D, Saltzman JR, et al. Peroral endoscopic repair of staple-line dehiscences in Roux-en-Y gastric bypass: a less invasive approach. Gastroenterology. 2004;126:A810. [Google Scholar]
- 12.Thompson CC, Slattery J, Bundga ME, et al. Peroral endoscopic reduction of dilated gastrojejunal anastomosis after Roux-en-Y gastric bypass: a possible new option for patients with weight regain. Surg Endosc. 2006;20:1744–8. doi: 10.1007/s00464-006-0045-0. [DOI] [PubMed] [Google Scholar]
- 13.Herron DM, Birkett DH, Thompson CC, et al. Gastric bypass pouch and stoma reduction using a transoral endoscopic anchor placement system: a feasibility study. Surg Endosc. 2008;22:1093–9. doi: 10.1007/s00464-007-9623-z. [DOI] [PubMed] [Google Scholar]
- 14.Mullady DK, Lautz DB, Thompson CC. Treatment of weight regain after gastric bypass surgery when using a new endoscopic platform: initial experience and early outcomes (with video). Gastrointest Endosc. 2009;70:440–4. doi: 10.1016/j.gie.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 15.Horgan S, Jacobsen G, Weiss GD, et al. Incisionless revision of post-Roux-en-Y bypass stomal and pouch dilation: multicenter registry results. Surg Obes Relat Dis. 2010;6:290–5. doi: 10.1016/j.soard.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Mikami D, Needleman B, Narula V, et al. Natural orifice surgery: initial US experience utilizing the StomaphyX device to reduce gastric pouches after Roux-en-Y gastric bypass. Surg Endosc. 2010;24:223–8. doi: 10.1007/s00464-009-0640-y. [DOI] [PubMed] [Google Scholar]
- 17.Thompson CC, Chand B, Chen YK, et al. Endoscopic suturing for transoral outlet reduction increases weight loss after Roux-en-Y gastric bypass surgery. Gastroenterology. 2013;145:129–37. doi: 10.1053/j.gastro.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Jirapinyo P, Slattery J, Ryan MB, et al. Evaluation of an endoscopic suturing device for transoral outlet reduction in patients with weight regain following Roux-en-Y gastric bypass. Endoscopy. 2013;45:532–6. doi: 10.1055/s-0032-1326638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.