Abstract
Type 2 diabetes can be a problem for captive chimpanzees. Accurate blood glucose (BG) readings are necessary to monitor and treat this disease. Thus, obtaining voluntary samples from primates through positive reinforcement training (PRT) is critical. The current study assessed the voluntary participation of 123 chimpanzees in BG sampling and investigated factors that may contribute to individual success. All subjects participate in regular PRT sessions as part of a comprehensive behavioral management program. Basic steps involved in obtaining BG values include: voluntarily presenting a finger/toe; allowing digit disinfection; holding for the lancet device; and allowing blood collection onto a glucometer test strip for analysis. We recorded the level of participation (none, partial, or complete) when each chimpanzee was first asked to perform the testing procedure. Nearly 30% of subjects allowed the entire procedure in one session, without any prior specific training for the target behavior. Factors that affected this initial successful BG testing included sex, personality (chimpanzees rated higher on the factor “openness” were more likely to participate with BG testing), and past training performance for “present-for-injection” (chimpanzees that presented for their most recent anesthetic injection were more likely to participate). Neither age, rearing history, time since most recent anesthetic event nor social group size significantly affected initial training success. These results have important implications for captive management and training program success, underlining individual differences in training aptitude and the need for developing individual management plans in order to provide optimal care and treatment for diabetic chimpanzees in captivity.
Keywords: positive reinforcement training (PRT), personality, diabetes, animal welfare
INTRODUCTION
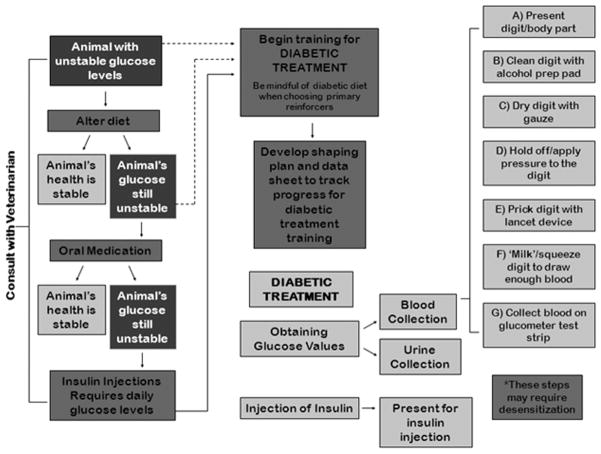
The diagnosis of type 2 (or adult onset) diabetes mellitus in captive nonhuman primates (NHP) has been occurring with increasing frequency. Cases of diabetes have been reported in over 24 different species of NHP [see Hansen, 1996], including the chimpanzee (Pan troglodytes). Factors linked to the onset of type 2 diabetes include obesity [Hansen and Bodkin, 1993], stress [Sapolsky, 1998], genetic predispositions [humans: Dahlquist et al., 1989; Weiss et al., 2000], and aging [humans: CDC, 2011; chimpanzees: McTighe et al., 2011]. High calorie diets and physical inactivity, hence obesity, are common among captive animals [Goodchild and Schwitzer, 2008; D’Eath et al., 2009]. The potential for stress in captive environments may also be high, due to exposure to loud, unpredictable, and uncontrollable noise; restricted ranging opportunities; non-species-typical group compositions; and reduced foraging times, among other things [see Morgan and Tromborg, 2007 for an overview]. Additionally, captive animals, in general, tend to live longer than wild animals due to many factors including veterinary care, lack of predation risk and habitat scarcity, as well as an abundance of food. In fact, chimpanzees in the wild live an average of 40–45 years, whereas, in captivity they can live much longer [Cawthon-Lang, 2006; Lambeth et al., 2013]. Combined, the factors associated with captivity (obesity, stress, and longer lifespan) suggest that captive primates could be at high risk for developing type 2 diabetes. Managers of captive animals should be cognizant of, and prepared to address, this risk. Currently, the prevention of type 2 diabetes in captive primate colonies focuses primarily on (1) early detection of pre-diabetes via annual health checks and (2) weight management techniques, including lower calorie diets and the provision of additional opportunities for physical activity [see Lambeth et al., 2011]. However, once a subject develops type 2 diabetes, managing the disease is very complex and time consuming, involving daily capillary blood sample collections for blood glucose (BG) readings and associated insulin injections. For a comprehensive depiction of the diabetic treatment process overall, from diagnosis through effective management, refer to Figure 1. Figure 2 shows, in detail, the steps involved in getting a single BG reading.
Fig. 1.
Diabetic treatment program; from diagnosis through maintenance.
Fig. 2.
Steps within the blood glucose testing process.
The use of positive reinforcement training (PRT) techniques with NHP to facilitate voluntary cooperation with daily husbandry, veterinary, or scientific procedures is widely used/recommended [Home Office, 1989; Laule et al., 2003; McCann et al., 2007; Veeder et al., 2009] and is known to decrease any fear or stress generally associated with such procedures [Prescott and Buchanan-Smith, 2003]. Indeed, Graham et al. [2011] found that the use of restraint (physical and chemical) for treatment was significantly reduced, if not almost completely eliminated, after the implementation of a training program for voluntary injections in experimentally- induced diabetic rhesus (Macaca mulatta) and cynomolgus macaques (M. fascicularis) [Graham et al., 2011]. Monkeys that were involved in the training program showed significantly lower rates of chronic stress, as assessed via thymus histology, than traditionally restrained macaques [Graham et al., 2011].
When attempting to determine the proper amount of insulin required for treatment, accurate measurements of glucose levels are imperative. Lambeth et al. [2006] demonstrated that BG levels were significantly higher when chimpanzees were anesthetized non-voluntarily, compared to when they voluntarily presented for an injection of anesthetic. Thus, in order to obtain accurate BG values for diabetic care, it is important that blood samples be acquired voluntarily, using PRT techniques.
The PRT program at The University of Texas MD Anderson’s Michale E. Keeling Center for Comparative Medicine and Research (KCCMR) was established in the early 1990s. Our chimpanzees therefore, have an extensive repertoire of trained behaviors including, but not limited to: targeting; retrieving and giving objects; shifting between enclosures for basic husbandry management; presenting body parts for inspection and medical treatment, including acupuncture; cooperative feeding; voluntarily presenting for anesthetic injections; voluntarily allowing urine or semen collection; and voluntary venipuncture [Laule et al., 1996; Bloomsmith et al., 1998; Lambeth et al., 2006; Coleman et al., 2008; Magden et al., 2013; Schapiro, 2013]. This foundation of successfully trained behaviors helps the animals perform new, complicated behaviors, as they can generalize, or “learn to learn”; while allowing for bonds to form between the animal and the trainer, an essential part of the overall PRT paradigm [Pryor, 1984; Ramirez, 1999]. For more complete discussions of PRT techniques, see Laule et al. [2003], Laule and Whittaker [2007], Pryor [1984], Ramirez [1999], and Schapiro et al. [2013].
Recent studies have shown that there is inter-individual variability in training success. Coleman et al. [2005] and Clay et al. [2008] both found that rhesus macaques categorized as more “inhibited” took longer to reach training criteria. Veeder et al. [2009] found that shifting success in sooty mangabeys (Cercocebus atys atys) differed significantly based on dominance rank. Coleman [2011] emphasized the importance of examining these differences and utilizing them to form individualized management plans for captive NHPs.
With the identification of a small number of type 2 diabetic chimpanzees in our colony, our training program was expanded to include voluntary capillary blood sample collection from a finger or toe in an attempt to proactively address any potential onset of the disease in the future. It is often easier to address behavioral issues before they become problems, than it is to respond to them once they have become problematic. Our first step was to test all of the chimpanzees at the KCCMR, to assess which animals, if any, would allow the BG testing procedures in a single PRT session, without prior, focused training for these specific behaviors. We subsequently analyzed the data to determine whether sex, group size, age, rearing history, personality, and voluntary presentation for an anesthetic injection were factors that contributed to the initial participation and success of BG testing without prior, specific training for this procedure.
It should be noted that while the majority of previous studies assessing individual differences in trainability focused on the overall learning of a behavior throughout subsequent training sessions [e.g., Veeder et al., 2009; Coleman et al., 2005; Perlman et al., 2010], the current study assessed individual differences at a baseline level, examining differences within the first PRT session. It stands to reason that individuals that fully complete the behavior within a single session, logically, would then reach the criteria for successful training more quickly than those chimpanzees that did not. However, this was not specifically tested. Hence, this study assesses initial training success or baseline trainability, but not trainability as discussed in other studies [Coleman et al., 2005; Veeder et al., 2009; Perlman et al., 2010]. As personnel time is often an issue for training programs [Prescott and Buchanan-Smith, 2003], this baseline trainability test is thought to be a useful assessment tool that is quick and may be applicable at many facilities.
As little data currently exist that examine factors affecting trainability, the aim of this study was to assess which factors (sex, group size, age, rearing history, personality, previous training success), if any, affect the initial training success of diabetic treatment training, specifically BG testing. This is a very important health concern for captive chimpanzees in which stress-free, voluntary compliance is of the utmost importance and the use of PRT is essential.
METHODS
Subjects
The subjects for this study included 123 (61F, 62M) chimpanzees (P. troglodytes) housed at the KCCMR in Bastrop, Texas. The chimpanzees ranged in age from 9 to 49 years old and lived in enriched indoor/outdoor enclosures in social groups ranging in size from 2 to 13 individuals. Animals, including diabetics and pre-diabetics, which had already been trained for BG testing, were explicitly excluded from this sample.
Procedures
Each of the subjects participated in a single assessment session with their designated staff trainer while they remained in their normal social group; no animals were separated for this study. Testing of all subjects was completed within a 6- week period (August–September 2010). Each chimpanzee was asked to follow the steps presented in Figure 2 and the performance of each subject was recorded. Each session utilized PRT techniques, including successive approximations and differential reinforcement [see Ramirez, 1999 or Pryor, 1984], to elicit the ultimate goal; the successful completion of steps A–G; of the BG testing process. The number of subjects that completed each step (A–G) of the BG testing process during the test session was then calculated. For a subset of individuals with data available (N = 53), the average length of a session was calculated using the overall session time divided by the number of animals in the social group for each session.
Analyses
Performance of each chimpanzee was recorded as one of three categories: “Complete Participation” (animal successfully completed steps A–G), “Partial Participation” (animal successfully completed any combination of steps between A and F, but not including G), and “No Participation” (animal did not participate in any steps).
The rearing history of the subjects was divided into three categories: (1) wild-born; (2) captive-born, mother- reared; and (3) captive-born, nursery-reared. Individuals were considered “mother-reared” if they had been with their mother for at least the first year of life. The chimpanzees were also categorized according to whether or not they cooperated by presenting for an anesthetic injection during their most recent health check (between September 2009 and September 2010): (1) cooperation; (2) no cooperation. When receiving an anesthetic injection for an annual health check, each animal is always asked to present an arm or leg voluntarily for the anesthetic injection (cooperation) and if they refuse, they are then anesthetized by non-voluntary means, such as darting [no cooperation; Lambeth et al., 2006], while always having the opportunity to present. In addition, we assessed whether the length of time between the most recent anesthetic event and BG testing affected initial training success.
Personality ratings were collected and analyzed for each of the subjects as part of an earlier study focused on assessing the personalities of the chimpanzees housed at the KCCMR [Freeman et al., 2013]. The chimpanzees were rated on 41 traits by 21 people who had between 6 months and 21 years of experience working with the animals. Each trait was listed with an associated Likert scale ranging from one (least descriptive of chimpanzee) to seven (most descriptive of chimpanzee). Intraclass correlation coefficents were used to determine the reliability of the traits. All but one of the traits, predictable, were revealed to be reliable. The remaining 40 traits were used in a varimax-rotation principal components analysis (PCA). The PCA revealed six factors which are listed in Table 1 with each of the traits within each factor [Table 1 and Freeman et al., 2013]. The factors included: Openness, Extraversion, Dominance, Reactivity/Undependable, Methodical, and Agreeableness, with average factor scores ranging between 2.52 and 5.83. For additional details on the personality assessments and analyses, see Freeman et al. [2013].
TABLE 1.
A list of personality traits and the factors they load onto
| REACTIVITY | DOMINANCE | EXTROVERSION | OPENNESS | AGREEABLENESS | METHODICAL |
|---|---|---|---|---|---|
| Irritable | Fearfula | Solitarya | Human oriented | Protective | Self-caring |
| Temperamental/moody | Timida | Depresseda | Inquizative/curious | Considerate | Methodical |
| Deceptive | Cautiousa | Active | Inventive | ||
| Impulsive | Dominant | Playful | Intelligent | ||
| Defiant | Dependenta | Sexual | Affectionate/friendly | ||
| Mischevious | Anxiousa | Affiliative | Persistent | ||
| Jealous/attention-seeking | Bold | ||||
| Manipulative | Relaxed | ||||
| Stingy | |||||
| Bullying | |||||
| Aggressive | |||||
| Excentric | |||||
| Socially Inept | |||||
| Calma | |||||
| Excitable | |||||
| Autistic |
Denotes negative loadings [Freeman et al., 2013].
Statistics
To determine the effect of the independent variables on initial training success in BG testing (no, partial and complete participation) a cumulative odds ordinal logistic regression.
(Generalized Linear Model) was run. The independent variables included: sex (male, female), rearing history (wild, captive/mother, captive/nursery), level of cooperation for most recent anesthetic injection (cooperation, no cooperation), age, interaction between age and rearing history (age*rearing), all six personality factors, group size, and months since most recent anesthetic event. Personality rating data (the six factor scores obtained from the PCA analysis) included: Openness, Extraversion, Dominance, Reactivity/ Undependable, Methodical, and Agreeableness.
RESULTS
The average length of the testing session was 5.76 minutes per animal (N = 53). A summary of subjects’ level of participation for BG testing, including sex and rearing history demographics, can be found in Table 2, with nearly 30% (44 of the 123 subjects) offering complete participation during the first session (P >0.05).
TABLE 2.
A summary of subjects’ level of participation listed as %(N) in BG testing including sex and rearing history demographics
| Complete participation | Partial participation | No participation | ||
|---|---|---|---|---|
| Wild born | 35.5% (11) | 58.1% (18) | 6.5% (2) | 25.2% (31) |
| Mother reared | 18.7% (14) | 73.3% (55) | 8.0% (6) | 61.0% (75) |
| Nursery reared | 58.8% (10) | 35.3% (6) | 5.9% (1) | 13.8% (17) |
| Male | 33.3% (21) | 61.9% (79) | 4.8% (3) | 51.2% (63) |
| Female | 23.0% (14) | 67.2% (41) | 9.8% (6) | 49.6% (61) |
| Total | 28.5% (35) | 64.2% (79) | 7.3% (9) | 123 |
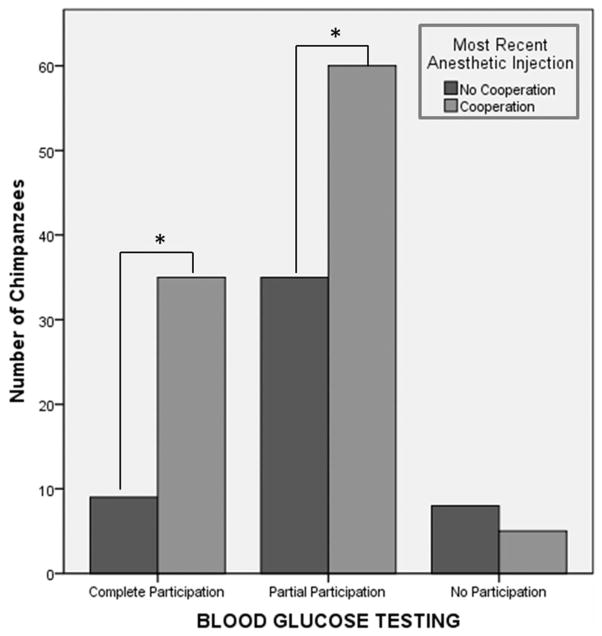
Level of participation for initial BG testing was significantly affected by sex, the personality factor of openness, and success on most recent anesthetic event. The odds ratio of participating in initial BG testing for males versus females was 5.417 (95% CI: 1.634–17.959), a statistically significant effect (Wald χ2 = 7.632, df = 1, P = 0.006). Furthermore, the odds ratio of participating in initial BG testing for subjects that cooperated on their most recent anesthetic event (N = 82) versus those that did not (N = 41) is 0.213 (95% CI: 0.073–0.620), a statistically significant effect, Wald χ2 = 8.061, df = 1, P = 0.005. To further investigate this relationship, individual chi-square analyses conducted post hoc showed that individuals that either completely or partially participated in the BG testing scan were significantly more likely to have voluntarily presented for their most recent anesthetic injection (“Complete”: x2 = 15.36, df = 1, P <0.01; “Partial”: x2 = 6.58, df = 1, P = 0.01; “No”: x2 = 0.692, df = 1, P = 0.405), see Figure 3.
Fig. 3.
Graph showing level of cooperation on most recent anesthetic injection with current blood glucose testing scan success [*P ≤ 0.017].
Of the six personality factors examined (see Table 1), only the factor of “openness” was found to be significantly related to the level of participation for BG testing. Specifically, an increase in the personality rating of openness was associated with an increase in the odds of participating in initial BG testing, with an odds ratio of 20.279 (95% CI: 4.266–96.404), which is statistically significant, Wald χ2 = 14.316, df = 1, P <0.001.
Level of participation for BG testing (complete, partial, or no) was not significantly affected by group size, months since most recent anesthetic event, age, rearing, the interaction of age and rearing (age*rearing), nor the personality factors of agreeableness, conscientiousness, reactivity, dominance, or extroversion.
DISCUSSION
Besides the obvious medical implications, the captive management concern with chimpanzees developing type 2 diabetes is the monitoring and treatment of the disease. Effective treatment requires training complex and time-consuming behaviors (i.e., BG testing, present for injections). Obviously, preventing the development of diabetes is the best management option which may be accomplished through weight management [see Lambeth et al., 2011], as well as yearly health screenings for early detection (see Fig. 1). However, if the onset of type 2 diabetes does occur, it is imperative to be able to voluntarily perform BG testing to obtain accurate results and to provide optimal care, so understanding the factors that may contribute to training success is important. The current study assessed factors that potentially affect initial BG training success. Of our chimpanzee population, all of whom have extensive training experience, 29% voluntarily performed all of the behaviors necessary for the novel behavior of BG testing in a single session that lasted on average less than 6 min.
Individual chimpanzees differed considerably in their responses during the test session (no participation, partial participation, or complete participation), which warranted further investigation into possible factors contributing to the successful performance of this task. Sex, the personality factor of openness, and level of cooperation for the most recent anesthetic injection for annual health checks did appear to influence initial testing participation. However, the remaining factors: age, rearing history, the interaction of age and rearing history, group size, time since last anesthetic event, the personality factors of agreeableness, conscientiousness, reactivity, dominance, or extroversion, did not significantly affect the level of participation in initial BG testing. The implications of this study go beyond just diabetic training success and could potentially be applied to all NHP PRT programs.
As all of the chimpanzees in the current study were trained within their social groups in full view of other group members (in random/opportunistic order), audience effects and social learning are two potential methodological concerns. There is evidence in NHP literature showing conspecific audience effects in many contexts, including sexual behavior [chimpanzee: Townsend and Zuberbuhler, 2009; rhesus macaque: Overduin-de Vries et al., 2012], vocalizations [chimpanzee: Slocombe and Zuberbuhler, 2007] and parental care [Semple et al., 2009], where individuals will change their behavior based on who is present from their social group. However, previous studies have found that audience effects are related to dominance, kinship, and group composition [Townsend and Zuberbuhler, 2009; Slocombe and Zuberbuhler, 2007], none of which were assessed in the present study. Equally, chimpanzees have been shown to use a variety of different social learning mechanisms [see Whiten et al., 2004 for review], both in the wild [Whiten et al., 1999; Boesch, 2003] and in captivity [Whiten et al., 1996; Dean et al., 2012]. Perlman et al. [2010] found that chimpanzees that watched a video of another chimpanzee successfully urinating on command were reliably trained on this same behavior significantly faster than control subjects that did not watch the video. It is conceivable that social learning and/or audience effects occurred during the BG testing scan, however, testing this was beyond the scope of the current study. The current study did look at group size to assess if having more conspecifics present during the testing session affected overall outcome and found that it did not. Further research into the link between social learning and audience effects in relation to training may be beneficial and could potentially be applied to aid training programs.
Sex differences were found between initial BG training success levels, where males were more likely to have participated in the initial BG training. Studies in humans have shown that males excel at tasks which require manipulation of visual images, whereas females excel at tasks requiring long-term memory retrieval [Halpern and LaMay, 2000]. With this knowledge, it is perhaps not surprising that males were more likely to do better than females, as this was a novel task (i.e., not requiring long-term memory retrieval) with visual components.
While there was a trend for captive-born, mother-reared chimpanzees to exhibit the lowest levels of participation (see Table 2), rearing condition did not significantly affect level of participation in initial BG testing. This is in contrast to Bloomsmith et al. [2006] who found that, in response to novel environments, young, captive chimpanzees that were nursery-reared showed more exploratory behavior than mother-reared peers. One possible explanation for these diverging findings could be an interaction effect between age and rearing history, as wild-caught individuals are generally much older than captive-born chimpanzees. In an attempt to address this issue, the current study looked at age as well as the interaction of age and rearing and found that none of these variables predict initial BG testing success. It should be noted that in the current study, the trend toward greater participation for both the “wild-born” and “nursery-reared” conditions include chimpanzees that were essentially raised by humans, (as wild-born individuals were typically taken from their mothers at a young age). This suggests that human-reared individuals are perhaps more comfortable with humans, and therefore more likely to participate in novel training experiences, such as BG testing.
This human-centered theory is further supported by the finding that the personality factor of “openness” was a significant predictor of level of initial BG testing participation (specifically, those that participated more were also rated higher on the openness scale). One of the descriptive traits that loads positively on the personality factor of “openness” is the adjective “human-oriented” (see Table 1). Pederson et al. [2004] described “openness” as inventive or inquisitive and predicted that this factor would be positively related to exploration and manipulation in chimpanzees. There is limited literature exploring a relationship between personality and successful PRT; however, emerging evidence suggests a link. Coleman et al. [2005] found that female rhesus monkeys that were either “exploratory” or “moderate” on a novelty test, had higher training success than subjects that were categorized as “inhibited,” demonstrating that a simple test of temperament can be a useful tool to predict training success in rhesus macaques. Clay et al. [2008] found similar results when comparing responses to a novelty test and training success. Studies utilizing novel object tests often use the term “curiosity” to define differences in response to novelty [Uher and Asendorpf, 2008; Ehrlich, 1970; Watson and Ward, 1996] and, in humans, curiosity can be considered the motivation behind exploratory behavior [Berlyne, 1960]. Thus, it is perhaps not surprising that the current study found that openness was associated with cooperation for this initial, novel training task, since the adjective “curious” loads on openness. Our findings support the suggestion that assessments of personality, or at least the personality factor of openness, can be useful in predicting training success in chimpanzees.
While yearly health checks are necessary to provide proper care for captive animals, each sedation process requires trust between the chimpanzee and the trainer. Trainers at KCCMR work with the animals as much as possible to ensure this trust is in place. However, as our trainers conducted both the current study, as well as each subject’s most recent anesthetic injection (whether for annual health check or for clinical intervention), it is conceivable that this could be a confounding influence, where a subject’s trust in their trainer is diminished following a sedation event. If this were the case, we would expect that individuals that were tested more closely to an anesthetic event would be less likely to have participated in the current task, as they would not have had time to rebuild trust with the trainer, or recover from any regression in the behavior. However, we found that length of time since last anesthetic event, which ranged from 1 to 11 months, was not related to BG testing participation, suggesting that the animal-trainer relationship was steady throughout and is not a factor affecting initial BG training success.
When attempting to identify additional factors that may affect training success, the results show that those that voluntarily presented for their most recent anesthetic injection were more likely to have participated in initial BG testing (see Fig. 3). This difference is potentially due to training exposure: it is commonly accepted that generalization, or “learning to learn,” can speed up the training process [Ramirez, 1999; Perlman et al., 2010]. Another consideration could be that animals in a regular PRT program are establishing and maintaining strong bonds with their trainers, thus trusting them enough to try new behaviors. Although it is not surprising that voluntary cooperation for other trained behaviors was linked to voluntary cooperation with the current behavior, these findings do emphasize the value of having a working PRT program as part of the overall captive management system. This is especially important when working with captive NHP, and is imperative when working with captive chimpanzees [IOM report, 2011]. Establishing a pool of animals that have been successfully trained to perform a number of useful behaviors, including voluntary presentation of an arm/leg for an injection, is a vital, controllable factor that can positively affect the rapid treatment of diabetes, or other diseases, in captive chimpanzees.
Recent studies have identified the importance of assessing and utilizing individual differences to enhance the effectiveness of behavioral management strategies for NHP [Coleman et al., 2005; Uher and Asendorpf, 2008; Coleman, 2011; Gottlieb et al., 2013a,b; Hannibal et al., 2013]. Perlman et al. [2010] even suggested that animals could be scored on a “trainability index” in which the number of trained behaviors an individual can reliably perform would fall onto a continuous scale and be used to help predict future success. This scale could then be used by behavioral managers to aid in managing training programs [Perlman et al., 2010]. Based on the current study, as well as others [Coleman et al., 2005; Veeder et al., 2009; Coleman, 2011], there is clearly an emerging need for the development of a comprehensive “trainability index” that incorporates all factors affecting training success and that could be used as a tool for individualized behavioral management and PRT programs. This comprehensive trainability index could provide a scale that takes into account many factors that potentially affect training success, including sex, age, rearing history, personality, previous training success (quantity and quality), dominance, cognitive abilities, and so on. With this information, managers of captive animals could design individualized plans, according to each animal’s needs—potentially allowing more time to desensitize animals rated lower on the scale—thus setting each animal up for success. For example, if there are two overweight chimpanzees at a facility with unstable BG levels (suggestive of pre-diabetes), both should be put on a weight management plan and trained for BG testing and diabetic treatment in order to be prepared for the possible onset of diabetes (see Fig. 1). Currently, the behavioral management plans for each of these individuals would be identical. However, with the addition of a comprehensive trainability index to current management practices, the treatment/training plan for each animal could be optimized for the care of each specific animal. Overall, managers should be aware of not only the health status, but also the “trainability” status/needs of each animal under their care.
It should be expressly stated that testing for BG is by no means trainable within a single PRT session; this study, however, adds some insight into potential factors that affect the baseline, or starting point, of such training. In the future, it would also be interesting to assess consecutive sessions in the process of BG testing training for these chimpanzees. Perhaps, once the novelty of the behavior is gone, certain factors will no longer be a predictor of BG testing success or different factors may emerge. This would also make the study more comparable to other published studies assessing individual variation in trainability [e.g., Coleman et al., 2005; Veeder et al., 2009; Perlman et al., 2010]. Overall, this study has important implications for how to approach the animal training for the management of type 2 diabetes in captive chimpanzees by assessing the factors that significantly affect level of participation in the training of required behaviors. Diabetes is a real concern in the management of captive chimpanzees; however, strides can be taken to attempt to minimize the risk of onset, set PRT programs up for successful treatment, and optimize the care provided to each animal.
CONCLUSIONS
PRT is an effective tool for eliciting voluntary cooperation for the management of diabetes in chimpanzees. Factors affecting successful participation in BG testing include sex, personality factor “openness,” and previous training success.
Having a repertoire of trained behaviors to serve as a baseline from which animals can “generalize,” appears to increase compliance of BG testing and is the only factor within the control of animal trainers.
Training programs may benefit from using personality assessments to assist in understanding how chimpanzees might respond to training (i.e., those that scored high on the personality factor “openness” might be easier to train).
These results highlight the need to develop a comprehensive “trainability index” that could be applied to individual animals, incorporating all factors likely to affect training success, which could then be used as a tool for developing individualized behavioral management plans based on the needs of each animal.
Acknowledgments
Grant sponsor: NIH/NCRR; grant number: U42-OD011197.
The authors would like to thank the chimpanzee care and management staffs at the KCCMR, as well as the veterinarians, particularly Dr. Patrick Hanley. Thank you also to Mary Beth Sarnowski, Jessica Rogge, Katrina Sherenco, Mary Catherine Mareno and Dr. Larry Williams for comments and suggestions on the content of the paper. Also, thank you to two anonymous reviewers for their comments on the original manuscript. NIH/NCRR U42-OD011197 supports the KCCMR chimpanzee colony. All animal research was performed in compliance with the guidelines for use of animals in research published in Animal Behavior [43: 185–188, 1992] and the KCCMR has been continuously accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care—International since 1979.
References
- Berlyne DE. Conflict, arousal and curiosity. New York: McGraw-Hill; 1960. [Google Scholar]
- Bloomsmith MA, Stone AM, Laule GE. Positive reinforcement training to enhance the voluntary movement of group-housed chimpanzees within their enclosures. Zoo Biol. 1998;17:333–341. [Google Scholar]
- Bloomsmith MA, Baker KC, Ross SR, Lambeth SP. Early rearing conditions and captive chimpanzee behavior: some surprising findings. In: Sackett G, Ruppenthal G, editors. Nursery Rearing of Nonhuman Primates in the 21st Century. New York, NY: Kluwer Academic Publishers; 2006. pp. 289–312. [Google Scholar]
- Boesch C. Is culture a golden barrier between humans and chimpanzees? Evol Anthropol. 2003;12:82–91. [Google Scholar]
- Cawthon Lang KA. “Primate Info Net.” Primate Factsheets: Chimpanzee (Pan troglodytes) [accessed December 5, 2011];Taxonomy Morphology Ecology. 2006 Apr 13; http://pin.primate.wisc.edu/factsheets/entry/chimpanzee.
- (CDC) Centers for Disease Control and Prevention. National Diabetes Fact Sheet: national estimates and general information on diabetes and prediabetes in theUnited States 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- Clay AW, Bloomsmith MA, Marr MJ, Maple TL. Temperament predicts training success in Rhesus macaques (Macaca mulatta) Am J Primatol. 2008;70:20–69. [Abstract] [Google Scholar]
- Coleman K, Tully LA, McMillan J. Temperament correlates with training success in adult rhesus macaques. Am J Primatol. 2005;65:63–71. doi: 10.1002/ajp.20097. [DOI] [PubMed] [Google Scholar]
- Coleman K, Pranger L, Maier A, et al. Training rhesus macaques for venipuncture using positive reinforcement techniques: a comparison with chimpanzees. J Am Assoc Lab Anim Sci. 2008;47:37–41. [PMC free article] [PubMed] [Google Scholar]
- Coleman K. Individual differences in temperament and behavioral management practices for nonhuman primates. Appl Anim Behav Sci. 2011;137:3–4. 106–113. doi: 10.1016/j.applanim.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlquist G, Blom L, Tuvemo T, et al. The Swedish childhood diabetes study -results from a nine year case register and a one year case-referent study indicating that type 1 (insulin-dependent) diabetes mellitus is associated with both type 2 (non-insulin dependent) diabetes mellitus and auto. Diabetologia. 1989;32:2–6. doi: 10.1007/BF00265396. [DOI] [PubMed] [Google Scholar]
- Dean LG, Kendal RL, Schapiro SJ, Thierry B, Laland KN. Identification of the social and cognitive processes underlying human cumulative culture. Science. 2012;335:1114–1118. doi: 10.1126/science.1213969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Eath RB, Tolkamp BJ, Kyriazakis I, Lawrence AB. ‘Freedom from hunger’ and preventing obesity: the animal welfare implications of reducing food quantity or quality. Anim Behav. 2009;77:275–288. [Google Scholar]
- Ehrlich A. Response to novel objects in three lower primates: greater galago, slow loris, and owl monkey. Behaviour. 1970;37:55–63. [Google Scholar]
- Freeman HD, Brosnan SF, Hopper LM, et al. Developing a comprehensive and comparative questionnaire for measuring personality in chimpanzees using a simultaneous top-down/bottom-up design. Am J Primatol. 2013;75:1042–1053. doi: 10.1002/ajp.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild S, Schwitzer C. The problem of obesity in captive lemurs. Int Zoo News. 2008;55:353–357. [Google Scholar]
- Gottlieb DH, Capitanio JP, McCowan B. Risk factors for stereotypic behavior and self-biting in rhesus macaques (Macaca mulatta): animal’s history, current environment, and personality. Am J Primatol. 2013a;75:995–1008. doi: 10.1002/ajp.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DH, Maier A, Coleman K. The relationship between environmental enrichment, temperament, and stereotypy in captive rhesus macaques (Macaca mulatta). American Journal of Primatology: Abstracts of the 36th Annual Meeting of the American Society of Primatologists; 2013b. in press. [Google Scholar]
- Graham ML, Rieke EF, Mutch LA, et al. Successful implementation of cooperative handling eliminates the need for restraint in a complex non-human primate disease model. J Med Primatol. 2011;41:89–106. doi: 10.1111/j.1600-0684.2011.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern DF, LaMay ML. The smarter sex: a critical review of sex differences in intelligence. Educ Psychol Rev. 2000;12:2. [Google Scholar]
- Hannibal D, Minier D, Capitanio J, McCowan B. Effect of temperament on the behavioral conditioning of individual rhesus monkeys (Macaca mulatta) [ABSTRACT] Am J Primatol. 2013;75:S1. [Google Scholar]
- Hansen BC. Diabetes mellitus: a fundamental and clinical text. In: LeRoith D, Taylor SI, Olefsky JM, editors. Diabetes mellitus: a fundamental and clinical text. Philadelphia: Lippincott Williams and Wilkins; 1996. pp. 1059–1074. [Google Scholar]
- Hansen BC, Bodkin NL. Primary prevention of diabetes mellitus by prevention of obesity in monkeys. Diabetes. 1993;42:1809–1814. doi: 10.2337/diab.42.12.1809. [DOI] [PubMed] [Google Scholar]
- Home Office. Code of practice for the housing and care of animals used in scientific procedures. London: Her Majesty’s Stationery Office; 1989. [Google Scholar]
- IOM Report. Chimpanzees in Biomedical and Behavioral Research: Assessing the Necessity. 2011 [PubMed] [Google Scholar]
- Lambeth SP, Hau J, Perlman JE, Martino M, Schapiro SJ. Positive reinforcement training affects hemotologic and serum chemistry values in captive chimpanzees. Am J Primatol. 2006;68:245–256. doi: 10.1002/ajp.20148. [DOI] [PubMed] [Google Scholar]
- Lambeth SP, Bernacky BJ, Hanley P, Schapiro SJ. Weight management in a captive colony of chimpanzees. Am J Primatol. 2011;73:40. [Abstract] [Google Scholar]
- Lambeth SP, Schapiro SJ, Bernacky BJ, Wilkerson GK. Establishing ‘quality of life’ parameters using behavioural guidelines for humane euthanasia of captive non-human primates. Anim Welf. 2013;22:429–435. doi: 10.7120/09627286.22.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laule GE, Thurston RH, Alford PL, Bloomsmith MA. Training to reliably obtain blood and urine samples from a diabetic chimpanzee (Pan troglodytes) Zoo Biol. 1996;15:587–591. [Google Scholar]
- Laule GE, Bloomsmith MA, Schapiro SJ. The use of positive reinforcement training techniques to enhance the care, management, and welfare of primates in the laboratory. J Appl Anim Welf Sci. 2003;6:163–173. doi: 10.1207/S15327604JAWS0603_02. [DOI] [PubMed] [Google Scholar]
- Laule G, Whittaker M. Enhancing nonhuman primate care and welfare through the use of positive reinforcement training. J Appl Anim Welf Sci. 2007;10:31–38. doi: 10.1080/10888700701277311. [DOI] [PubMed] [Google Scholar]
- Magden ER, Haller RL, Thiele EJ, et al. Acupuncture as an adjunct therapy for osteoarthritis in chimpanzees (Pan troglodytes) J Am Assoc Lab Anim Sci. 2013;52:475–480. [PMC free article] [PubMed] [Google Scholar]
- McCann C, Buchanan-Smith H, Jones-Engel L, et al. Report of the captive care committee of the International primatological society. 2. 2007. IPS International guidelines for the acquisition, care and breeding of nonhuman primates; pp. 1–76. [Google Scholar]
- McTighe MS, Hansen BC, Ely JJ, Lee DR. Determination of hemoglobin A1c and fasting blood glucose reference intervals in captive chimpanzees (Pan troglodytes) J Am Assoc Lab Anim Sci. 2011;50:165–170. [PMC free article] [PubMed] [Google Scholar]
- Morgan KN, Tromborg CT. Sources of stress in captivity. Appl Anim Behav Sci. 2007;102:262–302. [Google Scholar]
- Overduin-de Vries AM, Massen JJM, Spruijt BM, Sterck EM. Sneaky monkeys: an audience effect of male rhesus macaques (Macaca mulatta) on sexual behavior. Am J Primatol. 2012;74:217–228. doi: 10.1002/ajp.21988. [DOI] [PubMed] [Google Scholar]
- Pederson AK, King JE, Landau VI. Chimpanzee (Pan troglodytes) personality predicts behavior. J Res Pers. 2004;39:534–549. [Google Scholar]
- Perlman JE, Horner V, Bloomsmith MA, Lambeth SP, Schapiro SJ. Positive reinforcement training, social learning, and chimpanzee welfare. In: Lonsdorf E, Ross SR, Matsuzawa T, editors. The mind of the chimpanzee: ecological and experimental perspectives. Chicago: University of Chicago Press; 2010. pp. 320–331. [Google Scholar]
- Prescott MJ, Buchanan-Smith H. Training primates using positive reinforcement techniques. J Appl Anim Welf Sci. 2003;6:157–161. doi: 10.1207/S15327604JAWS0603_01. [DOI] [PubMed] [Google Scholar]
- Pryor K. Don’t Shoot the Dog. New York, NY: Simon and Schuster; 1984. [Google Scholar]
- Ramirez K. Animal training: successful animal management through positive reinforcement. Chicago, IL: Shedd Aquarium; 1999. [Google Scholar]
- Sapolsky R. Why zebras don’t get ulcers. 3. New York, NY: Holt Paperbacks; 1998. [Google Scholar]
- Schapiro SJ. Refinements in studies using macaques and chimpanzees. P Cong Swed Vet Assn. 2013:203–206. [Google Scholar]
- Schapiro SJ, Coleman K, Akinyi M, et al. Nonhuman primate welfare in the research environment. New York: Laboratory Animal Welfare ACLAM; 2013. pp. 197–212. [Google Scholar]
- Semple S, Gerald MS, Suggs DN. Bystanders affect the outcome of mother-infant interactions in rhesus macaques. P Roy Soc B Bio. 2009;276:2257–2262. doi: 10.1098/rspb.2009.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocombe KE, Zuberbuhler K. Chimpanzees modify recruitment screams as a function of audience composition. Proc Natl Acad Sci USA. 2007;104:17228–17233. doi: 10.1073/pnas.0706741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend S, Zuberbuhler K. Audience effects in chimpanzee copulation calls. Commun Integr Biol. 2009;2:282–284. doi: 10.4161/cib.2.3.6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher J, Asendorpf JB. Personality assessment in Great Apes: Comparing ecologically valid behavior measures, behavior ratings, and adjective ratings. J Res Pers. 2008;42:821–838. [Google Scholar]
- Veeder CL, Bloomsmith MA, McMillan JL, Perlman JE, Martin AL. Positive reinforcement training to enhance the voluntary movement of group-housed sooty mangabeys (Cercocebus atys atys) J Am Assoc Lab Anim Sci. 2009;48:192–195. [PMC free article] [PubMed] [Google Scholar]
- Watson SL, Ward JP. Temperament and problem solving in the small-eared bushbaby (Otolemur garnettii) J Comp Psychol. 1996;110:377–385. [Google Scholar]
- Weiss P, Heinz AM, Scholz S, et al. Long-term follow-up of infants of mothers with type 1 diabetes: evidence for hereditary and nonhereditary transmission of diabetes and precursors. Diabetes Care. 2000;23:905–911. doi: 10.2337/diacare.23.7.905. [DOI] [PubMed] [Google Scholar]
- Whiten A, Custance DM, Gomez JC, Teixidor P, Bard KA. Imitative learning of artificial fruit processing in children (Homo sapiens) and chimpanzees (Pan troglodytes) J Comp Psychol. 1996;110:3–14. doi: 10.1037/0735-7036.110.1.3. [DOI] [PubMed] [Google Scholar]
- Whiten A, Goodall J, McGrew WC, et al. Cultures in chimpanzees. Nature. 1999;399:682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- Whiten A, Horner V, Litchfield CA, Marshal-Pescini S. How do apes ape? Learn Behav. 2004;32:36–52. doi: 10.3758/bf03196005. [DOI] [PubMed] [Google Scholar]