Abstract
BACKGROUND
The emerging role of vitamin D in immunology and autoimmune disorders has been a worldwide interest in the last decade. Systemic lupus erythematosus (SLE) patients are particularly at a delicate position predisposing them to suffer from vitamin D deficiency due to the multiple risk factors accompanying the disease. Whether vitamin D deficiency is also involved as a risk factor for developing SLE and affecting its course is a considerable concern.
OBJECTIVES
The objective of this study was to estimate the prevalence of vitamin D deficiency in SLE patients and its relation to disease.
MATERIALS AND METHODS
In our observational cross-sectional study, serum levels of vitamin D [25(OH)D] in 60 SLE patients and 30 age- and sex-matched healthy controls were assessed and estimated for deficiency and insufficiency at 10 and 30 ng/mL, respectively. Disease activity was evaluated by SLE disease activity index (SLEDAI), irreversible organ damage by Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SLICC/ACR DI), and severity by Severity of Disease Index. Fatigue was measured by visual analog scale.
RESULTS
Significantly lower levels of 25(OH)D were found in SLE patients (17.6 ± 6.9 ng/mL) in comparison to controls (79.0 ± 28.7 ng/mL), with a statistically high significant difference (t = −11.2, P < 0.001). High prevalence of vitamin D insufficiency and deficiency was detected as 73.3% and 23.3%, respectively. Vitamin D had a highly significant negative correlation with SLEDAI (r = −0.495, P < 0.001), SLICC (r = −0.431, P < 0.05), and fatigue (r = −0.436, P < 0.05).
CONCLUSION
Vitamin D deficiency and insufficiency were found to be prevalent in SLE patients in our study and related to disease activity and fatigue. If needed, routine screening and consequent repletion of vitamin D are recommended in SLE patients. Restoring adequate vitamin D levels in SLE patients should be more explored as a potential yet simple measure to their usual management to improve their condition.
Keywords: systemic lupus erythematosus, vitamin D, SLEDAI, SLICC, disease activity
Introduction
Vitamin D has a crucial role in calcium metabolism. Nowadays, a new era for vitamin D has emerged since it has been involved in immune modulation1,2 and in the risk for developing autoimmune diseases such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE).3–5
The conversion of 7-dehydrocholesterol into previtamin D3 in the skin, by the solar ultraviolet B radiation, is considered the main source of vitamin D, whereas only a smaller amount is obtained from food.6
Vitamin D receptors (VDRs) have been identified on immune cell membranes, thus, together with existing 1α-hydroxylase in these cells, clearly underlining the importance of vitamin D for immune regulation.7,8 In addition, VDR genetic polymorphisms may affect their functionality and have an impact on immune regulation in autoimmunity.9
Newly identified targets for vitamin D reveal multiple molecular pathways of anti-inflammatory actions for 1,25(OH)D3 in several immune cells. These include inhibition of prostaglandin (PG) synthesis and biological actions; inhibition of p38 stress kinase activation; inhibition of nuclear factor κB (NF-κB) signaling, which results in the attenuation of the synthesis of pro-inflammatory cytokines such as interleukin-8 (IL-8) via upregulation of the expression of insulin-like growth factor binding protein-3 (IGFBP-3); and inhibition of angiogenesis due to suppressive effects on the expression of pro-angiogenic factors such as hypoxia-inducible factor 1 (HIF-1) and vascular endothelial growth factor.10
Vitamin D deficiency has been implicated in various rheumatic disorders. The risk of developing RA was found to be inversely related to vitamin D intake.11 RA cases with low plasma 25(OH)D were found by some researchers to have higher risk of disease activity,12 while others found no associations of serum 25(OH)D levels with disease activity.13
SLE is a chronic inflammatory multisystem disease mainly affecting women of childbearing age. It is characterized by a very large spectrum of clinical manifestations accompanied by prototypic abnormalities of the immune system.14
One aspect that is particularly pronounced is fatigue and musculoskeletal symptoms, which is experienced by up to 90% of SLE patients and is considered their most disabling disease symptom by ∼50% of patients.15 This is often multifactorial in origin and can be mediated through disease-related factors, comorbid conditions, or environmental factors.16
Commonly frequented SLE musculoskeletal symptoms and pain can be caused by vitamin D deficiency. Hence, the concomitant presence of such deficiency might be overlooked in these patients in spite of having multiple risk factors for its occurrence. Chronic steroid use may alter vitamin D metabolism, by reducing the level of dihydroxyvitamin D3, although there is contradictory evidence about it.8 Additionally, hydroxychloroquine, often used in SLE, is suspected to decrease vitamin D2 conversion into the more biologically active vitamin D3.17 Renal involvement is common in SLE, which can interfere with 1-hydroxylation that is essential to make 25-OH vitamin D active.18 SLE photosensitivity and the resultant avoidance of sun exposure also contribute to such deficiency.19 Moreover, antivitamin D antibodies were observed in an SLE patient’s subset and in antiphospholipid syndrome and were particularly associated with anti-dsDNA antibodies.20
Regardless of the fact that vitamin D deficiency is a cause or consequence or both in SLE, its prevalence has been an addressed issue in many studies. Its presence is almost undeniable though it is expected to differ in prevalence between various populations. Its effect on disease course in terms of activity and severity represents an interesting area of research as it might allow a simple way to ameliorate disease.
We aimed to estimate the prevalence of vitamin D deficiency in SLE Egyptian patients and its relation to various disease parameters.
Materials and Methods
This is a cross-sectional study that included 60 patients with SLE, fulfilling the updated American College of Rheumatology (ACR) criteria.21 The patients were selected from the inpatients of Internal Medicine, Rheumatology, and Rehabilitation departments and outpatient clinics. A total of 30 age- and sex-matched healthy subjects were also included in this study and served as the control group. All the patients were on steroids and (immunomodulator) drugs. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki (1964) and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
- Group I (patients):
- This group was further subdivided according to the presence of nephritis into two subgroups:
- a: 40 patients without lupus nephritis (LN).
- b: 20 patients with LN.
- Group II (control):
- A total of 30 normal healthy subjects were also included as a control group; they were matched for age and sex with SLE patients.
Exclusion criteria
Patients with end-stage renal disease, sepsis, antiphospholipid syndrome, and diabetes and those on vitamin D therapy were excluded.
Clinical assessment
Full medical history was taken with emphasis on renal, mucocutaneous, and musculoskeletal manifestations and history of drug administration.
Patients were clinically assessed through both general and local examinations. Fatigue scale was performed where patients were asked to reflect in a 0–10 visual analog scale (VAS), the degree of fatigue ranging from 0 = no fatigue to 10 = intense fatigue. SLE disease activity index (SLEDAI) was done within the last 10 days. The activity categories are no activity (SLEDAI = 0), mild activity (SLEDAI = 1–5), moderate activity (SLEDAI = 6–10), high activity (SLEDAI = 11–19), and very high activity (SLEDAI20).22 Also disease-related damage (Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index [SLICC/ACR DI]) was performed, where there are 41 items covering 12 systems. It includes specific comorbidities with SLE, to be scored as damage.23 In addition, Lupus Severity of Disease Index (Lupus SDI) was calculated to assess 10 items, where each of the first four receives one point and each of the following six items receives two points with possible maximum score being 13. The severity is considered high if ≥ 4.24
Laboratory assessment
The following laboratory investigations were done: complete blood counts, erythrocyte sedimentation rate (ESR) by Westergren method, C-reactive protein (CRP) by ELISA technique, anti-dsDNA by ELISA technique, serum level of C3, C4 serum anticardiolipin either IgG or IgM using ELISA, prothrombin time, partial thromboplastin time, international normalized ratio, blood urea nitrogen (BUN), serum creatinine (mg/dL), complete urine analysis with 24-hour urine test for protein excretion, and protein/creatinine ratio levels. The vitamin D 25(OH) D levels were determined by the Liaison 25 OH Vitamin D Assay (DiaSorin Inc.), a direct, competitive chemiluminescent immunoassay in which human serum is used.
Renal histopathological assessment for LN
The renal tissue was obtained from all lupus patients with proteinuria <500 mg/24 hours and evaluated by light microscopy by one pathologist. The biopsies were graded according to the classification of LN by the International Society of Nephrology/Renal Pathology Society,25 in which normal glomeruli are designated as Class I LN, mesangial hypercellularity represents Class II, and a state of LN showing focal or diffuse segmental or global endo- or extracapillary glomerulonephritis, with or without mesangial alterations, is classified as Class III (focal LN) and Class IV (diffuse LN), respectively. Membranous nephritis and advanced sclerosis are categorized as Class V and Class VI, respectively. Activity and chronicity scores are calculated according to the study by Austin et al.26
The statistical analysis was performed by IBM SPSS Statistics (V. 19.0, IBM Corp., 2010), which was used for data analysis. Data were expressed as mean ± SD for quantitative measures.
The following tests were performed:
Comparison of two independent mean groups for parametric data using Student’s t-test.
Comparison between two independent groups for nonparametric data using Wilcoxon rank-sum test.
Ranked Spearman correlation test to study the possible association between two variables among each group.
Regression analysis.
Results
Demographic, clinical, and laboratory data of the studied group
This study included 60 SLE patients and 30 normal subjects serving as a control group. The patients group included 52 females (86.7%) and 8 males (13.3%); their age ranged from 16.0 to 59.0 years with a mean ± SD age of 29.6 ± 10.0 years and their disease duration ranged from 1 to 14.0 years with a mean ± SD of 4.4 ± 0.6 years. They were further classified into two subgroups: Group a, 40 patients without LN (66.7%), and Group b, 20 patients known to have LN (33.3%). The control group included 26 females (86.7%) and 4 males (13.3%), their age ranged from 17 to 53.0 years with a mean ± SD age of 31.0 ± 9.1 years.
All patients (100%) had different forms of skin affection. Eighteen patients (30.0%) presented with vasculitis rash in the form of pitting of finger bulbs. Six patients (10.0%) presented with lung affection. Twenty patients (33.3%) had CNS affection in the form of headache, cognitive dysfunction, and peripheral neuritis, another 34 patients (56.7%) had joint affection in the form of arthralgia or arthritis. 20% of patients had serositis in the form of pericardial or pleural effusion. 26.7% of patients had ophthalmic affection in the form of retinal vasculitis or papilledema. Twenty-two patients (36.7%) had renal symptoms in the form of lower limb edema, puffy eyelids, loin pain, and hypertension.
Among 60 patients, 16.7% of patients had leukopenia (<4000/cmm), 56.7% of patients had anemia (<12 g/dL), 13.3% of patients had thrombocytopenia (<150,000/cmm), and 66.7% of patients had CRP > 6 mg/dL, while 90% of patients had ESR > 12 mm/hour. All patients had positive ANA and 90% had anti-dsDNA (>75 IU/mL); 33.3% of patients had C3 < 75 mg/dL, while 36.7% of patients had C4 < 12 mg/dL. Serum creatinine was >1.3 mg/dL in 8 patients (13.3%), and BUN was >20 mg/dL in 16 patients (26.7%).
Among 60 patients, according to SLEDAI, there were 6 patients with mild grade (10%), 26 patients with moderate grade (43.3%), 14 patients with high grade (23.3%), and 14 patients with very high grade (23.3%). Other clinical indices are presented in Table 1.
Table 1.
Clinical indices in SLE patients.
| RANGE | MEAN ± SD | |
|---|---|---|
| SLEDAI | 2–36 | 13.9 ± 1.8 |
| SLICCL/ACR DI | 0–5 | 1.0 ± 0.3 |
| SDI | 2–9 | 3.2 ± 0.3 |
Abbreviations: SLEDAI, SLE disease activity index; SLICC/ACR DI, Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index; SDI, Severity of Disease Index.
All patients were on steroid and hydroxychloroquine. The other drugs received by patients were as follows: azathioprine by 28 patients (46.7%), cyclophosphamide by 14 patients (23.3%), and mycophenolate mofetil by 4 patients (6.7%). No significant correlation was found between any of the drugs and serum vitamin D levels.
Renal biopsy study for LN patients
Among 20 patients with LN, 2 patients had Class II LN, 8 patients had Class III, 8 patients had Class IV, and 2 patients had Class V. While the activity index of renal biopsy in those patients with LN ranged from 2 to 14 with a mean ± SD of 7.5 ± 1.2, and chronicity index ranged from 0 to 4 with a mean ± SD of 2.4 ± 0.5.
Comparison between SLE patients and controls showed a highly significant difference with regard to serum level of 25(OH)D as presented in Table 2, whereas comparison between patients with LN with a mean ± SD of 16.6 ± 7.3 and those without LN 18.5.7 ± 7.1 showed no significant difference (t = 0.696 and P > 0.05).
Table 2.
Comparison between patients and control regarding vitamin D.
| PATIENTS GROUP | CONTROL GROUP | t | P | SIG. | |
|---|---|---|---|---|---|
| MEAN ± SD | MEAN ± SD | ||||
| Vitamin D (ng/mL) | 17.6 ± 6.9 | 79.0 ± 28.7 | −11. 2 | <0.001 | HS |
Abbreviations: HS, highly significant; P, level of significance; SD, standard deviation; t, t-test.
In our study, only two patients (3.3%) had normal levels of vitamin D in serum, 44 had insufficiency (73.3%), and 14 had deficiency (23.3%).
Significant inverse correlations between serum vitamin D and disease activity, severity, and fatigue were found as shown in Figures 1 and 2.
Figure 1.
Correlation between vitamin D and VAS.
Figure 2.
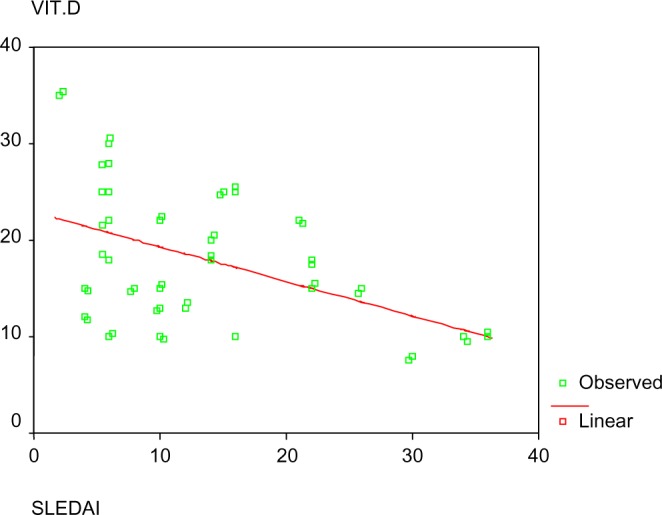
Correlation between vitamin D and SLEDAI.
In this study, there was a highly significant positive correlation between vitamin D and fatigue, SLICC (r = 0.502, P < 0.005), and CRP (r = 0.357, P < 0.005). There was a significant positive correlation with ESR (r = 0.323, P < 0.05), C4 (r = 0.324, P < 0.05), SDI (r = 0.419, P < 0.05), and anti-dsDNA (r = 0.413, P < 0.05) and a significant negative correlation with vitamin D level (r = −0.436, P < 0.05), while there was no significant correlation between fatigue with SLEDAI, disease duration, and P/C ratio as presented in Table 3.
Table 3.
Correlation between vitamin D, clinical indices, pathological indices, disease duration, and laboratory data in SLE patients.
| PARAMETER | r | P | SIG |
|---|---|---|---|
| SLEDAI | −0.495 | <0.01** | HS |
| SLICC/ACR DI | −0.431 | <0.05* | S |
| SDI | −0.264 | >0.05 | NS |
| VAS | −0.436 | <0.05* | S |
| Anti-dsDNA | −0.398 | <0.05* | S |
| Disease Duration | 0.174 | >0.05 | NS |
| P/C ratio | −0.341 | >0.05 (0.066) | NS |
| ESR | −0.323* | 0.012 | S |
| CRP | −0.357** | 0.005 | HS |
| C3 | −0.092 | 0.485 | NS |
| C4 | 0.324* | 0.011 | S |
After standardization of all clinical variants, regression analysis study showed a significantly inverse correlation between vitamin D and VAS and a highly significant correlation between vitamin D and SLEDAI score (Table 4).
Table 4.
Regression analysis of clinical and laboratory data with vitamin D as dependent variable.
| PARAMETER | UNSTANDARDIZED COEFFICIENTS | STANDARDIZED COEFFICIENTS | t | P | |
|---|---|---|---|---|---|
| B | STANDARD ERROR | ||||
| Age | −0.1 6 3 | 0.117 | −0.228 | −1.392 | 0.178 |
| VAS | −1.8 11 | 0.746 | −0.443 | −2.429 | 0.024 |
| Disease Duration | 0.355 | 0.395 | 0.176 | 0.898 | 0.379 |
| Anti-dsDNA | 1.305 | 0.007 | 0.664 | 1.756 | 0.094 |
| SDI | 0.573 | 1.233 | 0.139 | 0.465 | 0.647 |
| SLICC | −2.13 2 | 1.53 4 | −0.442 | −1.390 | 0.179 |
| SLEDAI | −0.682 | 0.249 | −0.940 | −2.733 | 0.012 |
| Creatinine | 3.036 | 1.980 | 0.344 | 1.533 | 0.140 |
Note: Dependent variable: vitamin D.
Discussion
Vitamin D deficiency has been an interesting area to explore in autoimmune disorders since the discovery of its involvement in immune system. SLE patients in particular have a list of multiple risk factors predisposing them to this deficiency. On the other hand, many researchers suggested it as a risk factor itself for developing SLE. Numerous previous studies analyzing its prevalence in SLE patients and its relation with disease activity and severity and finding positive versus negative results made it even more interesting to investigate it in our current study.
In this study, we found a prevalence of vitamin D insufficiency in 73% and deficiency in 23% of our Egyptian patients. Abou-Raya et al.27 conducted a study on Egyptian SLE patients and found that at baseline the overall prevalence of suboptimal and deficient 25(OH)D serum levels among patients with SLE was 69%. However, Hamza et al.28 studied Egyptian pediatric SLE patients and found that 60% were insufficient and 13.3% were deficient. Moreover, our results agree with the study by Ruiz-Irastorza et al.19, who reported that 75% of SLE patients had vitamin D levels <30 ng/mL and 15% had <10 ng/mL, despite the fact that their population resides in a south European country with plenty of sunny days. The difference between our results might be due to vitamin D supplementation in some patients in the previous study while none in the current study received any at the time of entry. Similarly, Kamen et al.18 found vitamin D insufficiency and critical deficiency in 67% and 17.8%, respectively, of the SLE patients in their study. These baseline results within a cohort of newly diagnosed SLE patients suggested vitamin D deficiency as a possible risk factor for the development of the disease. Short disease duration in that study decreased the likelihood that vitamin D deficiency could have resulted from the disease and hence suggesting its presence as a cause rather than a consequence. This provided guidance for future investigations looking at a potential role for vitamin D in the prevention and/or treatment of SLE.19 A remarkable note is that the same ethnic disparities seen in vitamin D deficiency prevalence are also seen in the prevalence of SLE.29
Since the specific impact of vitamin D deficiency or insufficiency on SLE is more important than just its presence, it was a main scope in our work. Our present cross-sectional study showed important correlations with various studied parameters.
In the current study, an inverse relationship was found between vitamin D level and SLE activity measured by SLEDAI (r = −0.495, P < 0.001). This is in accordance with other cross-sectional studies such as the studies by Borba et al.30, reporting a strong negative correlation (P = 0.0005), and Yeap et al.31 (P = 0.033), both using SLEDAI. The former reported SLEDAI to be associated with low vitamin D levels and high cytokines levels such as IL-6 and TNF and also suggested bone remodeling infection consequent to decreased vitamin D, whereas the latter suggested better BMD response to treatment in patients with higher levels of vitamin D. On the other hand, Ruiz-Irastorza et al.19 failed to report this relation. Several other studies were concomitant to our finding though measuring activity was slightly different by SLEDAI-2 K like Amital et al.6, who concluded that there is an inverse relationship between level of vitamin D and disease activity in SLE and further questioned whether to routinely supplement those patients with vitamin D.32 This relationship can be explained by the inhibitory effect that vitamin D was found to exert on Th1 immunity and autoantibodies production in basic studies.33,34
An inverse correlation was also found between serum vitamin D level and irreversible organ damage measured by the SLICC/ACR DI (r = −0.431, P < 0.05). Such a relationship could not be verified in two studies that used the same index. In spite no correlation was observed by those researchers, the most recent study of the two highlighted that vitamin D insufficiency is highly prevalent among SLE patients and that sunscreen use and obesity increase the risk. They further stated that clinicians should be aware of these factors and supplement SLE patients at risk for vitamin D deficiency accordingly.19,35
On the other hand, Lupus SDI and vitamin D showed negative correlation but did not reach significance.
Fatigue was found to be prevalent in 97.7% of our studied SLE patients. When measured by VAS, it showed significant negative correlation with vitamin D (r = −0.436, P < 0.05) and significant positive correlations with SLICC, SDI, and anti-dsDNA antibodies (r = 0.502, P < 0.001; r = 0.419, P < 0.05; and r = 0.413, P < 0.05, respectively). In accordance with the current work, Ruiz-Irastorza et al.19 stated the association between vitamin D deficiency and fatigue in SLE patients and highlighted it as a predictor for higher values of VAS. They further explained that by the fact that vitamin D deficiency is a well-identified cause of myalgia and weakness.19 Another interesting work in 2010 studied the relationship between changes in 25(OH)D levels from baseline (after supplementation) and changes in fatigue (measured by VAS) and found inverse significant correlations between 25(OH)D levels and the VAS (P = 0.001) at baseline and between changes in 25(OH)D levels and changes in the VAS in patients with baseline vitamin D < 30 ng/mL (P = 0.017); they hence suggested that increasing 25(OH)D levels may have a beneficial effect on fatigue.36
In the study by Carvalho et al.20, antivitamin D antibodies have been observed in a subset of SLE patients and showed a strong association with anti-dsDNA antibodies. We did not study antivitamin D antibodies, but in our study, we found a negative correlation between serum levels of vitamin D and anti-dsDNA (r = −0.398, P < 0.05), which could be explained by the antibodies against vitamin D in SLE demonstrated in the former mentioned study.
Although some studies found a negative relation between vitamin D levels and corticosteroids in terms of dose28 and cumulative dose,37 in our study no significant correlation was found between dose and 25(OH)D levels. This is in accordance with the study by Fragoso et al.38, who found no correlation between vitamin D level and neither steroid use nor antimalarials in SLE. We also did not find any significant correlation with any of the drugs used by our patients, including antimalarials. On the other hand, the role of HCQ in vitamin D metabolism is relatively complex. Ruiz-Irastorza et al.19 found that patients on antimalarial treatment had higher levels of 25(OH)D and were less likely to have critically low vitamin D levels; they further explained that it inhibits the 1α-hydroxylation of 25(OH)D, thus decreasing the levels of the most active form of vitamin D.39
Patients with autoimmune diseases such as multiple sclerosis, RA, and SLE show low 25-OH vitamin D serum levels. Some studies suggested that adequate vitamin D levels diminished the risk for developing various autoimmune diseases.3,40,41 Others proposed “preventive” treatment using vitamin D for high-risk individuals for developing autoimmune diseases.42 In particular, SLE patients have multiple risk factors for vitamin D deficiency and disease activity and severity might be correlated with lower 25-OH vitamin D serum levels. Treatment of vitamin D deficiency could be particularly important in SLE patients, especially in view of the possible immunomodulatory effects exerted by vitamin D.
The major limitation of this study was the sample size, as well as the duration of the study. Another difficulty was the inability to study the influence of vitamin D supplementation on various clinical manifestations and disease activity.
In conclusion, we found high prevalence of vitamin D deficiency in SLE Egyptian patients. It is associated with higher degree of fatigue and disease activity in those patients. Its effect on disease severity (organ damage) still needs further studies. Also, since the replenishment of adequate level of vitamin D is considered as an easy therapeutic measure (if proven to be both needed and efficient), it may provide a relatively low cost, simple, and safe approach to improve outcome in SLE patients.
So, it is time to routinely give vitamin D supplementation to SLE patients after periodic measurement of its level. Our next step is to subcategorize the patients according to age, sex, and their vitamin D level and to study the effect of vitamin D supplementation on their disease activity and fatigue over a period of time.
Acknowledgments
A preliminary version of this research was the basis of a poster presentation at the Eular Congress 2015, and the abstract was subsequently published in the Annals of the Rheumatic Diseases.
Footnotes
ACADEMIC EDITOR: Christopher Chang, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1016 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: NMA, RME-M, AS, RHE-k. Analyzed the data: NMA, RME-M, AS. Wrote the first draft of the manuscript: NMA, RME-M, AS. Contributed to the writing of the manuscript: SAM. Agree with manuscript results and conclusions: NMA, RME-M, AS. Jointly developed the structure and arguments for the paper: KFA-h, AAAZ. Made critical revisions and approved final version: SAM, KFA-h, AAAZ. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Kamen DL, Tangpricha V. Review Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med. 2010;88:441–50. doi: 10.1007/s00109-010-0590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White JH. Review Vitamin D metabolism and signaling in the immune system. Rev Endocr Metab Disord. 2012;13:21–29. doi: 10.1007/s11154-011-9195-z. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;11:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4.Cantorna MT. Vitamin D and autoimmunity: is vitamin D status an environmental factor affecting autoimmune disease prevalence? Proc Med. 2000;11:230–3. doi: 10.1046/j.1525-1373.2000.22333.x. [DOI] [PubMed] [Google Scholar]
- 5.Cutolo M, Otsa K, Paolino S, Yprus M, Veldi T, Seriolo B. Vitamin D involvement in rheumatoid arthritis and systemic lupus erythaematosus. Ann Rheum Dis. 2009;11:446–7. doi: 10.1136/ard.2008.093476. [DOI] [PubMed] [Google Scholar]
- 6.Amital H, Szekanecz Z, Szucs G, et al. Serum concentrations of 25-OH vitamin D in patients with systemic lupus erythematosus (SLE) are inversely related to disease activity: is it time to routinely supplement patients with SLE with vitamin D? Ann Rheum Dis. 2010;69:1155–7. doi: 10.1136/ard.2009.120329. [DOI] [PubMed] [Google Scholar]
- 7.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new etiological and therapeutic considerations. Ann Rheum Dis. 2007;66:1137–42. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutolo M, Otsa K. Review: vitamin D, immunity and lupus. Lupus. 2008;17:6–10. doi: 10.1177/0961203307085879. [DOI] [PubMed] [Google Scholar]
- 9.Smolders J, Peelen E, Thewissen M, et al. The relevance of vitamin D receptor gene polymorphisms for vitamin D research in multiple sclerosis. Autoimmun Rev. 2009;8:621–6. doi: 10.1016/j.autrev.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Cutolo M. Further emergent evidence for the vitamin D endocrine system involvement in autoimmune rheumatic disease risk and prognosis. Ann Rheum Dis. 2013;72:473–5. doi: 10.1136/annrheumdis-2012-202538. [DOI] [PubMed] [Google Scholar]
- 11.Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum. 2004;50:72–7. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 12.Sabbagh Z, Markland J, Vatanparast H. Vitamin D status is associated with disease activity among rheumatology outpatients. Nutrients. 2013;5:2268–75. doi: 10.3390/nu5072268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pakchotanon R, Chaiamnuay S, Narongroeknawin P, Asavatanabodee P. bThe association etween serum vitamin D Level and disease activity in Thai rheumatoid arthritis patients. Biomed Res Int. 2015;1:913804–9. doi: 10.1111/1756-185X.12222. [DOI] [PubMed] [Google Scholar]
- 14.Chizzolini C, Cohen CD, Eisenberger U, et al. Towards the Swiss systemic lupus erythematosus cohort study (SSCS) Rev Med Suisse. 2009;5:808–11. [PubMed] [Google Scholar]
- 15.Zonana-Nacach A, Roseman JM, McGwin G, Jr, et al. Systemic lupus erythematosus in three ethnic groups. VI: Factors associated with fatigue within 5 years of criteria diagnosis. LUMINA Study Group. Lupus in minority populations: nature vs nurture. Lupus. 2000;9:101–9. doi: 10.1191/096120300678828046. [DOI] [PubMed] [Google Scholar]
- 16.Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria for Fatigue Review measurement of fatigue in systemic lupus erythematosus: a systematic review. Arthritis Rheum. 2007;57:1348–57. doi: 10.1002/art.23113. [DOI] [PubMed] [Google Scholar]
- 17.O’Leary TJ, Jones G, Yip A, Lohnes D, Cohanim M, Yendt ER. The effects of chloroquine on serum 1,25-dihydroxyvitamin D and calcium metabolism in sarcoidosis. N Engl J Med. 1986;315:727–30. doi: 10.1056/NEJM198609183151203. [DOI] [PubMed] [Google Scholar]
- 18.Kamen DL, Cooper GS, Bouali H, Shaftman SR, Hollis BW, Gilkeson GS. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev. 2006;5:114–7. doi: 10.1016/j.autrev.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz-Irastorza G, Egurbide MV, Olivares N, Martinez-Berriotxoa A, Aguirre C. Vitamin D deficiency in systemic lupus erythematosus: prevalence, predictors and clinical consequences. Rheumatology (Oxford) 2008;47:920–3. doi: 10.1093/rheumatology/ken121. [DOI] [PubMed] [Google Scholar]
- 20.Carvalho JF, Blank M, Kiss E, Tarr T, Amital H, Shoenfeld Y. Antivitamin D, vitamin D in SLE: preliminary results. Ann N Y Acad Sci. 2007;1109:550–7. doi: 10.1196/annals.1398.061. [DOI] [PubMed] [Google Scholar]
- 21.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 22.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index of lupus patients. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 23.Gladman DD, Goldsmith CH, Urowitz MB, et al. The Systemic Lupus Erythematosus International Collaborating Clinics/American College of Rheumatology index for systemic lupus erythematosus international comparison. J Rheumatol. 2000;27:373–6. [PubMed] [Google Scholar]
- 24.Katz JD, Senecal JL, Rivest C, Goulet JR, Rothfield NA. Simple severity of disease index for systemic lupus erythematosus. Lupus. 1993;2:119–23. doi: 10.1177/096120339300200210. [DOI] [PubMed] [Google Scholar]
- 25.Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521–30. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 26.Austin HA, Muenz LR, Joyce KM, et al. Prognostic factors in lupus nephritis. Contribution of renal histologic data. Am J Med. 1983;75:382–91.3. doi: 10.1016/0002-9343(83)90338-8. [DOI] [PubMed] [Google Scholar]
- 27.Abou-Raya A, Abou-Raya S, Helmii M. The effect of vitamin D supplementation on inflammatory and hemostatic markers and disease activity in patients with systemic lupus erythematosus: a randomized placebo-controlled trial. J Rheumatol. 2013;40:265–72. doi: 10.3899/jrheum.111594. [DOI] [PubMed] [Google Scholar]
- 28.Hamza RT, Awwad KS, Ali MK, Hamed AI. Reduced serum concentrations of 25-hydroxy vitamin D in Egyptian patients with systemic lupus erythematosus: relation to disease activity. Med Sci Monit. 2011;17:CR711–8. doi: 10.12659/MSM.882131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilkeson G, James J, Kamen D, et al. The United States to Africa lupus prevalence gradient revisited. Lupus. 2011;20:1095–103. doi: 10.1177/0961203311404915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borba VZ, Vieira JG, Kasamatsu T, Radominski SC, Sato EI, Lazaretti-Castro M. Vitamin D deficiency in patients with active systemic lupus erythematosus. Osteoporos Int. 2009;20:427–33. doi: 10.1007/s00198-008-0676-1. [DOI] [PubMed] [Google Scholar]
- 31.Yeap SS, Othman AZ, Zain AA, Chan SP. Vitamin D levels: its relationship to bone mineral density response and disease activity in premenopausal Malaysian systemic lupus erythematosus patients on corticosteroids. Int J Rheum Dis. 2012;15:17–24. doi: 10.1111/j.1756-185X.2011.01653.x. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds JA, Haque S, Berry JL, et al. 25-hydroxyvitamin D deficiency is associated with increased aortic stiffness in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2012;51:544–51. doi: 10.1093/rheumatology/ker352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1,25-dihydroxy vitamin D3: preferential Inhibition of Th1 Functions. J Nutr. 1995;125:1704S–8S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- 34.Linker-Israeli M, Elstner E, Klinenberg JR, Wallace DJ, Koeffler HP. Vitamin D3 and its synthetic analogs inhibit the spontaneous in vitro immunoglobulin production by SLE-derived PBMC. ClinImmunol. 2001;99:82–93. doi: 10.1006/clim.2000.4998. [DOI] [PubMed] [Google Scholar]
- 35.Muñoz-Ortego J, Torrente-Segarra V, Prieto-Alhambra D, Salman-Monte TC, Carbonell-Abello J. Prevalence and predictors of vitamin D deficiency in non-supplemented women with systemic lupus erythematosus in the Mediterranean region: a cohort study. Scand J Rheumatol. 2012;41:472–5. doi: 10.3109/03009742.2012.697189. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz-Irastorza G, Gordo S, Olivares N, Egurbide MV, Aguirre C. Changes in vitamin D levels in patients with systemic lupus erythematosus: effects on fatigue, disease activity, and damage. Arthritis Care Res (Hoboken) 2010;62:1160–5. doi: 10.1002/acr.20186. [DOI] [PubMed] [Google Scholar]
- 37.Toloza S, Cole D, Gladman D, Ibañez D, Urowitz MB. Vitamin D insufficiency in a large female SLE cohort. Lupus. 2010;19:13–9. doi: 10.1177/0961203309345775. [DOI] [PubMed] [Google Scholar]
- 38.Fragoso TS, Dantas AT, Marques CD, et al. 25-Hydroxyivitamin D3 levels in patients with systemic lupus erythematosus and its association with clinical parameters and laboratory tests. Rev Bras Reumatol. 2012;52:60–5. [PubMed] [Google Scholar]
- 39.Barnes TC, Bucknall RC. Vitamin D deficiency in a patient with systemic lupus erythematosus. Rheumatology. 2004;43:393–4. doi: 10.1093/rheumatology/keh050. [DOI] [PubMed] [Google Scholar]
- 40.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–73. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 41.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitaminD3, and the immune system. Am J Clin Nutr. 2004;80:1717S–20S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 42.Harel M, Shoenfeld Y. Predicting and preventing autoimmunity, myth or reality? Ann NY Acad Sci. 2006;1069:322–45. doi: 10.1196/annals.1351.031. [DOI] [PubMed] [Google Scholar]


