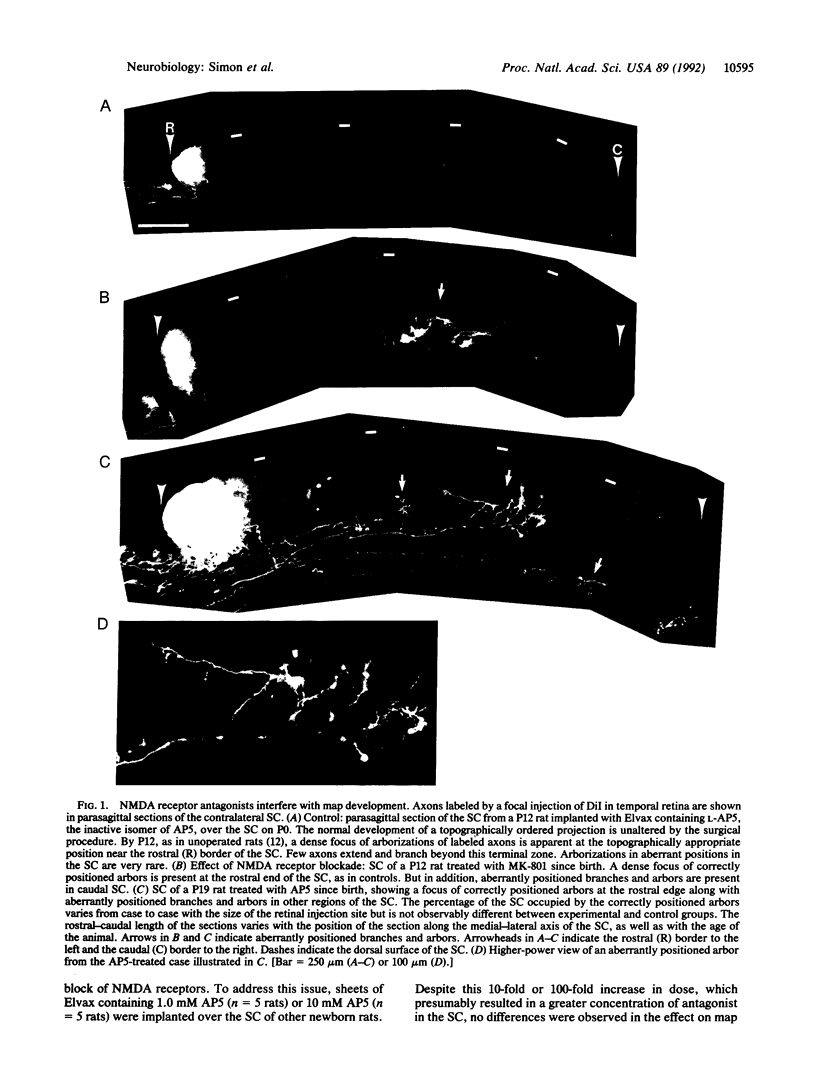
Abstract
The topographic ordering of retinal connections in the rat superior colliculus emerges during early postnatal life from an initially diffuse projection. Disruption of N-methyl-D-aspartate (NMDA) receptor activity in the superior colliculus during this period interferes with map remodeling. In rats chronically treated with NMDA receptor antagonists during the first two postnatal weeks, aberrant axons remain and arborize at topographically incorrect sites. These results indicate that, at a stage preceding visually evoked activity, normal NMDA receptor function is important for the development of an ordered neural map in the mammalian brain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artola A., Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987 Dec 17;330(6149):649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- Bear M. F., Kleinschmidt A., Gu Q. A., Singer W. Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. J Neurosci. 1990 Mar;10(3):909–925. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt S. M., Lund R. D., Land P. W. Prenatal development of the optic projection in albino and hooded rats. Brain Res. 1983 Jan;282(2):149–168. doi: 10.1016/0165-3806(83)90093-7. [DOI] [PubMed] [Google Scholar]
- Chiaia N. L., Fish S. E., Bauer W. R., Bennett-Clarke C. A., Rhoades R. W. Postnatal blockade of cortical activity by tetrodotoxin does not disrupt the formation of vibrissa-related patterns in the rat's somatosensory cortex. Brain Res Dev Brain Res. 1992 Apr 24;66(2):244–250. doi: 10.1016/0165-3806(92)90086-c. [DOI] [PubMed] [Google Scholar]
- Cline H. T., Constantine-Paton M. NMDA receptor antagonists disrupt the retinotectal topographic map. Neuron. 1989 Oct;3(4):413–426. doi: 10.1016/0896-6273(89)90201-8. [DOI] [PubMed] [Google Scholar]
- Cline H. T., Debski E. A., Constantine-Paton M. N-methyl-D-aspartate receptor antagonist desegregates eye-specific stripes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4342–4345. doi: 10.1073/pnas.84.12.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol Sci. 1990 Jul;11(7):290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M., Cline H. T., Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- Fox K., Daw N., Sato H., Czepita D. Dark-rearing delays the loss of NMDA-receptor function in kitten visual cortex. Nature. 1991 Mar 28;350(6316):342–344. doi: 10.1038/350342a0. [DOI] [PubMed] [Google Scholar]
- Fujisawa H. Mode of growth of retinal axons within the tectum of Xenopus tadpoles, and implications in the ordered neuronal connection between the retina and the tectum. J Comp Neurol. 1987 Jun 1;260(1):127–139. doi: 10.1002/cne.902600110. [DOI] [PubMed] [Google Scholar]
- Funke K., Eysel U. T., FitzGibbon T. Retinogeniculate transmission by NMDA and non-NMDA receptors in the cat. Brain Res. 1991 May 3;547(2):229–238. doi: 10.1016/0006-8993(91)90966-y. [DOI] [PubMed] [Google Scholar]
- Gu Q. A., Bear M. F., Singer W. Blockade of NMDA-receptors prevents ocularity changes in kitten visual cortex after reversed monocular deprivation. Brain Res Dev Brain Res. 1989 Jun 1;47(2):281–288. doi: 10.1016/0165-3806(89)90183-1. [DOI] [PubMed] [Google Scholar]
- Hablitz J. J., Langmoen I. A. N-methyl-D-aspartate receptor antagonists reduce synaptic excitation in the hippocampus. J Neurosci. 1986 Jan;6(1):102–106. doi: 10.1523/JNEUROSCI.06-01-00102.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm J. O., Langdon R. B., Sur M. Disruption of retinogeniculate afferent segregation by antagonists to NMDA receptors. Nature. 1991 Jun 13;351(6327):568–570. doi: 10.1038/351568a0. [DOI] [PubMed] [Google Scholar]
- Harris W. A. Axonal pathfinding in the absence of normal pathways and impulse activity. J Neurosci. 1984 Apr;4(4):1153–1162. doi: 10.1523/JNEUROSCI.04-04-01153.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris W. A. The effects of eliminating impulse activity on the development of the retinotectal projection in salamanders. J Comp Neurol. 1980 Nov 15;194(2):303–317. doi: 10.1002/cne.901940203. [DOI] [PubMed] [Google Scholar]
- Holt C. E. Does timing of axon outgrowth influence initial retinotectal topography in Xenopus? J Neurosci. 1984 Apr;4(4):1130–1152. doi: 10.1523/JNEUROSCI.04-04-01130.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt C. E., Harris W. A. Order in the initial retinotectal map in Xenopus: a new technique for labelling growing nerve fibres. Nature. 1983 Jan 13;301(5896):150–152. doi: 10.1038/301150a0. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A., Bear M. F., Singer W. Blockade of "NMDA" receptors disrupts experience-dependent plasticity of kitten striate cortex. Science. 1987 Oct 16;238(4825):355–358. doi: 10.1126/science.2443978. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Nakamura H., Yasuda M. Disturbance of refinement of retinotectal projection in chick embryos by tetrodotoxin and grayanotoxin. Brain Res Dev Brain Res. 1990 Dec 1;57(1):29–35. doi: 10.1016/0165-3806(90)90181-w. [DOI] [PubMed] [Google Scholar]
- Linden R., Perry V. H. Massive retinotectal projection in rats. Brain Res. 1983 Aug 1;272(1):145–149. doi: 10.1016/0006-8993(83)90371-2. [DOI] [PubMed] [Google Scholar]
- Maffei L., Galli-Resta L. Correlation in the discharges of neighboring rat retinal ganglion cells during prenatal life. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2861–2864. doi: 10.1073/pnas.87.7.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M., Wong R. O., Baylor D. A., Shatz C. J. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991 May 17;252(5008):939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991 Nov 7;354(6348):31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Nakamura H., O'Leary D. D. Inaccuracies in initial growth and arborization of chick retinotectal axons followed by course corrections and axon remodeling to develop topographic order. J Neurosci. 1989 Nov;9(11):3776–3795. doi: 10.1523/JNEUROSCI.09-11-03776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northmore D. P., Masino T. Recovery of vision in fish after optic nerve crush: a behavioral and electrophysiological study. Exp Neurol. 1984 Apr;84(1):109–125. doi: 10.1016/0014-4886(84)90009-8. [DOI] [PubMed] [Google Scholar]
- O'Rourke N. A., Fraser S. E. Dynamic changes in optic fiber terminal arbors lead to retinotopic map formation: an in vivo confocal microscopic study. Neuron. 1990 Aug;5(2):159–171. doi: 10.1016/0896-6273(90)90306-z. [DOI] [PubMed] [Google Scholar]
- Ramoa A. S., Alkondon M., Aracava Y., Irons J., Lunt G. G., Deshpande S. S., Wonnacott S., Aronstam R. S., Albuquerque E. X. The anticonvulsant MK-801 interacts with peripheral and central nicotinic acetylcholine receptor ion channels. J Pharmacol Exp Ther. 1990 Jul;254(1):71–82. [PubMed] [Google Scholar]
- Rauschecker J. P., Egert U., Kossel A. Effects of NMDA antagonists on developmental plasticity in kitten visual cortex. Int J Dev Neurosci. 1990;8(4):425–435. doi: 10.1016/0736-5748(90)90075-d. [DOI] [PubMed] [Google Scholar]
- Reh T. A., Constantine-Paton M. Retinal ganglion cell terminals change their projection sites during larval development of Rana pipiens. J Neurosci. 1984 Feb;4(2):442–457. doi: 10.1523/JNEUROSCI.04-02-00442.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi D. S., Murphey R. K. Map formation in the developing Xenopus retinotectal system: an examination of ganglion cell terminal arborizations. J Neurosci. 1985 Dec;5(12):3228–3245. doi: 10.1523/JNEUROSCI.05-12-03228.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer W. J., Udin S. B. N-methyl-D-aspartate antagonists prevent interaction of binocular maps in Xenopus tectum. J Neurosci. 1989 Nov;9(11):3837–3843. doi: 10.1523/JNEUROSCI.09-11-03837.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. T. Long-term potentiation and activity-dependent retinotopic sharpening in the regenerating retinotectal projection of goldfish: common sensitive period and sensitivity to NMDA blockers. J Neurosci. 1990 Jan;10(1):233–246. doi: 10.1523/JNEUROSCI.10-01-00233.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz C. J. Impulse activity and the patterning of connections during CNS development. Neuron. 1990 Dec;5(6):745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- Shatz C. J., Stryker M. P. Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science. 1988 Oct 7;242(4875):87–89. doi: 10.1126/science.3175636. [DOI] [PubMed] [Google Scholar]
- Silberstein G. B., Daniel C. W. Elvax 40P implants: sustained, local release of bioactive molecules influencing mammary ductal development. Dev Biol. 1982 Sep;93(1):272–278. doi: 10.1016/0012-1606(82)90259-7. [DOI] [PubMed] [Google Scholar]
- Simon D. K., O'Leary D. D. Development of topographic order in the mammalian retinocollicular projection. J Neurosci. 1992 Apr;12(4):1212–1232. doi: 10.1523/JNEUROSCI.12-04-01212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D. K., O'Leary D. D. Limited topographic specificity in the targeting and branching of mammalian retinal axons. Dev Biol. 1990 Jan;137(1):125–134. doi: 10.1016/0012-1606(90)90013-9. [DOI] [PubMed] [Google Scholar]
- Stuermer C. A. Retinotopic organization of the developing retinotectal projection in the zebrafish embryo. J Neurosci. 1988 Dec;8(12):4513–4530. doi: 10.1523/JNEUROSCI.08-12-04513.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuermer C. A., Rohrer B., Münz H. Development of the retinotectal projection in zebrafish embryos under TTX-induced neural-impulse blockade. J Neurosci. 1990 Nov;10(11):3615–3626. doi: 10.1523/JNEUROSCI.10-11-03615.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udin S. B., Fawcett J. W. Formation of topographic maps. Annu Rev Neurosci. 1988;11:289–327. doi: 10.1146/annurev.ne.11.030188.001445. [DOI] [PubMed] [Google Scholar]
- Weidman T. A., Kuwabara T. Postnatal development of the rat retina. An electron microscopic study. Arch Ophthalmol. 1968 Apr;79(4):470–484. doi: 10.1001/archopht.1968.03850040472015. [DOI] [PubMed] [Google Scholar]
- Wigström H., Gustafsson B. On long-lasting potentiation in the hippocampus: a proposed mechanism for its dependence on coincident pre- and postsynaptic activity. Acta Physiol Scand. 1985 Apr;123(4):519–522. doi: 10.1111/j.1748-1716.1985.tb07621.x. [DOI] [PubMed] [Google Scholar]