Abstract
Background
Squamous cell carcinoma (SCC) is the most common malignancy of the oral cavity. A relationship between the human papilloma virus (HPV) infection and the prognosis of oral cavity SCC (OCSCC) has been discussed before.
Objectives
We investigated the prevalence rate of HPV status in patients with OCSCC, and its effects on clinicopathological characteristics of tumors and patients’ prognosis.
Patients and Methods
Sections of formalin-fixed, paraffin-embedded tissue blocks from 114 histopathologically confirmed OCSCC cases were investigated in this study. Polymerase chain reaction (PCR) was applied to evaluate the HPV status in the samples.
Results
Fifteen (13.16%) cases were identified as HPV positive. The detected viral subtypes in this study were the subtypes 6 and 11. The stage and especially lymph node stage was significantly higher in the HPV positive group compared to the HPV negative group (P = 0.04). Disease free survival (DFS) was remarkably lower in the HPV positive group compared to the HPV negative group (13.9 vs. 49.9 months, P = 0.02). Overall survival (OS) was also significantly inferior in the HPV positive group (15.7 vs. 49.6 months, P = 0.01). In the current study, no significant differences were observed between two groups in relation to the variables of age, gender, tumors site, tumor size, tumor grading and also the recurrence rate.
Conclusions
The observed higher mortality rate among the HPV positive group indicates the poorer prognosis of this group in comparison with the HPV negative patients. The incidence rate of HPV infection was low in the studied samples; however, interaction of subtypes 6 and 11 of HPV in poorer prognosis of the patients and a carcinogenic role of HPV in OCSCC cannot be ruled out.
Keywords: Oral Cavity Squamous Cell Carcinoma; Human Papilloma Virus, Polymerase Chain Reaction; Prognosis; Iran
1. Background
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide, with an average 5-year survival rate of around 50% (1). Despite improvements in the therapeutic modalities, the prognosis of these patients has not been significantly improved in the last three decades.
Alcohol and both smoked and smokeless tobacco use are associated with increased risk of developing malignancy of the oral cavity and pharynx (2).
Recently, the role of oncogenic viruses especially HPV in the pathogenesis of SCC has attracted the researchers’ attention. HPVs belong to the family of DNA Papovaviridae. Over 100 types of HPV have been identified (3). HPV viral proteins, E6 and E7, play major roles in the cervical as well as orophararyngeal carcinogenesis. This proteins act through inactivation of tumor suppressor genes, P53 and retinoblastoma (Rb) genes, resulting in cell immortalization and transformation (4). Perhaps one of the most important developments in the head and neck oncology over the past decade is the recognition of HPV status in oropharyngeal SCCs (OPSCCs). It is obvious that HPV-ralated OPSCC has unique epidemiological and clinical entity and it had better distinguishes from the OCSCCs because of unknown role of HPV in non-oropharyngeal head and neck cancers (5-7).
Scapoli et al. in 2009 (8), Laco et al. in 2011 (9), and Lingen et al. in 2013 (10) considered low etiologic role for HPV status in OCSCCs. Laco et al. mentioned that probably other risk factors that are still unknown play roles in the pathogenesis of OCSCCs.
In recent years, much attention has been paid to the prognostic role of HPV status in HNSCC. Various studies have reported conflicting results. Some researchers have found that patients with HPV detected in their cancers, have better prognosis compared to those without HPV (11-15). Nevertheless there are studies that have found no difference or poorer prognosis for HPV related tumors (16-22).
2. Objectives
In this study, we have investigated the prevalence rate of HPV status in patients with OCSCC, and its significance in the clinicopathological characteristics of tumors and patients’ prognosis.
3. Patients and Methods
132 patients with diagnosis of OCSCC were included in this study between 2001 and 2013. Their records were collected from the files of department of pathology, faculty of dentistry and Omid hospital, Mashhad University of Medical Sciences, Iran. Age, gender, anatomical region, TNM stage, histological grade, therapeutic interventions, events including local, distant relapse and death were extracted from the medical records database. The Haematoxylin and Eosin stained sections from all specimens were reviewed by two pathologists to confirm the histology and grading.
Histological grading was assessed according to the WHO guidelines and tumor staging was reported according to the American Joint Committee on Cancer Staging Criteria (AJCC Sixth Edition, 2002). The study protocol was approved by the Ethical Committee of the Faculty of Dentistry.
3.1. Detection of HPV DNA Was Performed by PCR
5 to 10 μm sections were cut from each paraffin block. Cleaning off the microtome blade with xylene and ethanol after cutting each paraffin block was taken for preventing and monitoring contamination of samples. The tumor samples were deparaffinized using a series of xylene and ethanol washes. In this study, DNA extraction was performed manually using enzymatic method. To extract DNA, the tissue sample was mixed with 400 μL digestion buffer which consisted of Tris-Cl (100 mM, pH 7.5) and Tween-20 (0.055) and proteinase K 3μL (20 mg/mL; Frementase, Germany), followed by incubation at 56°C for 3 hour. After the complete digestion of tissue, the mixture was incubated at 100°C in Hotblock (AccuBlock™ Digital Dry Baths; USA) to inactivate proteinase K. The samples were then centrifuged at 5000 rpm, and the supernatant containing DNA was used for PCR amplification (96-well thermal cycler; Applied Biosystem, USA). The quality of DNA was determined by amplification of housekeeping gene (β-Globin) using the GH20/PC04 primers (Cinnagen, Iran).
A consensus sequence from the L1 region of the HPV genome was amplified using GP5+/GP6+ primers (Cinnagen, Iran) (2). GP5:5’-TTT GTT ACT GTG GTA GAT ACT AC-3’ and GP6:5’-GAA AAA TAA ACT GTA AAT CAT ATT C-3’ were used as the primer set.
HPV typing (HPV 6, 11, 16, 18 and 31) was also performed using HPV type specific primers (Cinnagen, Iran) (19). PCR products were separated in 2% agarose gels and visualized by Green Veiwer staining (Parstous, Iran).
3.2. Statistical Analysis
All analyses were performed using data processing program SPSS/PC version 16.0 for Windows (SPSS Inc., Chicago, IL, USA). Chi square, Mann -Whitney, Student’s t test and Fisher’s exact tests were used to assess the statistical significant differences between HPV status and clinicopathological parameters. The OS and DFS rates were estimated by the Kaplan-Meier method, and differences in survival rates were analyzed with the log-rank test. Relative risk and prognostic independence was assessed by the multivariate Cox proportional hazards model. P values less than 0.05 were considered statistically significant.
4. Results
4.1. Characteristics of Cases
Ten cases were excluded from the study because amplifiable DNA was not obtained. Moreover, eight patients were excluded because their clinicopathological databases were not complete. Remaining 114 OCSCC cases comprised of 58 men and 56 women. The mean age of patients was 58.6 years (range 19 - 85 years).The follow-up period ranged from 6 months to 8 years. Median follow up was 23.7 months.
The HPV sequences were detected in 15 patients (13.16%): 5 cases with HPV11 (33.3%), one case with HPV6 (6.7%) and two cases with both HPV6 and 11 (13.3%). In 7 patients (46.7%) the HPV subtypes remained undetermined. HPV16, 18, 31and 33 were not detected in any of the cases. The most common site of involvement in both groups was the tongue.
Patients’ characteristics and tumor features of the HPV positive and negative OCSCC cases are compared in Table 1. No significant differences were observed between the two groups in the variables of age, gender, tumor site, size and histological grading. Fifty five HPV-negative patients (55.6%) were N0, while five HPV-positive patients (33.3%) were N0. In addition, while twenty two HPV-negatives (22.2%) were N2, eight HPV-positive patients (53.3%) were N2. The median lymph node involvement was significantly higher in HPV positive group compared to HPV negative group (P = 0.045). As a result, significantly more stage IVA was seen among the HPV-positive tumors compared to the HPV-negative ones (66.7% vs. 35.4%, P = 0.04).
Table 1. The Clinicopathological Characteristics of HPV-Positive and HPV-Negative Patients.
| Characteristic | HPV Positive (N = 15) | HPV Negative (N = 99) | P Value |
|---|---|---|---|
| Sex | 0.838 | ||
| Male | 8 (53.3) | 50 (51) | |
| Female | 7 (46.7) | 49 (49) | |
| Age, y | 0.509 | ||
| < 50 | 3 (20.0) | 27 (27.3) | |
| 70 - 50 | 6 (40.0) | 42 (42.4) | |
| > 70 | 6 (40.0) | 30 (30.3) | |
| Location | 0.879 | ||
| Tongue | 10 (66.6) | 65 (65.7) | |
| Buccal mucosa | 0 (0.0) | 18 (18.2) | |
| Alveolar ridge | 3 (20.0) | 9 (9.1) | |
| Palate | 1 (6.7) | 3 (3.0) | |
| Floor of the mouth | 1 (6.7) | 2 (2.0) | |
| Lip | 0 (0.0) | 2 (2.0) | |
| Grade | 0.274 | ||
| 1 | 6 (40.0) | 59 (59.6) | |
| 2 | 9 (60.0) | 33 (33.3) | |
| 3 | 0 (0.0) | 7 (7.1) | |
| T classification | 0.406 | ||
| T1 | 3 (20.0) | 3 3(33.3) | |
| T2 | 6 (40.0) | 32 (32.3) | |
| T3 | 2 (13.3) | 12 (12.1) | |
| T4 | 4 (26.7) | 22 (22.2) | |
| N classification | 0.045 | ||
| N0 | 5 (33.3) | 55 (55.6) | |
| N1 | 1 (6.7) | 18 (18.2) | |
| N2 | 8 (53.3) | 22 (22.2) | |
| N3 | 0 (0.0) | 1 (1.0) | |
| NX | 1 (6.7) | 3 (3.0) | |
| Stage | 0.04 | ||
| I | 3 (20.0) | 25 (25.3) | |
| II | 1 (6.7) | 17 (17.2) | |
| III | 1 (6.7) | 22 (22.2) | |
| IV | 10 (66.7) | 35 (35.4) | |
| Smoking history | 0.99 | ||
| Never | 7 (46.6) | 42 (42.5) | |
| Ever | 4 (26.7) | 29 (29.3) | |
| Unknown | 4 (26.7) | 28 (28.2) |
Most of the HPV positive and negative cases were treated with surgery and adjuvant radiation therapy (Table 2). Local recurrence was the most common type of relapse. Thirty four out of 99 HPV-negative patients (34.3%) died from their disease compared with 11 out of 15 in the HPV-positive cases (73.3%). Chi square test revealed a significant relation between the rate of death and HPV positive status (P = 0.004).
Table 2. Comparison of the Two Groups According to Treatment Protocols and Outcomesa.
| HPV Positive (N = 15) | HPV Negative (N = 99) | P Value | |
|---|---|---|---|
| Treatment protocol | 0.67 | ||
| Only surgery | 3 (25) | 14 (15.8) | |
| Surgery + Adjuvant radiation therapy | 8 (66.7) | 65 (73) | |
| Surgery + Adjuvant chemoradiation | 1 (8.3) | 5 (5.6) | |
| Neoadjuvant (radiation therapy ± chemotherapy) | 0 | 5 (5.6) | |
| Events | 0.89 | ||
| Recurrence free | 10 (66.7) | 61 (61.6) | |
| Local recurrence | 3 (22) | 24 (24.2) | |
| Regional recurrence | 2 (13.3) | 8 (8.1) | |
| Locoregional recurrence | 0 | 3 (3) | |
| Distant metastasis | 0 | 2 (2) | |
| Second primary cancer | 0 | 1 (1) | |
| Patient’s status | 0.004 | ||
| Alive | 4 (26.7) | 65 (65.7) | |
| Dead | 11 (73.3) | 34 (34.3) | |
| Median disease free survival rate, mo | 13.9 | 49.9 | 0.029 |
| Median overall survival rate, mo | 15.7 | 49.6 | 0.017 |
aValues are expressed as No. (%).
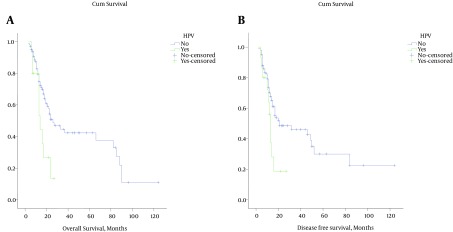
Survival and HPV DNA status: the median DFS was 13.9 months among the HPV-positive and 49.9 months among the HPV-negative patients with a significant difference (P = 0.029). Also the median OS of 15.7 months in the HPV-positive group compared to 49.6 months in the HPV-negative group was significantly better (P = 0.017). The Kaplan-Meier survival curves for the HPV-positive and negative cases are shown in Figure 1. We used multivariate analysis involving Cox proportional hazards model to determine which factors were jointly predictive of OS and DFS. The HPV status (HR = 2.10, CI = 1.02 to 4.28, P = 0.041) and Stage (HR = 1.92, CI = 1.36 to 2.70, P < 0.001) were significantly predictive of OS. In addition, both HPV status (HR = 2.80, CI = 1.35 to 5.79, P = 0.005) and stage of disease (HR = 1.95, CI = 1.37 to 2.79, P < 0.001) were significantly predictive of DFS.
Figure 1. Kaplan-Meier Survival Curves for Overall and Disease Free Survival With Respect to HPV Status.
5. Discussion
The participation of HPV in the oral and oropharyngeal carcinogenesis was first proposed in 1983 by Syrjanen et al. (23) and then supported by several other studies. According to many studies, the HPV prevalence rate in oral cancer is varied between 0% - 100% (6, 19, 24-26). This widespread variability can be in part due to differences in methodology of HPV detection ways and differences in sample types, populations, and anatomic sites tested (27, 28). Moreover, various studies have reported conflicting results for the probable role of HPV on the patient's prognosis (15, 16, 29).
In the current study, HPV DNA was detected in 15 out of 114 patients (13.6%). Regarding the sample size, the majority of small to medium sized studies (< 100 patients) have revealed a wide variation in HPV prevalence rates (varying between 0% and 100%); however, larger studies generally tend to show lower HPV prevalence rates (varying between 1.4% and 48.8%). Termine et al. (27) have mentioned the sample size as an important factor in the reportedly heterogeneous prevalence rate of HPV, which is in accordance with our findings.
The HPV positivity rate of 13.6% in our study is almost similar to those other reports of oral cavity cancers which are ranged from 12% to 70% for OPSCC (2, 15, 22, 30) versus 3% to 15% for OCSCC (2, 20, 31).
While there is another report that mentions a 25% incidence rate of HPV among tongue cancers in Iran and yet all of them subtypes of 18 and 16 (32), in the current study, 15 cases (13.16%) were identified as HPV positive and the detected viral subtypes were subtypes 6 and 11. However, it is not still clear whether these subtypes result in an infection driven carcinogenesis or they are just a transient infection (5, 33).
Pannone et al. mentioned that HPV 6, which was known as “low risk” or “non-oncogenic” subtype to the cervix, was present in a larger number of head and neck cancers. HPV 6 and 11 have been found in some tonsillar and laryngeal carcinomas and in the malignant transformation of benign laryngeal papillomas, HPV 11 has been most commonly implicated. HPV 6 and 11 have also been on suspicion of malignancies such as verrucous carcinoma of the oral cavity (34, 35). HPV 6 and 11 were also observed in other studies on cancers of the head and neck (36, 37).
Studies on OPSCC often suggested that the incidence of HPV in this region is more prevalent (5, 33, 38). It is also mentioned that HPV-mediated OPSCCs are more likely late stage, poorly differentiated, and with large lymph nodes in the neck, and in spite of that they have less risk of recurrence and death (2, 5, 24, 33, 39-41). Some studies indicate that such HPV-positive tumors are more radio-sensitive and have better overall survival rate (33, 42, 43). These patients tend to be younger and often male with less consumption of alcohol and smoking (33, 41, 44). There is also data indicating that patients with HPV-positive OPSCC are associated with a higher number of sexual partners (33, 34).
In SCCs limited to the oral cavity, usually the HPV prevalence rate is lower and HPV16 is also the most common type detected (2, 28).
Some studies have suggested that HPV does not play a significant role in the etiology of OCSCC (8-10) and also does not play a role in the progression toward malignancy, even though some studies have demonstrated the role of HPV16 and 18 in oral carcinogenesis (45-47). Notably, because of relatively low frequency of HPV in OCSCCs, only few studies have attamped to correlate HPV status with clinical outcome (5, 24, 46, 47).
In most of the studies of HNSCCs, the demonstrated clinicopatological features about HPV positive cases are usually related to the oropharyngeal samples; therefore, little is known about the influence of HPV on clinical course of patients with the oral cavity cancer because of the lower HPV prevalence.
No data currently supports the idea that HPV is significantly associated with improved outcome for patients with oral cancer. Only few published studies on patients with oral cavity carcinoma specifically examined the impact of HPV on outcome. Kaminagakura studied 114 patients and found a nonsignficant trend towards improved survival for 22 HPV-positive patients (47). Sugiyama et al. demonstrated a nonsignficant trend towards improved overall survival for HPVpositive oral cavity cancer patients (48). Smith found no association with HPV and outcome for patients with oral carcinoma, based on either serology or tumor HPV detection (49). Also in Isayeva’s cohort study of 89 patients with oral cavity carcinoma, no significant association was found for patients with either HPV16/18 E6E7 RNA and time to disease progression or disease specific survival (5).Therefore, future studies on oral cavity SCC should be powered to address the important clinical issue of HPV status.
In the present study, the mean age of the cases was 58.6 years, and there was no statistical difference between the HPV-positive and the HPV-negative groups based on age. Klozar et al. (13) and Marques-silva et al. (29) have come up with similar results. However, in some studies, the HPV-associated patients who suffer from oropharyngeal cancers tend to be younger (33, 39, 42, 44). In our study, 53.3% of HPV-positive patients were males and 46.7% were females, and there was no meaningful relation between gender and the HPV status. Such findings have been observed in some other studies (29), although some studies on oropharynx lesions have reported that the HPV-positive cases are predominantly male (33, 41, 42, 44). In the present study, the most common tumor site was tongue, and there was no meaningful statistical relation between tumor location and the HPV status. Similar findings have been reported in other studies (19, 29).
According to the tumor grade, although 60 % of the HPV-positive patients were grade 2 and 59.6% of HPV-negative patients were grade1, no meaningful statistical difference was found between HPV status and histological grading of the tumor (16, 19).
Although in most of the oropharyngeal cancer studies advanced tumor stages have been observed in the HPV-positive cases (18, 33, 39, 41, 44, 50), there are some studies which have not seen any difference (16, 19, 32). In this study 55.6 % of the HPV-negative patients and only 33.3% of the HPV-positive patients were N0. Still a majority of 53.3% of the HPV-positive patients were N2. Moreover, 66.7% of the HPV positive groups and only 35.4% of the HPV negative ones were stage IVA. Hoffmann et al. (51) have reported similar findings, although in some studies no difference have been observed (13) and in most of the HPV-related OPSCC cases higher lymph node involvement have been observed (18, 33, 39).
In the current study local recurrence was the most commen type of relapse, and there was no statistically significant difference according to HPV status. In most of the HPV-related OPSCC studies low reccurence rate have been observed (2, 50), although Weinberger et al. have noted that HPV status has no prognostic value for local recurrence alone (52).
In our study, 34.4% of the HPV-negative patients and 73.3% of the HPV-positive patients died of disease. That statistically meaningful relation was observed between HPV status and patients death. Less mortality risk in HPV-positive cases have been observed in some oropharyngeal studies (2, 50). But as far as we know, no study on oral cavity cancers has especially dealt with the mortality rate of HPV-positive patients.
We found that HPV-negative patients survived significantly longer than HPV-positive patients when both the OS and the DFS were measured. Similar findings have been reported in other studies based on tumors with higher stages of disease (16, 22). While the majority of oropharyngeal studies have reported an improved prognosis of HPV positive tumors (2, 5, 18, 19, 33, 39-41, 44, 50, 53), there are still other studies which have reported no difference (2, 5, 18, 19).
In our study, there was no meaningful relation between HPV status and smoking history. There are other studies which indicate HPV-related OPSCC patients, have a lower intake of tobacco and alcohol (33, 39, 44, 50).
5.1. Conclusion
Currently, little is known about the influence of HPV on the clinical course and survival of patients with OCSCC. Although the findings of this study revealed relatively low prevalance of HPV DNA in OCSCCs and also the well known high risk subtypes were not observed, the carcinogenic role of this virus and its less common variant of HPV 6 and 11, which we did see in the current study, cannot be totally ignored in the oral cavity cancers. Regarding high mortality rate of these variants of HPV-positive tumors, further investigations with higher sample volume using more developed methods are still required.
Acknowledgments
None declared.
Footnotes
Authors’ Contribution: Roham Salek and Nasrollah Saghravanian designed the study, collected the materials, analyzed the data and wrote the paper. Maryam zamanzadeh collected the materials, contributed in data entry, literature review and writing the paper. Monavar Afzal Aghaee analyzed the data. Zahra Meshkat contributed to laboratory operation. All authors read and approved the final manuscript.
Financial Disclosure: None declared.
Funding/Support: The research results given in this paper were obtained from doctoral thesis by a Grant supported from the Vice Chancellor of Mashhad University of Medical Sciences, Iran.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Herrero R, Castellsague X, Pawlita M, Lissowska J, Kee F, Balaram P, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95(23):1772–83. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 3.Campisi G, Giovannelli L. Controversies surrounding human papilloma virus infection, head & neck vs oral cancer, implications for prophylaxis and treatment. Head Neck Oncol. 2009;1:8. doi: 10.1186/1758-3284-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Syrjanen SM, Syrjanen KJ. New concepts on the role of human papillomavirus in cell cycle regulation. Ann Med. 1999;31(3):175–87. doi: 10.3109/07853899909115976. [DOI] [PubMed] [Google Scholar]
- 5.Isayeva T, Li Y, Maswahu D, Brandwein-Gensler M. Human papillomavirus in non-oropharyngeal head and neck cancers: a systematic literature review. Head Neck Pathol. 2012;6 Suppl 1:S104–20. doi: 10.1007/s12105-012-0368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeuss MS, Miller CS, White DK. In situ hybridization analysis of human papillomavirus DNA in oral mucosal lesions. Oral Surg Oral Med Oral Pathol. 1991;71(6):714–20. doi: 10.1016/0030-4220(91)90280-p. [DOI] [PubMed] [Google Scholar]
- 7.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121(8):1813–20. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 8.Scapoli L, Palmieri A, Rubini C, Martinelli M, Spinelli G, Ionna F, et al. Low prevalence of human papillomavirus in squamous-cell carcinoma limited to oral cavity proper. Mod Pathol. 2009;22(3):366–72. doi: 10.1038/modpathol.2008.180. [DOI] [PubMed] [Google Scholar]
- 9.Laco J, Vosmikova H, Novakova V, Celakovsky P, Dolezalova H, Tucek L, et al. The role of high-risk human papillomavirus infection in oral and oropharyngeal squamous cell carcinoma in non-smoking and non-drinking patients: a clinicopathological and molecular study of 46 cases. Virchows Arch. 2011;458(2):179–87. doi: 10.1007/s00428-010-1037-y. [DOI] [PubMed] [Google Scholar]
- 10.Lingen MW, Xiao W, Schmitt A, Jiang B, Pickard R, Kreinbrink P, et al. Low etiologic fraction for high-risk human papillomavirus in oral cavity squamous cell carcinomas. Oral Oncol. 2013;49(1):1–8. doi: 10.1016/j.oraloncology.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–9. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 12.Chiba I, Shindoh M, Yasuda M, Yamazaki Y, Amemiya A, Sato Y, et al. Mutations in the p53 gene and human papillomavirus infection as significant prognostic factors in squamous cell carcinomas of the oral cavity. Oncogene. 1996;12(8):1663–8. [PubMed] [Google Scholar]
- 13.Klozar J, Kratochvil V, Salakova M, Smahelova J, Vesela E, Hamsikova E, et al. HPV status and regional metastasis in the prognosis of oral and oropharyngeal cancer. Eur Arch Otorhinolaryngol. 2008;265 Suppl 1:S75–82. doi: 10.1007/s00405-007-0557-9. [DOI] [PubMed] [Google Scholar]
- 14.Elango KJ, Suresh A, Erode EM, Subhadradevi L, Ravindran HK, Iyer SK, et al. Role of human papilloma virus in oral tongue squamous cell carcinoma. Asian Pac J Cancer Prev. 2011;12(4):889–96. [PubMed] [Google Scholar]
- 15.Chien CY, Su CY, Fang FM, Huang HY, Chuang HC, Chen CM, et al. Lower prevalence but favorable survival for human papillomavirus-related squamous cell carcinoma of tonsil in Taiwan. Oral Oncol. 2008;44(2):174–9. doi: 10.1016/j.oraloncology.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Brandwein M, Zeitlin J, Nuovo GJ, MacConnell P, Bodian C, Urken M, et al. HPV detection using "hot start" polymerase chain reaction in patients with oral cancer: a clinicopathological study of 64 patients. Mod Pathol. 1994;7(7):720–7. [PubMed] [Google Scholar]
- 17.Haraf DJ, Nodzenski E, Brachman D, Mick R, Montag A, Graves D, et al. Human papilloma virus and p53 in head and neck cancer: clinical correlates and survival. Clin Cancer Res. 1996;2(4):755–62. [PubMed] [Google Scholar]
- 18.Paz IB, Cook N, Odom-Maryon T, Xie Y, Wilczynski SP. Human papillomavirus (HPV) in head and neck cancer. An association of HPV 16 with squamous cell carcinoma of Waldeyer's tonsillar ring. Cancer. 1997;79(3):595–604. doi: 10.1002/(sici)1097-0142(19970201)79:3<595::aid-cncr24>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.Snijders PJ, Scholes AG, Hart CA, Jones AS, Vaughan ED, Woolgar JA, et al. Prevalence of mucosotropic human papillomaviruses in squamous-cell carcinoma of the head and neck. Int J Cancer. 1996;66(4):464–9. doi: 10.1002/(SICI)1097-0215(19960516)66:4<464::AID-IJC9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 20.Ha PK, Pai SI, Westra WH, Gillison ML, Tong BC, Sidransky D, et al. Real-time quantitative PCR demonstrates low prevalence of human papillomavirus type 16 in premalignant and malignant lesions of the oral cavity. Clin Cancer Res. 2002;8(5):1203–9. [PubMed] [Google Scholar]
- 21.Oliveira LR, Ribeiro-Silva A, Costa JP, Simoes AL, Matteo MA, Zucoloto S. Prognostic factors and survival analysis in a sample of oral squamous cell carcinoma patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(5):685–95. doi: 10.1016/j.tripleo.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Koo BS, Kang S, Park K, Kim H, Lee KR, et al. HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int J Cancer. 2007;120(7):1418–25. doi: 10.1002/ijc.22464. [DOI] [PubMed] [Google Scholar]
- 23.Syrjanen KJ, Pyrhonen S, Syrjanen SM, Lamberg MA. Immunohistochemical demonstration of human papilloma virus (HPV) antigens in oral squamous cell lesions. Br J Oral Surg. 1983;21(2):147–53. doi: 10.1016/0007-117x(83)90060-4. [DOI] [PubMed] [Google Scholar]
- 24.Smith EM, Hoffman HT, Summersgill KS, Kirchner HL, Turek LP, Haugen TH. Human papillomavirus and risk of oral cancer. Laryngoscope. 1998;108(7):1098–103. doi: 10.1097/00005537-199807000-00027. [DOI] [PubMed] [Google Scholar]
- 25.Young SK, Min KW. In situ DNA hybridization analysis of oral papillomas, leukoplakias, and carcinomas for human papillomavirus. Oral Surg Oral Med Oral Pathol. 1991;71(6):726–9. doi: 10.1016/0030-4220(91)90282-h. [DOI] [PubMed] [Google Scholar]
- 26.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–75. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 27.Termine N, Panzarella V, Falaschini S, Russo A, Matranga D, Lo Muzio L, et al. HPV in oral squamous cell carcinoma vs head and neck squamous cell carcinoma biopsies: a meta-analysis (1988-2007). Ann Oncol. 2008;19(10):1681–90. doi: 10.1093/annonc/mdn372. [DOI] [PubMed] [Google Scholar]
- 28.Castro TP, Bussoloti Filho I. Prevalence of human papillomavirus (HPV) in oral cavity and oropharynx. Braz J Otorhinolaryngol. 2006;72(2):272–82. doi: 10.1016/S1808-8694(15)30068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marques-Silva L, Farias LC, Fraga CA, de Oliveira MV, Cardos CM, Fonseca-Silva T, et al. HPV-16/18 detection does not affect the prognosis of head and neck squamous cell carcinoma in younger and older patients. Oncol Lett. 2012;3(4):945–9. doi: 10.3892/ol.2012.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charfi L, Jouffroy T, de Cremoux P, Le Peltier N, Thioux M, Freneaux P, et al. Two types of squamous cell carcinoma of the palatine tonsil characterized by distinct etiology, molecular features and outcome. Cancer Lett. 2008;260(1-2):72–8. doi: 10.1016/j.canlet.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 31.Nagpal JK, Patnaik S, Das BR. Prevalence of high-risk human papilloma virus types and its association with P53 codon 72 polymorphism in tobacco addicted oral squamous cell carcinoma (OSCC) patients of Eastern India. Int J Cancer. 2002;97(5):649–53. doi: 10.1002/ijc.10112. [DOI] [PubMed] [Google Scholar]
- 32.Seraj JM, Yazdani N, Ashtiani ZO, Seraj SM, Hasheminasab SM, Memar B, et al. TP53 gene expression in HPV-positive oral tongue SCC and its correlation with nodal metastasis. Pathol Res Pract. 2011;207(12):758–61. doi: 10.1016/j.prp.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Snow AN, Laudadio J. Human papillomavirus detection in head and neck squamous cell carcinomas. Adv Anat Pathol. 2010;17(6):394–403. doi: 10.1097/PAP.0b013e3181f895c1. [DOI] [PubMed] [Google Scholar]
- 34.Pannone G, Santoro A, Papagerakis S, Lo Muzio L, De Rosa G, Bufo P. The role of human papillomavirus in the pathogenesis of head & neck squamous cell carcinoma: an overview. Infect Agent Cancer. 2011;6:4. doi: 10.1186/1750-9378-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goon PK, Stanley MA, Ebmeyer J, Steinstrasser L, Upile T, Jerjes W, et al. HPV & head and neck cancer: a descriptive update. Head Neck Oncol. 2009;1:36. doi: 10.1186/1758-3284-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmieri A, Scapoli L, Martinelli M, Pezzetti F, Girardi A, Spinelli G, et al. Incidence of low risk human papillomavirus in oral cancer: a real time PCR study on 278 patients. Int J Immunopathol Pharmacol. 2011;24(2 Suppl):83–7. doi: 10.1177/03946320110240S215. [DOI] [PubMed] [Google Scholar]
- 37.Chang KC, Su IJ, Tsai ST, Shieh DB, Jin YT. Pathological features of betel quid-related oral epithelial lesions in taiwan with special emphasis on the tumor progression and human papillomavirus association. Oncology. 2002;63(4):362–9. doi: 10.1159/000066227. [DOI] [PubMed] [Google Scholar]
- 38.El-Mofty SK. HPV-related squamous cell carcinoma variants in the head and neck. Head Neck Pathol. 2012;6 Suppl 1:S55–62. doi: 10.1007/s12105-012-0363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lajer CB, von Buchwald C. The role of human papillomavirus in head and neck cancer. APMIS. 2010;118(6-7):510–9. doi: 10.1111/j.1600-0463.2010.02624.x. [DOI] [PubMed] [Google Scholar]
- 40.Mellin H, Friesland S, Lewensohn R, Dalianis T, Munck-Wikland E. Human papillomavirus (HPV) DNA in tonsillar cancer: clinical correlates, risk of relapse, and survival. Int J Cancer. 2000;89(3):300–4. [PubMed] [Google Scholar]
- 41.Badulescu F, Crisan A, Badulescu A, Schenker M. Recent data about the role of human papillomavirus (HPV) in oncogenesis of head and neck cancer. Rom J Morphol Embryol. 2010;51(3):437–40. [PubMed] [Google Scholar]
- 42.El-Mofty SK. Human papillomavirus (HPV) related carcinomas of the upper aerodigestive tract. Head Neck Pathol. 2007;1(2):181–5. doi: 10.1007/s12105-007-0021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gillespie MB, Rubinchik S, Hoel B, Sutkowski N. Human papillomavirus and oropharyngeal cancer: what you need to know in 2009. Curr Treat Options Oncol. 2009;10(5-6):296–307. doi: 10.1007/s11864-009-0113-5. [DOI] [PubMed] [Google Scholar]
- 44.D'Souza G, Zhang HH, D'Souza WD, Meyer RR, Gillison ML. Moderate predictive value of demographic and behavioral characteristics for a diagnosis of HPV16-positive and HPV16-negative head and neck cancer. Oral Oncol. 2010;46(2):100–4. doi: 10.1016/j.oraloncology.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ostwald C, Rutsatz K, Schweder J, Schmidt W, Gundlach K, Barten M. Human papillomavirus 6/11, 16 and 18 in oral carcinomas and benign oral lesions. Med Microbiol Immunol. 2003;192(3):145–8. doi: 10.1007/s00430-002-0161-y. [DOI] [PubMed] [Google Scholar]
- 46.Kristoffersen AK, Enersen M, Kverndokk E, Sunde PT, Landin M, Solheim T, et al. Human papillomavirus subtypes in oral lesions compared to healthy oral mucosa. J Clin Virol. 2012;53(4):364–6. doi: 10.1016/j.jcv.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 47.Kaminagakura E, Villa LL, Andreoli MA, Sobrinho JS, Vartanian JG, Soares FA, et al. High-risk human papillomavirus in oral squamous cell carcinoma of young patients. Int J Cancer. 2012;130(8):1726–32. doi: 10.1002/ijc.26185. [DOI] [PubMed] [Google Scholar]
- 48.Sugiyama M, Bhawal UK, Kawamura M, Ishioka Y, Shigeishi H, Higashikawa K, et al. Human papillomavirus-16 in oral squamous cell carcinoma: clinical correlates and 5-year survival. Br J Oral Maxillofac Surg. 2007;45(2):116–22. doi: 10.1016/j.bjoms.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 49.Smith EM, Rubenstein LM, Haugen TH, Pawlita M, Turek LP. Complex etiology underlies risk and survival in head and neck cancer human papillomavirus, tobacco, and alcohol: a case for multifactor disease. J Oncol. 2012;2012:571862. doi: 10.1155/2012/571862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Syrjanen S. The role of human papillomavirus infection in head and neck cancers. Ann Oncol. 2010;21 Suppl 7:vii243–5. doi: 10.1093/annonc/mdq454. [DOI] [PubMed] [Google Scholar]
- 51.Hoffmann M, Gorogh T, Gottschlich S, Lohrey C, Rittgen W, Ambrosch P, et al. Human papillomaviruses in head and neck cancer: 8 year-survival-analysis of 73 patients. Cancer Lett. 2005;218(2):199–206. doi: 10.1016/j.canlet.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 52.Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, et al. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24(5):736–47. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 53.Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92(4):805–13. doi: 10.1002/1097-0142(20010815)92:4<805::aid-cncr1386>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]