Highlights
-
•
A model scaling stoma behaviour at leaf level is proposed.
-
•
The model is tested by reproducing natural variations of light intensity.
-
•
Stomatal aperture and photosynthesis decrease as a function of sugar accumulation.
-
•
Leaf anatomy influences the rapidity of stomatal conductance.
-
•
The model dissects intrinsic water use efficiency into traits of interest.
Keywords: Stomatal conductance, Photosynthesis, Dynamics, Diurnal, Intrinsic water use efficiency
Abstract
Intrinsic water use efficiency (Wi), the ratio of net CO2 assimilation (A) over stomatal conductance to water vapour (gs), is a complex trait used to assess plant performance. Improving Wi could lead in theory to higher productivity or reduced water usage by the plant, but the physiological traits for improvement and their combined effects on Wi have not been clearly identified. Under fluctuating light intensity, the temporal response of gs is an order of magnitude slower than A, which results in rapid variations in Wi. Compared to traditional approaches, our new model scales stoma behaviour at the leaf level to predict gs and A during a diurnal period, reproducing natural fluctuations of light intensity, in order to dissect Wi into traits of interest. The results confirmed the importance of stomatal density and photosynthetic capacity on Wi but also revealed the importance of incomplete stomatal closure under dark conditions as well as stomatal sensitivity to light intensity. The observed continuous decrease of A and gs over the diurnal period was successfully described by negative feedback of the accumulation of photosynthetic products. Investigation into the impact of leaf anatomy on temporal responses of A, gs and Wi revealed that a high density of stomata produces the most rapid response of gs but may result in lower Wi.
Nomenclature
- A
Net CO2 assimilation
- AG
Gross CO2 assimilation
- Rd
Mitochondrial respiration
- gs
Stomatal conductance to water vapour
- gm
Mesophyll conductance to CO2
- gb
Boundary layer conductance to water vapour
- gt
Total conductance to CO2
- Wi
Intrinsic water use efficiency
- Ca
Atmospheric CO2 concentration
- Ci
CO2 concentration in the intercellular airspaces
- Cc
CO2 concentration at the site of carboxylation
- a
Stomatal pore area
- as
Steady state target of stomatal pore area
- amin
Minimum stomatal pore area
- amax
Maximum stomatal pore area
- αL
Slope of the relationship
- θL
Curvature factor of the curve
- ki
Time constant for an increase in a
- kd
Time constant for an decrease in a
- L
Percentage of efficiency
- SD
Stomatal density
- D
Diffusivity of water in air
- V
Molar volume of air
- l
Depth of stomatal pore
- Pa
The atmospheric pressure
- Sa
Factor representing the influence of the rate of accumulation of sugars
- Se
Factor representing the influence of the rate of export of sugars
- Vcmax
Maximum Rubisco activity
- Jmax
Maximum electron transport activity
- α
Proportion of light absorbed by PSII
1. Introduction
In order to meet the projected demand for cereal production by 2050, crop yields must improve by 1.16–1.31% each year; however, current estimates are well below this required rate [1]. The primary determinant of crop yield is the cumulative rate of photosynthesis over the growing season and is determined by the ability of the plant to capture light and CO2, use this energy to convert the CO2 to biomass, and how much of this biomass ends in usable yield. Improving photosynthetic efficiency is recognised as an important but unexploited avenue to increase yield potential in crop plants [2]. Increasing photosynthetic efficiency is accompanied by a higher CO2 demand, which can be limited by the resistance of CO2 diffusion into the leaf. Any attempt to decrease this resistance greatly increases the water loss by transpiration from the leaf.
Photosynthetic productivity is linked to water consumed by the plant and often measured as water use efficiency (WUE). WUE can be defined at different scales of time and space and, at the leaf level, it is often assessed as the ratio of CO2 fixed per unit of H2O transpired (E). Intrinsic water use efficiency (Wi) is defined when stomatal conductance to water vapour (gs) is used instead of E. The use of gs to describe the stomatal control on the rate of E facilitates the comparisons between different leaves and environmental conditions. The photosynthetic capacity of the leaf determines the net CO2 assimilation (A) as a function of the variation in the micro-climate surrounding the leaf. Over the diurnal period, A is mainly determined by the irradiance absorbed by the leaf and the limitation of CO2 imposed by stomatal control. Under field conditions, environmental variables that affect both photosynthesis and stomatal behaviour are rarely constant. For example, light intensity (and spectral quality) alters in time scales of seconds to hours to which A and gs must respond. The temporal response of A and gs to a fluctuating environment are asynchronous, with gs response often an order of magnitude slower than A, which results in rapid variations of Wi. Thus, it is important when describing the kinetic response of Wi to use an approach that considers responses by A and gs simultaneously.
Intrinsic water use efficiency (Wi) is dependent on the anatomy (e.g. stomatal size and density) and the physiology (e.g. behaviour) of stomata as well as the leaf biochemistry (e.g. activity of the Calvin cycle), all of which interact to determine the kinetics of CO2 and H2O gaseous exchange between the leaf and atmosphere. The dynamic nature of the interactions between the different components that determine Wi are not fully understood and need to be addressed if we are to successfully improve both A and Wi under dynamic field conditions.
It is possible to conceptualise the inherent complexity of gas exchange over a fluctuating light regime through modelling, which will improve our understanding of the Wi response by simulating a number of gas exchange scenarios (e.g. changes in light intensity and humidity) that would normally be difficult to assess in a reasonable amount of time using experimental approaches. Current models focus on predicting gs in steady state [3] and cannot be used to infer the impact of stomatal behaviour on A or Wi under dynamic conditions. Although temporal responses of gs have previously been described using a dynamic model [4], [5], the relationship between stomatal response and leaf level gas exchange was not clearly described. We propose to use a model that will take into consideration the anatomy and physiology of stomata to more accurately represent the stomatal control of Wi.
To scale stomatal responses to leaf level gs, the two most important stomatal characteristics are aperture and density [6], [7], [8]. A high stomatal density does not necessarily result in a higher gs as stomata ultimately control their aperture depending on the guard cell responses to the external (e.g. light intensity) and internal (e.g. mesophyll demand for CO2) stimuli [9]. To link stomatal behaviour to leaf level gas exchange responses, we propose a ‘big stoma’ approach that consists of simulating the response of one stoma that is representative of the heterogeneous response of many stomata and scaling the response to the leaf level. This approach was incorporated in an enhanced version of the multi compartments model described by Noe and Giersch [10] to predict A and Wi. Scaling up the dynamic of the stomatal response to the leaf level, with the improved model for CO2 diffusion inside the leaf, will help to dissect Wi into traits of interest and predict potential gains in Wi.
The objective of this study was to develop a new model combining our most recent knowledge of kinetics in stomatal behaviour and photosynthesis to describe the temporal response of Wi over the course of a day with natural dynamic variations in irradiance. All the parameters of the model described here incorporate a trait of interest for Wi and were adjusted using Bayesian inference. The model was validated using a dataset with a different irradiance pattern to assess the predictive power of the model. A sensitivity analysis was finally performed to show the interaction among the parameters and display the potential gain in Wi in the case of one or two parameters changing. We used the output of the model to understand how temporal responses in gs impacts A and Wi.
2. Material and methods
2.1. Dynamic modelling of photosynthesis and stomatal conductance
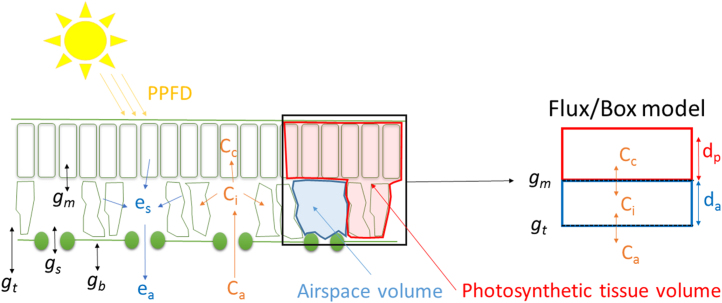
The model essentially consists of four differential equations describing the diffusion of CO2 between different compartments represented by the atmosphere, the intercellular air spaces and the photosynthetic tissues (Fig. 1). The exchanges between these compartments are dependent on the stomatal aperture and the resistance of diffusion in the mesophyll cells. In addition, the model took into account the limitation of photosynthesis and stomatal aperture that appeared during a period of light.
| (1) |
Fig. 1.
Schematic of the diffusion of gas exchange between the leaf and atmosphere compared to the conceptual version used in the model. The total conductance to water vapour (gt) controlling the gradient of H2O from leaf to atmosphere was composed by the stomatal conductance (gs) and the boundary layer conductance (gb). The gradient of CO2 from the atmosphere to the site of carboxylation was also dependent on mesophyll conductance (gm). The H2O concentrations inside and outside the leaf were represented by es and ea respectively. The CO2 concentrations in three compartments were considered: in the atmosphere (Ca), in the intercellular airspaces (Ci) and at the sites of carboxylation (Cc). The different compartments of the leaf were represented as a standardized volume defined by the thickness of the intercellular airspace (da) and the photosynthetic tissues (dp).
The first differential equation (Eq. (1)) described the temporal variations of the stomatal pore area (a) with as the steady state target followed by a and two time constants, ki and kd, for an increase or a decrease of a respectively. Considering the spatial heterogeneity of the stomatal response, a top-down approach was used, signifying that the model simulated the response of one stoma representative of the sum of the individual stomatal responses and scaled it to leaf level instead of trying to integrate the response of each stoma.
The steady state target of a (as) as a function of the light intensity (PPFD) was predicted using a non-rectangular hyperbola [4]:
| (2) |
with amin and amax the minimum and maximum stomatal pore area, αL the slope of the relationship, θL the curvature factor of the curve and L the percentage of efficiency (see below).
| (3) |
Eq. (3) described the variation of the CO2 concentration in the intercellular airspaces (Ci) with Ca the atmospheric CO2 concentration and Cc the CO2 concentration at the sites of carboxylation.
The resistance of CO2 diffusion from the air to the leaf was represented by the total conductance to CO2 (gt):
| (4) |
with gb the boundary layer conductance to water vapour estimated in the gas exchange chamber.
The stomatal conductance to water vapour (gs) was derived from a using the equation of Dow et al. [7]:
| (5) |
with SD the stomatal density, D the diffusivity of water in air, V the molar volume of air and l the depth of the stomatal pore.
The resistance of CO2 diffusion in the mesophyll cells (gm) was calculated following the equations described by Von Caemmerer et al. [11] and the values given for Arabidopsis thaliana. As the temperature of the leaf was regulated during the experiment, gm was considered stable.
The last part of the equation was used to convert the fluxes (μmol m−2 s−1) between the two compartments (leaf and atmosphere) into concentrations of CO2 (μmol mol−1) contained in the volume represented by the intercellular airspaces (See the Appendix in Noe and Giersch [10] for more information), with R the gas constant, Tl the leaf temperature, Pa the atmospheric pressure, and da the depth of the intercellular airspaces (da = leaf thickness (m) • airspaces (%)).
| (6) |
Eq. (6) described the variation of Cc with Rd the mitochondrial respiration during the day, AG the gross CO2 assimilation and L the efficiency coefficient of as and AG (see below). The gross assimilation was calculated using Cc at the current state of the solver and the equations of Farquhar et al. [12]. The net CO2 assimilation A was calculated at the end of the simulation as: A = AG − Rd. In the last part of the equation da is replaced with dp representing the depth of the photosynthetic tissues (dp = leaf thickness (m) •[1 − airspaces (%)]).
| (7) |
The last equation (Eq. (7)) described the negative feedback of sugar accumulation on as (Eq. (2)) and AG during a period of light, with Sa being a factor representing the influence of the rate of accumulation of sugars, and Se a factor representing the influence of the rate of export of sugars.
Originally in Noe and Giersch [10], this equation only described the negative feedback of sugar export on A. The equation was modified to allow recovery of the response of AG in the absence of light and tested using the diurnal data sets described below. The initial simulations showed that the model was able to reproduce the slow decrease of A during the day but not the similar decrease observed for gs. Eq. (2) was therefore modified to include a decrease in gs during the diurnal period similar to that for A.
2.2. Solving differential equations
The differential equations were solved using the function lsodes from the R package deSolve (v1.12). The initial absolute tolerance was set to 0.01 for all the outputs and decreased by 10 each time the solver failed to converge. The initial values of the differential equations were chosen based on the observed data. For the pore area, the initial aperture was calculated by solving Eq. (5) for the first recorded gs.
2.3. Parameterization of the model
Parameter values known to impact the diurnal variation of gas exchange were adjusted using the Bayesian inference (see below) to fit the observed data. During the diurnal period, gross CO2 assimilation (AG) was determined by maximum Rubisco activity (Vcmax), maximum electron transport activity (Jmax) and the proportion of light absorbed by PSII (α). The day respiration in presence of light (Rd) was used to calculate the net CO2 assimilation (A). The stomatal conductance to water vapour (gs) was determined as described above by amin, amax, αL and ki/kd. The diurnal variation of A and gs were linked by the negative feedback of sugar export described by Sa and Se.
Parameter values known to remain unchanged during the diurnal period of measurement or that could be assigned a priori were treated as constants in the model, to facilitate the adjustment, and were chosen from the literature or measured on Arabidopsis thaliana (see below). The Michaelis constants and the temperature responses of the Farquhar model were parameterized using values from Walker et al. [13]. The thickness of the leaf was set to 200 μm [14], [15] and the ratio of airspace in the leaf was set to 0.255 [14], [16]. The value of θL in equation 2 was chosen at 0.7 by analogy with the similar equation describing the electron transport rate [17]. The boundary layer conductance (gb) and atmospheric pressure (Patm) were set to the values provided by the LI-6400XT: 9.29 mol m−2 s−1 and 101.8 kPa respectively. Estimates of gb are computed as a function of leaf area and fan speed (LI-6400XT Instruction Manuals, page 3–93). The parameter values describing the membrane properties required to estimate gm followed that of Von Caemmerer et al. [18]. Stomatal density (SD) was assessed on plants grown under the same environmental conditions, on both faces of the leaf (section 1.9). As the model simulated the average response of both faces, the densities of both faces were added and set to 400 mm−2. In equation 5, 5.89 μm was used for the depth of the stomatal pore (l) [7], the diffusivity of water in air at 25 °C (D) was 24.2e−6 m2 s−1, the molar volume of air at 25 °C (V) was 0.02446533 m3 mol−1 [19].
2.4. Bayesian inference
The parameter values were estimated using Bayesian inference by comparing the model outputs to recorded gas exchange data [20]. The inference was performed on two objective functions as A and gs were adjusted simultaneously and in this case, there was a risk of bimodal posterior distributions. Monte Carlo Markov Chain (MCMC) using inter-chain adaptation were previously able to sample from bimodal distributions and this technique was thus used to adjust our model (INCA, [21], [22]). To remove any adaptive effect, the final algorithm used to sample the posterior distributions was the random walk metropolis (RWM) with the covariance matrix estimated previously with INCA as an input. The use of Bayesian Inference allowed us to use prior information available in the literature and shown in Table 1. The results of the inference were posterior estimates describing the probability density of the possible parameter values considering the error of prediction and the sensitivity of the model for these parameters and their inter-correlation. The posteriors were represented using quantiles of the posterior distribution at 2.5% and 97.5%, also called credible intervals (similar to a confidence interval). The medians were also shown as the most representative set of parameter values. Two chains with 30,000 iterations were thinned every 2 samples and tested for different criteria: the acceptance rate greater than 15% (to achieve a rapid mixing of the chain), the Effective Sample Size (ESS) > 100 (which is usually enough to describe 95% probability intervals) and the convergence using Monte Carlo Standard Error (MCSE) less than 6.27% (which allows the true mean to be within 5% of the area under a Gaussian distribution around the estimated mean; [23]). The algorithms used to perform the Bayesian inference were implemented using the R software (R Core Team, 2015, v3.2.2).
Table 1.
Parameters of the dynamic gas exchange model adjusted with Bayesian inference: mean and standard deviation (SD) of the prior values estimated from the literature (see footnotes) and quantiles at 2.5%, 50% and 97.5% of the credible interval of each posterior distribution estimated using Bayesian inference.
| unit | Prior |
Posterior |
||||
|---|---|---|---|---|---|---|
| Mean | SD | 2.5% | 50% | 97.5% | ||
| amin1 | μm2 | 3.56 | 0.2 | 4.03 | 4.16 | 4.27 |
| amax2 | μm2 | 100 | 10 | 72.15 | 93.24 | 113.87 |
| αL | 0 | 10 | 0.742 | 0.767 | 0.793 | |
| ki | s | 100 | 1e4 | 1290 | 1430 | 1580 |
| kd | s | 100 | 1e4 | 463 | 541 | 627 |
| Vcmax3 | μmolm−2s−1 | 65.5 | 22.6 | 89.8 | 99.3 | 128.8 |
| Jmax3 | μmolm−2s−1 | 102.4 | 21.9 | 180.5 | 186.4 | 193 |
| Rd | μmolm−2s−1 | 0 | 10 | 0.01 | 0.14 | 0.37 |
| α | 0 | 1 | 0.177 | 0.183 | 0.192 | |
| Se | 0 | 10 | 0.263e−6 | 8.486e−6 | 32.904e−6 | |
| Sa | 0 | 10 | 0.207e−6 | 0.280e−6 | 0.385e−6 | |
The accuracy of the model outputs compared to the observed data was assessed using the Root Mean Square Error (RMSE):
| (8) |
where xobs and xmod represents the observed and modelled values to be compared, and n the number of observations.
2.5. Sensitivity analysis
The sensitivity analysis was performed using the same data set and parameter values estimated previously using Bayesian inference. Each parameter value was altered by ±50%, the impact on the model outputs was summarized by using the daily mean of A and gs divided by the corresponding values obtained using the original parameters values. This resulted in a factor centred on 1 representing the positive or negative impact of the parameter alterations on A and gs (e.g. a factor of 1.1 corresponds to an increase of 10% in the original parameter value).
2.6. Plant material
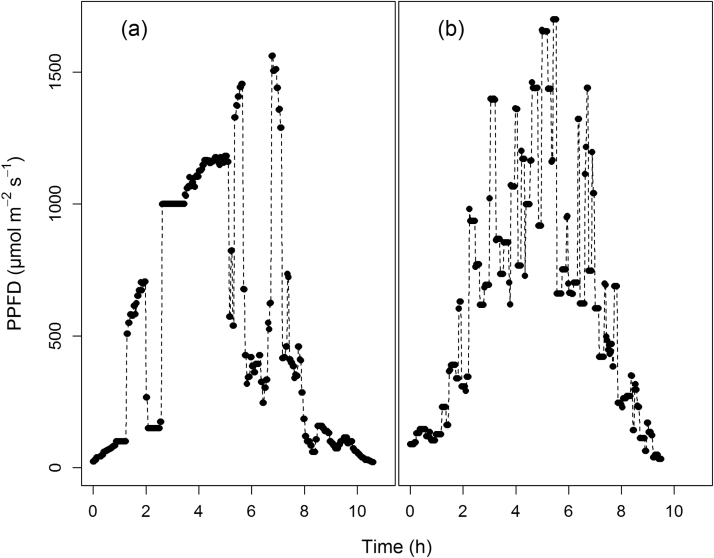
Arabidopsis thaliana ecotype Col-0 plants were grown in a controlled environment under fluctuating light conditions provided by a Heliospectra LED light source (Heliospectra AB, Göteborg, Sweden). The fluctuating light regime was reconstructed from natural variations in light intensity recorded during a day in July 2013 at the University of Essex, UK (Fig. 2a) assuming a constant spectral distribution. The growth environment was maintained at a relative humidity of 55–65%, a temperature of 21–22 °C and a CO2 concentration of 400 μmol mol−1. Plants were well watered, and positioning under the Heliospectra light was altered daily. Measurements were taken on the youngest fully expanded leaf.
Fig. 2.
Light regimes a recorded from natural variations at the University of Essex, UK, July 2013, and b simulated using a Gaussian altered with random variations.
2.7. Model simulations of step change of light
Using the set of parameter values previously estimated, the model was used as a simulator to assess the diversity of the temporal response of gs after a step change of light. The step change was performed by initially setting the light to an intensity of 100 μmol m−2 s−1 for 30 min followed by a step increase in intensity to 1000 μmol m−2 s−1 for 2 h. All other environmental conditions were kept constant, with the [CO2] maintained at 400 μmol mol−1 and a leaf temperature of 25 °C. Parameter values of SD, ki and αL were increased or decreased by a factor of 2 to illustrate their impact on temporal responses of gs, A and Wi. The rate of change of gs was estimated using a linear regression on the first 10 min of the variation. The impact of the temporal response of gs on A and Wi was characterised by calculating the percentage of variation of the mean A and Wi during the 2 h after the step change of light.
2.8. Leaf gas exchange
Photosynthesis gas exchange parameters (A and gs) were measured using a Li-Cor 6400XT portable gas exchange system (Li-Cor, Lincoln, Nebraska, USA), with dew point and vapour pressure deficit maintained via a Li-Cor 610 portable dew point generator. Throughout the measurement cuvette, conditions were maintained at a CO2 concentration of 400 ppm (corresponding to ambient growth), leaf temperature of 25 °C, and a leaf to air water vapour pressure deficit of 1 (±0.2) kPa. The largest fully expanded mature leaf was used, with A and gs allowed to stabilize under the controlled cuvette conditions for a minimum of 30 min. At this point, the automatic 12 h light program (mirroring that of the growth conditions, Fig. 2a) was started, with A and gs recorded every 2 min. Wi was calculated as A/gs. For the model validation, the same measurement conditions were used in the cuvette with the exception of the light that reproduced a different pattern of light intensity (Fig. 2b).
2.9. Stomatal density
Following the dental impression methods of Weyers & Johansen (1985), negative impressions of the ab- and ad-axial leaf surface were made using Xantoprene Polysiloxane precision material (Heraesus Kulzer Ltd). Positive impressions were produced by coating nail varnish on the dry polymer and used to count the number of stomata in 9 field of view using an Olympus BX60 light microscope (Olympus Europa, Southend-On-Sea, UK). The area of view was measured using an eyepiece graticule to express the number of stomata by mm−2. Stomatal impressions of the leaf surface were taken on the same leaves as gas exchange measurements were conducted.
3. Results
3.1. Bayesian inference
The two chains (describing the posterior distributions) resulting from the Bayesian inference successfully converged and were used to calculate the credible intervals of each parameter shown in Table 1. The credible interval of amin was significantly higher and not overlapping the prior distribution, which was not the case for amax, suggesting that the observed data were informative for amin but not for amax. The model estimated αL precisely, despite the fact that the prior distribution covered a large range of possible values, suggesting that the model was particularly sensitive to this parameter. The time constants, ki and kd, displayed a strong asymmetry with values almost 3 times higher for ki compared to kd. Vcmax and Jmax values were sensibly higher than the prior means estimated from the literature, however the prior distribution and the credible interval overlapped, suggesting that these values were still in the range observed in the literature.
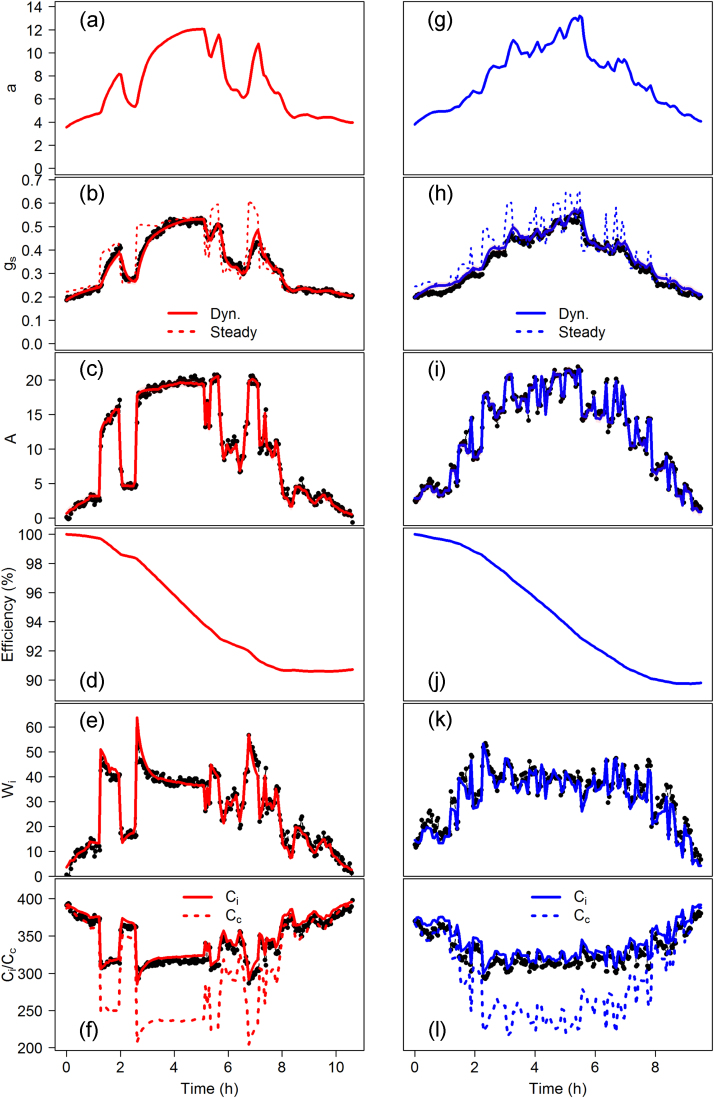
The medians of the posterior estimates shown in Table 1 were used to represent the most representative prediction of the model (Fig. 3a–f). Under a diurnal fluctuating light regime, our dynamic model accurately described gs (Fig. 3b, RMSE: 0.014 mol m−2 s−1; Suppl. Fig. S1a, R2:0.984) and A (Fig. 3c, RMSE: 0.6 μmol m−2 s−1; Suppl. Fig. S1b, R2:0.993). Consequently, the rapid variations of Wi were also accurately reproduced (Fig. 3e, RMSE: 2.58 μmol mol−1; Suppl. Fig. S1c, R2:0.97). By adjusting the model to the observed gs, our ‘big stoma’ approach allowed us to predict the average behaviour of the stomata with a variation of the pore area from 4 to 14 μm2 (Fig. 3a). Under these environmental conditions A and gs displayed a decrease in efficiency of approximately 8% at the end of the diurnal period (Fig. 3d), which was described by Se and Sa. The model seemed to be less sensitive to Se as the credible interval was much larger than the one of Sa. The model successfully describes the variation of Ci but also predicts the variations of Cc that showed a larger variation compared to Ci due to low gm (Fig. 3f).
Modelled (Mod.) vs observed (Obs.) stomatal conductance to water vapour (gs, mol m−2 s−1), net CO2 assimilation (A, μmol m−2 s−1) and intrinsic water use efficiency (Wi, μmol(CO2)/mol(H2O)) measured under: a–c light regime described in Fig. 2a for the model parameterization, d–f light regime describe in Fig. 2b for the model validation. Solid lines and dashed lines represented the 1:1 lines and linear regressions respectively. The coefficient of determination (R2) was derived from the linear regression.
Fig. 3.
Modelled (solid & dashed lines) and observed (black circles) diurnal variations in gas exchange under two different light regimes (Fig. 2). a–f Modelled data (red lines) were fitted to observations (black circles) using Bayesian inference to estimate the most credible set of parameter values under the light regime described in Fig. 2a. g-l Modelled data (blue lines) were simulated using the same set of parameter values and the light regime describe in Fig. 2b. The red and blue dashed line in b and h represented the steady state gs target (Steady) if conditions remained constant, whilst solid lines represent the dynamic outputs. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Model validation
To validate the model, the same plant and set of parameter values (Table 1) were used to predict A, gs and Wi under a different light regime (Fig. 2b). Our dynamic model was able to accurately predict the observed gas exchanges (Fig. 3g-l): gs (Fig. 3h, RMSE: 0.023 mol m−2 s−1; Suppl. Fig. S1d, R2:0.983), A (Fig. 3i, RMSE: 0.69 μmol m−2 s−1; Suppl. Fig. S1e, R2:0.988) and Wi (Fig. 3k, RMSE: 2.95 μmol mol−1; Suppl. Fig. S1f, R2:0.961). Compared to the rapidity of the stomatal response (ki: 24 min and kd: 9 min), the light variations were too fast (on average a change every 8 min) to observe the complete stomatal response and, as a consequence, a followed the general tendency of the light intensity (Fig. 3g). The efficiency of A and gs during the diurnal period showed a decrease of around 10% similar to that of the estimate during the other diurnal period (Fig. 3j).
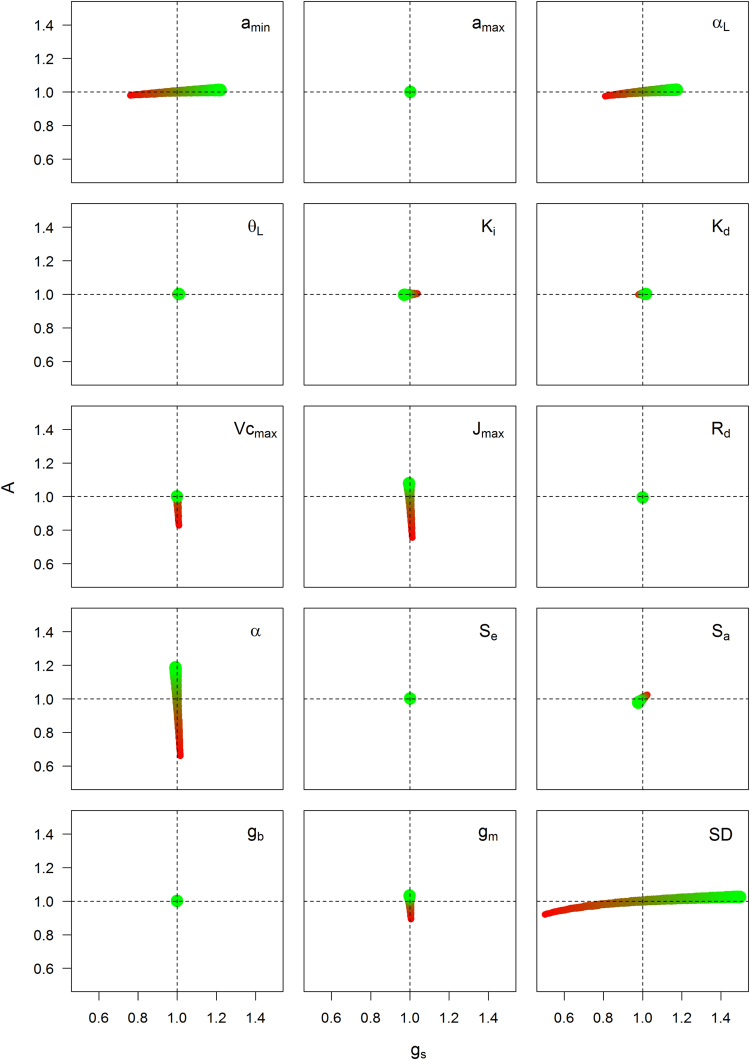
3.3. Sensitivity analysis
A sensitivity analysis of the model was undertaken to illustrate the impact of varying individual parameters on A and gs (Fig. 4). The parameters that displayed the most sensitivity for gs were (ordered by importance): SD, amin, αL, ki, and Sa. For A, the parameters were (ordered by importance): α, Jmax, Vcmax, gm, SD, amin, αL and Sa, showing the interdependence of A and gs. Even if the model was sensitive to the variation of a parameter, this variation would not necessarily improve Wi. For example, Vcmax only displayed variation in A that resulted in a decrease in Wi suggesting that under the conditions used here A is not limited by CO2 diffusion. The greatest impact on both A and gs was observed when SD was decreased, while increasing photosynthetic capacity (Vcmax and Jmax) by 50% only improved A by 10% under the environmental conditions used here.
Fig. 4.
Relative variation of the daily mean net assimilation (A) and daily mean stomatal conductance to water vapour (gs) using parameter values ranging ±50% around the estimated parameters from Table. 1. The size and the colour of the circles are proportional to the deviation of the parameter values (small and red circles correspond to decreased parameter values whilst large green circles correspond to increased parameter values). The length and direction are relative to the impact of the parameter on A and gs.The simulated daily mean of A and gs for each parameter set was divided by the mean values of A and gs determined using the parameters in Table 1. The (1,1) coordinate showed similar daily mean values of A and gs compared to values simulated using the parameter set described in Table 1. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Impact of stomatal characters on gs, A and Wi temporal responses
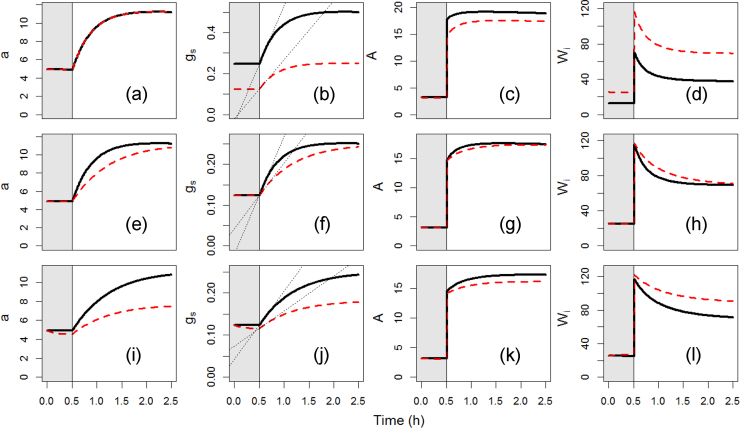
In order to determine the impact of stomatal characters on the temporal response of gs, A and Wi, the influence of the rapidity of the stomatal response (ki) and the steady states gs (SD, αL) to a step increase in light intensity were examined (Fig. 5). The initial slope of the gs response displayed large differences (e.g. 0.58–0.29 mol m−2 s−2, Fig. 5b) even if the rapidity of increasing stomatal aperture did not change (Fig. 5a). The different temporal responses of gs driven by SD, ki and αL limited A by 8.8% (Fig. 5c), 2% (Fig. 5g) and 6.1% (Fig. 5k) respectively. The impact on Wi was due to a larger decrease in gs rather than A following the step increase in light intensity, with an increase of Wi by 82% (Fig. 5d), 7.4% (Fig. 5h) and 19.2% (Fig. 5l) respectively.
Fig. 5.
Simulation of the temporal response of a, gs, A and Wi with different alterations on SD, ki, and αL under a step change of light from 100 (shaded area) to 1000 (white area) μmol m−2 s−1. a-d Impact of a variation of the stomatal density (SD) from 400 (black line) to 200 mm−2 (red dashed line). e-h Impact of a variation of the time constant for stomatal aperture (ki) from 1430 (black line) to 2860s (red line). i-l Impact of a variation of the slope of the relationship between the pore area and the light (αL) from 0.77 (black line) to 0.38 μm2/μmolm−2s−1(red line). The dotted lines on (b, f, j) represent the rates of gs variation for the first 10 min of the step change estimated by linear regression. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Over different diurnal light regimes, our new dynamic model was able to incorporate the rapid fluctuations in light and accurately predicted gs, A and Wi. To our knowledge, this is the first attempt to model stomatal behaviour and scale it up to leaf level to predict gas exchange under dynamic light regimes. This ‘big stoma’ approach to model gs provides a direct estimation of the rapidity of the stomatal response, whereas most current models consider only an instantaneous response [3]. Moreover, the simulated average pore area (a) predicted by the model was in a similar range to those previously reported in the literature [24], [25]. Previous studies have attempted to directly model temporal responses of gs [4], [5], but our approach models the speed of increasing pore area rather than the speed of gs increase, and is therefore more mechanistic and biologically relevant when describing the dynamics of gas exchange. Although we have validated the model using responses to fluctuations in light intensity, other environmental variables (e.g relative humidity) could be included in the future and would greatly improve the predictive power of the model.
Our model also incorporates negative feedback on A and gs that revealed a 10% decrease in both by the end of the diurnal period. Noe and Giersch [10] suggested that the accumulation of sugars from photosynthesis could negatively regulate A. The decrease in gs and therefore subsequent decrease in guard cell turgor, could be related to the decrease of A [26], [27], [28]. Such mechanisms for negative feedback are not well documented but are important for breeders, as any increase in photosynthetic capacity could lead to a great decrease in efficiency over the diurnal period. By accurately predicting diurnal gas exchange and dissecting Wi into the key parameters controlling dynamic responses, we believe our model will provide an important tool for future breeding strategies to identify targets for improved Wi under different environmental conditions.
Previous studies have shown spatial heterogeneity in stomatal distribution and behaviours [29], [30], [31], [32] that have not been included in the model. Instead, the model predicted the behaviour of a stoma that is representative of the sum of individual stomatal responses over the entire leaf surface. This simplification did not appear to impact on the quality of the predictions and it is difficult to assess the temporal and spatial heterogeneity in stomatal response; therefore, further investigations would be required to determine if and how such variations would impact model outputs.
Stomatal density (SD) was shown to be the most sensitive stomatal parameter for improving Wi, with decreases in SD resulting in a substantial reduction in gs but minimal impact on A. This is in agreement with the work of Franks et al. [33], who showed significant variation of Wi in Arabidopsis mutants with different SD. The Bayesian inference and the sensitivity analysis revealed that the model was not sensitive to changes in amax, as this value was not reached at any point over the diurnal period. In contrast, amin and αL were important determinants of Wi but are rarely reported or examined in this context in the literature. The model predicts that, under dark conditions, amin is statistically greater than zero resulting in significant nocturnal transpiration, which has been reported previously [34] and has implications for overall plant water use. As SD cannot change over the diurnal period, αL could be an important determinant of plant water use by adjusting the magnitudes of change in gs as a function of the environment.
Although the coefficient of light absorption by PSII (α) was an important determinant of A and Wi in the field, this parameter does not show a large diversity; therefore, Vcmax, Jmax and gm are the most promising targets for improving A. The model predicts that increasing gm could lead to similar improvements in A and Wi [35] as those observed when Vcmax and Jmax were increased through manipulating enzymes associated with the Calvin cycle (e.g. [36]).
The sensitivity analysis performed here should be seen as a tool to determine the key parameter(s) for improving Wi under specific environmental conditions (e.g. fluctuating light intensity). Indeed, the parameter values estimated with the Bayesian inference are specific for this study and the variation around the determined parameter may change between individuals and/or species. For example, an individual with different photosynthetic capacity (Vcmax, Jmax) or SD will exhibit different thresholds of A limitations (Fig. 5c) and the results of the sensitivity analysis may also vary. To generalize the sensitivity analysis, ranges of biologically possible parameter values and the interactions between them (e.g. relationship between size of the pore and SD) should be used; however, to our knowledge, this information is not yet fully available in the literature.
Our model simulations clearly showed that the temporal response of gs is the product of several parameters (SD, αL and ki/kd), and the rapidity of the stomatal response cannot be interpreted by comparing the initial slope of variation in gs, as is often reported in the literature [37], [38]. Changes in the initial slope of the gs response are correlated with SD, αL and ki/kd and therefore are not only indicative of the speed of changes in stomatal aperture. In terms of gas diffusion, stomatal size constrains the maximum pore area but the observed pore area also depends on the sensitivity of the guard cell (αL) responses to light intensity. As a result, it is theoretically possible to have large stomata that even at high light retain a small pore area. Considering the relationship between stomatal size and density [39], our results suggest that having a high density of small stomata will result in fast changes in gs but also in a high average gs value through the diurnal period, decreasing Wi.
The model simulations also revealed large variations in Wi (>80%) when the parameter values controlling the temporal response of gs were altered. Indeed, the decrease in A due to greater limitation of CO2 diffusion was minimal compared to the improvement in Wi. The importance of the temporal response of gs was highlighted previously [40], [41], but not the importance of the additive effects of parameter interactions on A and Wi as revealed by our model. Therefore, our model could be used to find the optimal parameter set that would decrease gs without impacting A and maximizing Wi to direct future breeding and research programmes to identify plants with reduced water usage, but the same or greater productivity.
5. Conclusion
We assessed and validated a new dynamic model of leaf gas exchange that accurately predicts gs, A, and Wi under fluctuating light regimes such as those experienced by plants in the field. The model uses a unique framework that takes into account leaf anatomy, biochemistry and the physiological responses of stomata on Wi. The model revealed important negative feedback controls on A and gs towards the end of the diurnal period that resulted in decreases in A with implications for overall plant productivity. Importantly, the model enabled Wi to be dissected into key parameters such as stomatal sensitivity to light (αL) and minimal pore area under dark conditions (amin) that have previous been neglected and could provide new and realistic targets for future improvements in crop water use efficiency.
Acknowledgements
This work supported SV-C through a BBSRC grant BB/1001187_1 to TL. NERC funding is acknowledged for PhD studentship to JSAM (Env-East DTP). MRB and YW were supported by BBSRC grants BB/L001276/1 and BB/L019205/1.
Footnotes
This article is part of a special issue entitled “Water-use Efficiency in plants”, published in Plant Science 251, 2016.
References
- 1.Hall A.J., Richards R.A. Prognosis for genetic improvement of yield potential and water-limited yield of major grain crops. Field Crop. Res. 2013;143:18–33. [Google Scholar]
- 2.Zhu X.-G., Long S.P., Ort D.R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 2010;61:235–261. doi: 10.1146/annurev-arplant-042809-112206. [DOI] [PubMed] [Google Scholar]
- 3.Damour G., Simonneau T., Cochard H., Urban L. An overview of models of stomatal conductance at the leaf level. Plant Cell Environ. 2010 doi: 10.1111/j.1365-3040.2010.02181.x. [DOI] [PubMed] [Google Scholar]
- 4.Kirschbaum M.U.F., Gross L.J., Pearcy R.W. Observed and modelled stomatal responses to dynamic light environments in the shade plant Alocasia macrorrhiza. Plant Cell Environ. 1988;11:111–121. [Google Scholar]
- 5.Vialet-Chabrand S., Dreyer E., Brendel O. Performance of a new dynamic model for predicting diurnal time courses of stomatal conductance at the leaf level. Plant Cell Environ. 2013;36:1529–1546. doi: 10.1111/pce.12086. [DOI] [PubMed] [Google Scholar]
- 6.Brown H.T., Escombe F. Static diffusion of gases and liquids in relation to the assimilation of carbon and translocation in plants. Philos. Trans. R. Soc. B Biol. Sci. 1900;193:223–291. [Google Scholar]
- 7.Dow G.J., Berry J.A., Bergmann D.C. The physiological importance of developmental mechanisms that enforce proper stomatal spacing in Arabidopsis thaliana. New Phytol. 2013 doi: 10.1111/nph.12586. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann P., Or D. Effects of stomata clustering on leaf gas exchange. New Phytol. 2015;207:1015–1025. doi: 10.1111/nph.13442. [DOI] [PubMed] [Google Scholar]
- 9.Lawson T., Morison J.I.L. Evol. Plant Physiol. Elsevier; 2004. Stomatal function and physiology; pp. 217–242. [Google Scholar]
- 10.Noe S.M., Giersch C. A simple dynamic model of photosynthesis in oak leaves: coupling leaf conductance and photosynthetic carbon fixation by a variable intracellular CO2 pool. Funct. Plant Biol. 2004;31:1195–1204. doi: 10.1071/FP03251. [DOI] [PubMed] [Google Scholar]
- 11.von Caemmerer S., Evans J.R. Temperature responses of mesophyll conductance differ greatly between species. Plant Cell Environ. 2015;38:629–637. doi: 10.1111/pce.12449. [DOI] [PubMed] [Google Scholar]
- 12.Farquhar G.D., von Caemmerer S. Physiol. Plant Ecol. II. Springer; Berlin, Heidelberg: 1982. Modelling of photosynthetic response to environmental conditions; pp. 549–587. [Google Scholar]
- 13.Walker B., Ariza L.S., Kaines S., Badger M.R., Cousins A.B. Temperature response of in vivo Rubisco kinetics and mesophyll conductance in Arabidopsis thaliana: comparisons to Nicotiana tabacum. Plant Cell Environ. 2013;36:2108–2119. doi: 10.1111/pce.12166. [DOI] [PubMed] [Google Scholar]
- 14.Tholen D., Boom C., Noguchi K., Ueda S., Katase T., Terashima I. The chloroplast avoidance response decreases internal conductance to CO2 diffusion in Arabidopsis thaliana leaves. Plant Cell Environ. 2008;31:1688–1700. doi: 10.1111/j.1365-3040.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 15.Wuyts N., Massonnet C., Dauzat M., Granier C. Structural assessment of the impact of environmental constraints on Arabidopsis thaliana leaf growth: a 3D approach. Plant Cell Environ. 2012;35:1631–1646. doi: 10.1111/j.1365-3040.2012.02514.x. [DOI] [PubMed] [Google Scholar]
- 16.Pyke K.A., Marrison J.L., Leech R.M. Temporal and spatial development of the cells of the expanding first leaf of Arabidopsis thaliana (L.) Heyn. J. Exp. Bot. 1991;42:1407–1416. [Google Scholar]
- 17.Von Caemmerer S. Steady-state models of photosynthesis. Plant Cell Environ. 2013;36:1617–1630. doi: 10.1111/pce.12098. [DOI] [PubMed] [Google Scholar]
- 18.von Caemmerer S., Evans J.R. Temperature responses of mesophyll conductance differ greatly between species. Plant Cell Environ. 2015;38:629–637. doi: 10.1111/pce.12449. [DOI] [PubMed] [Google Scholar]
- 19.Riechers G.H., Jones H.G. Plants and microclimate. Ecology. 1984;65:1701. [Google Scholar]
- 20.Van Oijen M., Rougier J., Smith R. Bayesian calibration of process-based forest models: bridging the gap between models and data. Tree Physiol. 2005;25:915–927. doi: 10.1093/treephys/25.7.915. http://www.ncbi.nlm.nih.gov/pubmed/15870058 [DOI] [PubMed] [Google Scholar]
- 21.Craiu R.V., Rosenthal J., Yang C. Learn from thy neighbor: parallel-chain and regional adaptive MCMC. J. Am. Stat. Assoc. 2009;104:1454–1466. [Google Scholar]
- 22.Solonen A., Ollinaho P., Laine M., Haario H., Tamminen J., Järvinen H. Efficient MCMC for climate model parameter estimation: parallel adaptive chains and early rejection. Bayesian Anal. 2012;7:715–736. [Google Scholar]
- 23.Flegal J.M., Haran M., Jones G.L. Markov Chain Monte Carlo: can we trust the third significant figure? Stat. Sci. 2008;23:250–260. [Google Scholar]
- 24.Kaiser H., Kappen L. Stomatal oscillations at small apertures: indications for a fundamental insufficiency of stomatal feedback-control inherent in the stomatal turgor mechanism. J. Exp. Bot. 2001;52:1303–1313. [PubMed] [Google Scholar]
- 25.Kaiser H., Kappen L. In situ observation of stomatal movements and gas exchange of Aegopodium podagraria L. in the understorey. J. Exp. Bot. 2000;51:1741–1749. doi: 10.1093/jexbot/51.351.1741. [DOI] [PubMed] [Google Scholar]
- 26.Lawson T., Lefebvre S., Baker N.R., Morison J.I.L., Raines C.A. Reductions in mesophyll and guard cell photosynthesis impact on the control of stomatal responses to light and CO2. J. Exp. Bot. 2008;59:3609–3619. doi: 10.1093/jxb/ern211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Busch F.A. Opinion: the red-light response of stomatal movement is sensed by the redox state of the photosynthetic electron transport chain. Photosynth. Res. 2014;119:131–140. doi: 10.1007/s11120-013-9805-6. [DOI] [PubMed] [Google Scholar]
- 28.Azoulay-Shemer T., Palomares A., Bagheri A., Israelsson-Nordstrom M., Engineer C.B., Bargmann B.O.R. Guard cell photosynthesis is critical for stomatal turgor production, yet does not directly mediate CO2- and ABA-induced stomatal closing. Plant J. 2015;83:567–581. doi: 10.1111/tpj.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weyers J.D.B., Lawson T. Heterogeneity in stomatal characteristics. Adv. Bot. Res. 1997:317–352. [Google Scholar]
- 30.Jones H.G. Use of thermography for quantitative studies of spatial and temporal variation of stomatal conductance over leaf surfaces. Plant Cell Environ. 1999;22:1043–1055. [Google Scholar]
- 31.Mott K.A., Woodrow I.E. Modelling the role of Rubisco activase in limiting non-steady-state photosynthesis. J. Exp. Bot. 2000:399–406. doi: 10.1093/jexbot/51.suppl_1.399. http://www.ncbi.nlm.nih.gov/pubmed/10938848 (51 Spec No) [DOI] [PubMed] [Google Scholar]
- 32.West J.D., Peak D., Peterson J.Q., Mott K.A. Dynamics of stomatal patches for a single surface of Xanthium strumarium L. leaves observed with fluorescence and thermal images. Plant Cell Environ. 2005;28:633–641. [Google Scholar]
- 33.Franks P.J., Doheny-Adams T.W., Britton-Harper Z.J., Gray J.E. Increasing water-use efficiency directly through genetic manipulation of stomatal density. New Phytol. 2015;2 doi: 10.1111/nph.13347. [DOI] [PubMed] [Google Scholar]
- 34.Caird M.A., Richards J.H., Donovan L.A. Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol. 2006;143:4–10. doi: 10.1104/pp.106.092940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flexas J., Díaz-Espejo A., Conesa M.A., Coopman R.E., Douthe C., Gago J. Mesophyll conductance to CO2 and Rubisco as targets for improving intrinsic water use efficiency in C3 plants. Plant Cell Environ. 2015 doi: 10.1111/pce.12622. [DOI] [PubMed] [Google Scholar]
- 36.Simkin A.J., McAusland L., Headland L.R., Lawson T., Raines C.A. Multigene manipulation of photosynthetic carbon assimilation increases CO2 fixation and biomass yield in tobacco. J. Exp. Bot. 2015;66:4075–4090. doi: 10.1093/jxb/erv204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drake P.L., Froend R.H., Franks P.J. Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013;64:495–505. doi: 10.1093/jxb/ers347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raven J.A. Speedy small stomata? J. Exp. Bot. 2014;65:1415–1424. doi: 10.1093/jxb/eru032. [DOI] [PubMed] [Google Scholar]
- 39.Hetherington A.M., Woodward F.I. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901–908. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- 40.Lawson T., Blatt M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014;164:1556–1570. doi: 10.1104/pp.114.237107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McAusland L., Vialet-Chabrand S., Davey P., Baker N.R., Brendel O., Lawson T. Effects of kinetics of light-induced stomatal responses on photosynthesis and water-use efficiency. New Phytol. 2016 doi: 10.1111/nph.14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flexas J., Ortuño M.F., Ribas-Carbo M., Diaz-Espejo A., Flórez-Sarasa I.D., Medrano H. Mesophyll conductance to CO2 in Arabidopsis thaliana. New Phytol. 2007:501–511. doi: 10.1111/j.1469-8137.2007.02111.x. [DOI] [PubMed] [Google Scholar]
- 43.Heckwolf M., Pater D., Hanson D.T., Kaldenhoff R. The Arabidopsis thaliana aquaporin AtPIP1;2 is a physiologically relevant CO2 transport facilitator. Plant J. 2011;67:795–804. doi: 10.1111/j.1365-313X.2011.04634.x. [DOI] [PubMed] [Google Scholar]
- 44.Cousins A.B., Ghannoum O., Von Caemmerer S., Badger M.R. Simultaneous determination of Rubisco carboxylase and oxygenase kinetic parameters in Triticum aestivum and Zea mays using membrane inlet mass spectrometry. Plant Cell Environ. 2010;33:444–452. doi: 10.1111/j.1365-3040.2009.02095.x. [DOI] [PubMed] [Google Scholar]
- 45.Sade N., Gallé A., Flexas J., Lerner S., Peleg G., Yaaran A. Differential tissue-specific expression of NtAQP1 in Arabidopsis thaliana reveals a role for this protein in stomatal and mesophyll conductance of CO2 under standard and salt-stress conditions. Planta. 2014;239:357–366. doi: 10.1007/s00425-013-1988-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Modelled (Mod.) vs observed (Obs.) stomatal conductance to water vapour (gs, mol m−2 s−1), net CO2 assimilation (A, μmol m−2 s−1) and intrinsic water use efficiency (Wi, μmol(CO2)/mol(H2O)) measured under: a–c light regime described in Fig. 2a for the model parameterization, d–f light regime describe in Fig. 2b for the model validation. Solid lines and dashed lines represented the 1:1 lines and linear regressions respectively. The coefficient of determination (R2) was derived from the linear regression.