Abstract
The aim of the present study was to analyze the changes of plasma and urinary prostaglandin E2 (PGE2) levels in preterm infants with symptomatic patent ductus arteriosus (sPDA) treated with oral ibuprofen and acetaminophen. A total of 87 preterm infants with sPDA admitted to the Neonatal Ward of the Affiliated Xuzhou Hospital of Medical College of Southeast University from October, 2012 to June, 2015 were selected and randomly divided into the ibuprofen group (n=43, 10 mg/kg ibuprofen administered orally as initial dose, followed by 5 mg/kg during the first 24 and 48 h later) and acetaminophen group (n=44, 15 mg/kg acetaminophen administered orally once every 6 h for three days). The levels of plasma and urinary PGE2 in the two groups were estimated before and after treatment. The treatment of sPDA infants with ibuprofen (ibuprofen group) or acetaminophen (acetaminophen group) caused a significant decrease in the plasma and urinary PGE2 levels in comparison with plasma and urinary PGE2 levels before treatment (P<0.05). Furthermore, plasma and urinary PGE2 levels in the acetaminophen group (45.0±36.9 ng/l) were significantly lower than those in the ibuprofen group (73.5±44.8 ng/l, P=0.002). The arterial duct closure rate was similar between the acetaminophen [31 (70.5%)] and ibuprofen groups [33 (76.7%), P=0.506]. The incidence of oliguria was less among sPDA infants of the acetaminophen group [1 (2.3%)] than observed among the sPDA infants of the ibuprofen group [6 (14.0%)]; however, this difference was not statistically significant (P=0.108). Additionally, the incidences of fecal occult blood positive rate, intraventricular hemorrhage, neonatal necrotizing enterocolitis and bronchopulmonary dysplasia were distributed similarly in the ibuprofen and acetaminophen groups (P>0.05). The levels of platelet, serum creatinine and alanine transaminase showed no significant changes between the ibuprofen and acetaminophen groups (P>0.05). Following treatment with ibuprofen or acetaminophen, the extent of decrease of plasma and urinary PGE2 was significantly higher among sPDA infants with oliguria (135.0±38.0 ng/l) than that observed in sPDA infants without oliguria (52.5±37.0 ng/l) (P=0.01). The study also found a significant correlation between plasma and urinary PGE2 levels (r=0.648, P=0.01) and the coefficient of variation of urinary PGE2 (0.427) was less than that of plasma PGE2 (0.539). The clinical efficacy of oral ibuprofen and acetaminophen in the treatment of preterm infants with sPDA was similar with low adverse events, whereas acetaminophen-induced PGE2 levels were less than the levels observed in the ibuprofen-treated group. The incidence of oliguria was also lower in the acetaminophen group compared to the ibuprofen group. In addition, monitoring urinary PGE2 levels was more suitable because of its non-invasiveness in the clinical setting than monitoring of plasma PGE2 in preterm infants with sPDA.
Keywords: ibuprofen, acetaminophen, patent ductus arteriosus, prostaglandin E2, infant, preterm
Introduction
The arterial catheter is located between the descending part of aortic arch and the pulmonary artery bifurcation, approaching the arteriae pulmonalis sinistra and it is an important channel for fetal circulation. Normally, the function of arterial catheter would close between 10–15 h after birth. The arterial catheter that continues to open after 10–15 h is defined as patent ductus arteriosus (PDA). The arterial ducts of premature infants due to the maldevelopment of arterial duct wall and the abnormal secretion of prostaglandin, always fail to close in time, thus resulting in left-to-right shunt. Due to their poor left ventricular systolic functions, premature infants are vulnerable to heart failure and pulmonary edema, and even symptomatic PDA (sPDA), which is also known as hemodynamically significant PDA (hsPDA) (1,2). Oral administration of indomethacin or ibuprofen to premature infants could significantly lower the level of prostaglandin E2 (PGE2), and promote constriction and closure of the arterial duct (3–5). Hammerman et al (6) reported that, acetaminophen caused arterial duct closure in 5 premature hsPDA infants that were not successfully treated with ibuprofen. In this context, to understand the effect of acetaminophen treatment on premature sPDA infants, we designed prospective randomized controlled trials (RCTs), aiming to utilize and develop plasma and urinary PGE2 levels as indicators of progress of arterial duct closure in a non-invasive manner.
Patients and methods
Patients
The patients of this RCT were premature infants who were admitted to the Neonatal Ward of Pediatrics Division of the Affiliated Xuzhou Hospital of Medical College of Southeast University between October, 2012 and June, 2015.
Sample size estimation
The sample size for this RCT was calculated based on the results of a previous study (5) and literature (4). Efficacy (1-false negative rate β) was set at 80% and the false-negative rate at α=0.05. According to our estimation, the sample size for each group was 39 patients.
Inclusion and exclusion criteria
The patients satisfying the following criteria were included in the presen study: i) gestational age, <37 weeks; ii) admitted to hospital within 24 h after birth; iii) sPDA diagnosis was made between 15 h to 10 days after birth and confirmed through echocardiogram to be sPDA if the patient showed at least three of the six clinical manifestations. These were: i) systolic or consecutive murmur in left border of sternum; ii) strengthened beat of anterior thorax; iii) locomotive pulse; iv) tachycardia in quiet state; v) unexplainable deterioration of respiratory condition; and vi) increased pulmonary vasculature shadows and enlarged heart or signs of pulmonary edema under chest X-ray examination. Diagnostic criteria of echocardiography was: i) left atrial: aortic root diameter ratio, (LA:Ao) >1.4; ii) pulmonary artery diastolic back flow (reflux); and iii) PDA catheter diameter >1.4 mm (1,7).
The patients were excluded from the study if: i) patients presented with any of the following medication contraindications such as thrombocytopenia (blood platelet count <50×109/l), hemorrhagic disease, oliguria (urine volume per 8 h <8 ml/kg), necrotizing colitis, intestinal perforation, high serum creatinine (>159.1 µmol/l), and alanine aminotransferase (>40 U/l) levels (1,2); ii) patients with congenital heart diseases such as ventricular septal defect, complex heart disease; and iii) patients with incomplete treatment or willing to depart from the study due to personal reasons.
A total of 95 patients met the inclusion criteria, of whom 8 patients were excluded for various reasons (reduced platelet count in 2 patients, 1 patient had sepsis with disseminated intravascular coagulation, 1 patient had oliguria, 1 patient had necrotizing colitis, 1 patient had complex heart disease and lack of required data for this study in 2 patients). Subsequently, 87 patients were included in the study.
Collection of data and analyses of parameters prior to treatment
Once the premature infants were admitted to hospital, information was collected and recorded including: i) gender, with or without use of a full course of hormone of the pregnant mother 7 days to 24 h before delivery; ii) presence or absence of infection in the pregnant mother; iii) premature rupture of membrane >18 h or less; iv) gestational age; v) delivery mode; vi) birth weight; vii) 5-min Apgar scoring <8 or >8; viii) with or without respiratory distress syndrome; and ix) number of days of positive pressure ventilation.
Tests such as platelet count, serum creatinine glutamic-pyruvic transaminase, and fecal occult blood were also carried out in patients.
Treatment of patients and analysis of parameters
The patients were randomly divided into the ibuprofen group (n=43) and acetaminophen group (n=44) based on a computer-generated random number table. The patients in the ibuprofen group were treated by oral administration of 10 mg/kg ibuprofen suspension initially, followed by 5 mg/kg during first 24 and 48 h later. Patients in the acetaminophen group were treated with oral administration of 15 mg/kg acetaminophen orally once in every 6 h for a total of 3 days. On the completion of 3 days, ultrasonic cardiogram, platelet count, serum creatinine, glutamic pyruvic transaminase, and fecal occult blood were re-examined. The incidence of intraventricular hemorrhage (IVH), neonatal necrotizing enterocolitis (NEC) and bronchopulmonary dysplasia (BPD) were also recorded.
Detection of PGE2 in blood and urine
The PGE2 level was estimated using a commercially availed ELISA kit following the manufacturer's instructions (VICMED Bioengineering Co., Ltd., China).
The present study conformed to ethical requirements of the Affiliated Xuzhou Hospital of Medical College of Southeast University. The parents of the patients were aware of the treatment and signed informed consent.
Statistical analysis
The χ2 test was applied to compare the collected information between the two groups. The t-test was applied to compare data having normal distribution, whereas, the Mann-Whitney U test method was utilized to compare data having skewed distribution between the groups. The paired-samples Student's t-test was used for intra-group comparison of the data having normal distribution before and after treatment. The Pearson correlation coefficient test was applied in the correlation analysis of the bivariate normal distribution data. Statistical analyses were carried out using IBM SPSS 20.0 software (Armonk, NY, USA). P<0.05 was considered of statistical significance.
Results
Clinical characteristics of sPDA infants of the ibuprofen and acetaminophen groups
The various clinical characteristics including weight at birth, arterial catheter diameter, and gestational week between the two groups of sPDA infants were not significantly different (P>0.05; Table I).
Table I.
Clinical characteristics of sPDA infants of the ibuprofen and acetaminophen groups.
| Characteristics | Ibuprofen group (n=43) | Acetaminophen group (n=44) | χ2/t/Z | P-value |
|---|---|---|---|---|
| Male, n (%) | 25 (58.1) | 27 (61.4) | χ2=0.094 | 0.759 |
| Hormone in full pregnancy course, n (%) | 28 (65.1) | 25 (56.8) | χ2=0.629 | 0.428 |
| Maternal infection, n (%) | 4 (9.3) | 6 (13.6) | χ2=0.089 | 0.766 |
| Premature rupture of membrane >18 h, n (%) | 8 (18.6) | 10 (22.7) | χ2=0.225 | 0.635 |
| Gestational age (weeks) | 33.4±2.1 | 33.6±2.1 | t=−0.491 | 0.625 |
| Cesarean delivery, n (%) | 24 (55.8) | 28 (63.6) | χ2=0.553 | 0.457 |
| Birth weight (g) | 2,091±657 | 2,219±606 | t=−0.946 | 0.347 |
| SGA, n (%) | 9 (20.9) | 6 (13.6) | χ2=0.811 | 0.368 |
| 5 min Apgar scoring <8, n (%) | 15 (34.9) | 18 (40.9) | χ2=0.335 | 0.563 |
| RDS, n (%) | 14 (32.6) | 12 (27.3) | χ2=0.290 | 0.590 |
| Positive pressure ventilation (days)a | 3.7 (1.9, 6.1) | 4.5 (3.0, 6.7) | Z=1.277 | 0.201 |
| Age at ultrasonic cardiogram examination (days) | 5.8±2.0 | 6.4±1.8 | t=−1.527 | 0.131 |
| Urine amount (ml/kg•h) | 2.52±0.54 | 2.48±0.76 | t=0.222 | 0.825 |
| Pulse pressure difference (mmHg) | 24.2±3.9 | 23.3±4.7 | t=0.880 | 0.381 |
| LA:Ao | 1.55±0.31 | 1.53±0.31 | t=0.323 | 0.748 |
| Arterial catheter diameter (mm) | 1.84±0.43 | 2.09±0.46 | t=−1.491 | 0.140 |
Positive pressure ventilation (d): range, 0–35; asymmetry coefficient was 3.181, kurtosis coefficient was 13.060, P<0.01, showed skewed distribution, presented by median (25th and 75th percentile). sPDA, symptomatic patent ductus arteriosus.
Effect of treatment in sPDA infants of the ibuprofen and acetaminophen groups
PDA closure rate, fecal occult blood positive rate, IVH, NEC, and BPD incidence rates were similar in the patients of the two groups (P>0.05). Oliguria was less frequent in the acetaminophen group than in the ibuprofen group, but this difference was insignificant (P=0.108; Table II).
Table II.
Effect of ibuprofen and acetaminophen treatment in sPDA infants.
| Ibuprofen group (n=43), n (%) | Acetaminophen group (n=44), n (%) | χ2 | P-value | |
|---|---|---|---|---|
| PDA occlusion | 33 (76.7) | 31 (70.5) | 0.442 | 0.506 |
| Oliguria | 6 (14.0) | 1 (2.3) | 2.587 | 0.108 |
| Positive stool OB | 4 (9.3) | 2 (4.5) | 0.205 | 0.651 |
| IVH | 4 (9.3) | 5 (11.4) | 0.000 | 1.000 |
| NEC | 5 (11.6) | 4 (9.1) | 0.001 | 0.971 |
| BPD | 6 (14.0) | 5 (11.4) | 0.132 | 0.716 |
PDA, patent ductus arteriosus; sPDA, symptomatic patent ductus arteriosus; IVH, intraventricular hemorrhage; NEC, neonatal necrotizing enterocolitis; BPD, bronchopulmonary dysplasia.
PGE2 level in sPDA infants of the ibuprofen and acetaminophen groups
The treatment of sPDA infants with ibuprofen or acetaminophen resulted in a significant decrease of plasma and urinary PGE2 levels compared with their levels before treatment (P<0.05). Furthermore, plasma and urinary PGE2 levels were significantly lower among ibuprofen group patients than the acetaminophen group (P<0.05; Table III). However, the descent range of the plasma [avg.12.6 (5.7, 19.5) ng/l] PGE2 level in the acetaminophen group [avg.12.6 (5.7, 19.5) ng/l] was significantly lower than that of the ibuprofen group [avg.18.5 (10.1, 33.8) ng/l], and the difference was statistically significant (Z=−2.158, P=0.031), and the descent range of urinary PGE2 of the acetaminophen group (45.0±36.9 ng/l) was lower than that of the ibuprofen group (73.5±44.8 ng/l) and the difference was statistically significant (t=3.244, P=0.002).
Table III.
PGE2, platelet, creatinine, and glutamic-pyruvic transaminase before and after treatment (mean ± standard deviation).
| Ibuprofen group (n=43) | Acetaminophen group (n=44) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Indicators | Before treatment | After treatment | t | P-value | Before treatment | After treatment | t | P-value | P-valuea |
| Plasma PGE2 (ng/l) | 70.0±35.7 | 47.3±24.7 | 7.091 | 0.000 | 74.2±35.5 | 59.9±32.9 | 7.298 | 0.000 | 0.046 |
| Urine PGE2 (ng/l) | 189.0±62.4 | 115.4±46.3 | 10.765 | 0.000 | 184.4±73.8 | 139.3±54.0 | 8.100 | 0.000 | 0.030 |
| Platelet (×109/l) | 192.4±94.6 | 224.4±88.0 | 1.807 | 0.078 | 183.8±107.7 | 195.0±84.3 | −0.506 | 0.615 | 0.115 |
| Serum creatinine (µmol/l) | 69.0±33.6 | 74.1±35.7 | 0.747 | 0.459 | 67.0±33.2 | 60.9±30.9 | 0.874 | 0.387 | 0.068 |
| Glutamic-pyruvic transaminase (U/l) | 15.4±7.4 | 16.8±4.9 | 1.309 | 0.198 | 15.9±11.2 | 17.4±6.6 | −0.815 | 0.419 | 0.635 |
Comparisons between the two groups after treatment. PGE2, prostaglandin E2.
The comparison on platelets, serum creatinine and glutamic-pyruvic transaminase between the two groups of patients before treatment (P>0.05) and after treatment (P>0.05) revealed no significant difference (Table III).
Descent range of PGE2 in PDA closed and PDA unclosed, oliguria and non-oliguria patients after treatment
The descent range of plasma and urinary PGE2 between PDA closed and PDA unclosed patients was not significantly different (P>0.05; Table IV). However, the descent range of plasma and urinary PGE2 in oliguria patients was higher than non-oliguria patients (P<0.05; Table IV).
Table IV.
Descent range of PGE2 in PDA closed and PDA unclosed, oliguria and non-oliguria patients.
| Descent range of PGE2 after treatment (ng/l) | PDA closed group (n=64) | PDA unclosed group (n=23) | Z/t | P-value | Oliguria group (n=7) | Nonoliguric group (n=80) | Z/t | P-value |
|---|---|---|---|---|---|---|---|---|
| Plasmaa | 13.7 (7.3, 25.6) | 15.7 (9.1, 25.3) | Z=0.067 | 0.946 | 35.0 (26.3, 49.8) | 13.3 (6.7, 20.8) | Z=−3.326 | 0.001 |
| Urineb | 61.1±44.7 | 53.7±39.2 | t=0.708 | 0.481 | 135.0±38.0 | 52.5±37.0 | t= 5.649 | 0.000 |
Median (25th and 75th percentile).
Mean ± standard deviation. PGE2, prostaglandin E2; PDA, patent ductus arteriosus.
Plasma and urinary PGE2 levels are highly correlated
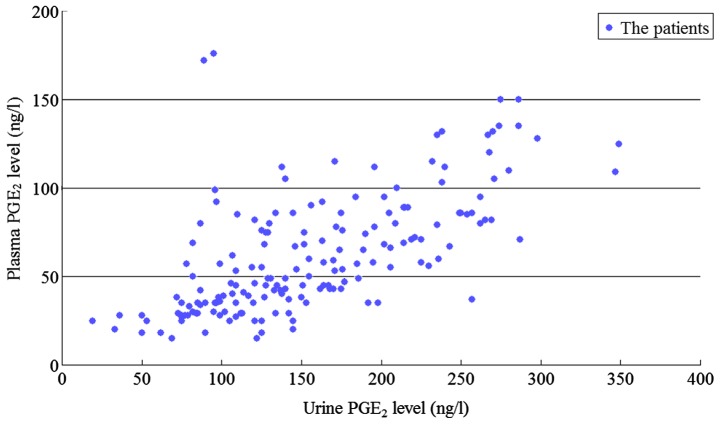
The plasma and urinary PGE2 levels were highly correlated (Pearson correlation coefficient r=0.648, P=0.01) as shown in Fig. 1 and the variable coefficient of urinary PGE2 (67.1/157.1=0.427) level was lower than the plasma PGE2 (33.9/62.9=0.539) level.
Figure 1.
Correlation of plasma and urinary PGE2 levels. PGE2, prostaglandin E2.
Incidence of adverse reactions among sPDA infants of acetaminophen and ibuprofen groups
The various incidences of adverse reactions including PDA occlusion, oliguria, and fecal occult blood observed are shown in Table II. None of the adverse reactions showed a significant association with administration of either acetaminophen or ibuprofen (P>0.05). Additionally, no significant changes were observed on the transcutaneous oxygen saturation, pulse rate, blood pressure, peripheral blood glucose, transcutaneous bilirubin, temperature, feeding, and bleeding tendency during the treatment period. The incidence of NEC that occurred several days later could not be confirmed to be associated with the drugs.
Discussion
General
Inside the uterus, low arterial oxygen partial pressure, prostaglandin and nitric oxide are the major factors that maintain the opening of the arterial catheter, of which PGE2 plays the key role (1,2). Cyclooxygenase (COX) is a key rate-limiting enzyme synthesized by prostaglandin, which has the active sites of COX and peroxidase. COX can catalyze arachidonic acid to transform into prostaglandin via its COX activity, and then catalyze the prostaglandin to transform into active PGE series via the activity of peroxidase. COX has two types of isozymes, COX-1 and COX-2. COX-3 has been quite widely used in recent years after first discovered by Chandrasekharan et al (8) in their study on the mechanism of action of acetaminophen. COX-3 was quite sensitive to the inhibiting effect of acetaminophen, thus being deemed as the effector target of acetaminophen. Ibuprofen, as the non-selective inhibitor of COX-1 and COX-2 could play the inhibiting role by blocking or modifying the COX active sites. However, the exact mechanism for the effect of acetaminophen on COX activity remains controversial. At present, two theories are quite prevalent: i) acetaminophen could selectively inhibit COX-3; and ii) acetaminophen has no affiliation to the active sites of COX, but it may restore the active oxydic COX into non-active COX and then obstruct its biological activity (9).
Influence of ibuprofen and acetaminophen treatment on PGE2 of premature infants with sPDA
In the current study, ibuprofen and acetaminophen treatment reduced the level of plasma and urinary PGE2. From this it could be inferred that the two drugs may reduce the PGE2 levels and promote the closure of sPDA. The present study has also shown that, the descent range of plasma and urinary PGE2 levels of the acetaminophen group was lower than those of the ibuprofen group. This may be because COX3 was selectively inhibited by acetaminophen activity of COX and peroxidase, although the activity was only 20% of COX-1 (9). Shetty et al (10) showed that, ibuprofen reduced the level of PGE2 in gingival crevicular fluid, but acetaminophen had no significant effect on the level of PGE2. No reports are available at present on the influence of acetaminophen on PGE2 level in premature infants with sPDA.
The action mechanisms of ibuprofen and acetaminophen on COX activity are different, the descended ranges of plasma and urinary PGE2 levels due to the drugs were different in this study, but their curative effects on promoting sPDA closure were similar and this curative effect was not related to the descent range of PGE2 level. A possible explanation for this may be that although the plasma prostaglandin level is an important factor in inhibiting the contraction of arterial duct, the closure of arterial catheter is affected by multiple factors including the maturity of arterial catheter, family genetic background and other unknown factors promoting and inhibiting the arterial duct contraction (1).
Association of ibuprofen and acetaminophen with adverse reactions and the descent range of PGE2 levels
Antonucci et al (4) in an earlier study reported that, ibuprofen treatment reduced the urinary PGE2 level and caused acute renal failure in 3 of 20 hsPDA patients. Antonucci et al (4) suggested that, ibuprofen inhibited COX thereby lowering the PGE2 level and this resulted in reduced peripheral blood vessel flow, increased oliguria, acute renal failure and NEC. In our study, the incidence of oliguria in acetaminophen group was significantly lower than that of the ibuprofen group. This may be because of the descending ranges of plasma and urinary PGE2 levels of acetaminophen group being lower than the ibuprofen group. Additionally, the descent ranges of plasma and urinary PGE2 levels of the oliguria group were more than those of the non-oliguria group. Our results substantiated the study of Antonucci et al (4). Although the descent range of plasma and urinary PGE2 between oliguria and non-oliguria patients showed a significant difference in the absolute values, the incidence of oliguria being very low among patients of the ibuprofen and acetaminophen groups may be due to the low sample size (sample size of this study was estimated on the basis of major research indicator ‘changes of plasma and urine PGE2 levels before and after treatment’).
Walker et al (11) showed that, indometacin induces NEC and PEG2 and its receptor plays a key role in regulating the intestinal blood flow. The more the reduction in the level of PEG2, the less was the intestinal blood flow. Dang et al (12) reported that, the incidence of gastrointestinal bleeding in patients administered with acetaminophen was significantly lower than the patients administered with ibuprofen. In our study, the incidence of positive fecal occult blood in the acetaminophen group was relatively lower than those observed in the ibuprofen group. We suggest that, acetaminophen selectively inhibited COX-3 and its inhibiting effect on COX-1 (associated with the synthesis of the protective prostaglandin in stomach and duodenum) was lower than that exerted by ibuprofen. Moreover, no COX-3 has been discovered in the small intestinal epithelial cells of human beings (13).
Correlation of plasma and urinary PGE2 levels
The findings of the present study showed that the plasma and urinary PGE2 levels were highly correlated, and the variable coefficient of urinary PGE2 level was lower than that of plasma PGE2. Thus, for monitoring PGE2 levels in sPDA infants, urine samples are optimal in the clinical setting, because it can be collected in a non-invasive manner.
Efficacy and safety of ibuprofen and acetaminophen in clinical setting
In 2011, Hammerman et al (6) first reported that, oral administration of 15 mg/kg acetaminophen caused closure of the arterial catheter in 5 sPDA infants who were unresponsive to ibuprofen treatment without any significant adverse reactions. Since then, the majority of studies (14–19) have used oral or intravenous administration of acetaminophen as a replacement therapy for the closure of arterial catheter in sPDA patients who were unresponsive by ibuprofen/indometacin treatments. Additionally, a low concentration of peroxide activated the activity of peroxidase, but a higher concentration of peroxide was needed to activate COX. Therefore, in the hypoxic environment, such as arterial duct endothelial cells, acetaminophen inhibits COX, rather than ibuprofen, in a better manner (9). This may be one of the reasons for the use of acetaminophen as a replacement therapy for patients who failed in the ibuprofen treatment. In the present study, we showed that, the effect of acetaminophen in promoting the closure of sPDA in premature infants was similar to that of ibuprofen, which was consistent with recent studies (14,20). A RCTs study which included 80 premature infants with sPDA (gestational age, ≤30 weeks; birth weight, ≤1,250 g) showed that, the arterial catheter closure rate among ibuprofen-treated patients was similar to that of acetaminophen-treated patients (21). Another non-inferiority RCTs study which included 160 premature infants with sPDA (gestational age, ≤34 weeks) showed that, the arterial catheter closure rate in acetaminophen-treated patients was not inferior to patients treated with ibuprofen (81.2 vs. 78.8%) (12).
Previous findings have shown that the tolerance for acetaminophen is good, and only few patients had their liver enzymes elevated (14). In this study, we showed that, oral administration of ibuprofen and acetaminophen exerted no significant influence on the platelet, serum creatinine, and glutamic-pyruvic transaminase and also there was no occurrence of NEC. This indicates that, oral administration of ibuprofen or acetaminophen at the dosage level described in this study is safe during a short-term administration of the drug.
Limitations of this study
The present study did not include a placebo control group and thus, we were not able to estimate the spontaneous arterial catheter closure rate and physiological changes of plasma and urinary PGE2 levels.
In conclusion, the clinical efficacy of oral ibuprofen and acetaminophen in the treatment of preterm infants with sPDA are relatively similar with low adverse events. Since a high correlation exists between plasma and urinary PGE2, the urinary PGE2 level can be used for predicting the occurrence of drug associated adverse reactions including oliguria, renal damage and gastrointestinal tract side effects in a non-invasive manner in preterm infants with sPDA.
References
- 1.Clyman RI. Patent ductus arteriosus in the preterm infant. In: Gleason CA, Devaskar S, editors. Avery's Diseases of the Newborn. 9th. Saunders; Philadelphia, PA: 2012. pp. 751–761. [DOI] [Google Scholar]
- 2.Hamrick SE, Hansmann G. Patent ductus arteriosus of the preterm infant. Pediatrics. 2010;125:1020–1030. doi: 10.1542/peds.2009-3506. [DOI] [PubMed] [Google Scholar]
- 3.Pacifici GM. Clinical pharmacology of indomethacin in preterm infants: implications in patent ductus arteriosus closure. Paediatr Drugs. 2013;15:363–376. doi: 10.1007/s40272-013-0031-7. [DOI] [PubMed] [Google Scholar]
- 4.Antonucci R, Cuzzolin L, Arceri A, Dessì A, Fanos V. Changes in urinary PGE2 after ibuprofen treatment in preterm infants with patent ductus arteriosus. Eur J Clin Pharmacol. 2009;65:223–230. doi: 10.1007/s00228-008-0586-3. [DOI] [PubMed] [Google Scholar]
- 5.Gao X, Hei M, Yang B, Zhu H, Zhang QG, Lei HG, Ren Y. The changes of plasma prostaglandins E2 at pre- and post-treatment in preterm infants with patent ductus arteriosus. Chin J Heart Heart Rhythm. 2015;3:102–108. [Google Scholar]
- 6.Hammerman C, Bin-Nun A, Markovitch E, Schimmel MS, Kaplan M, Fink D. Ductal closure with paracetamol: a surprising new approach to patent ductus arteriosus treatment. Pediatrics. 2011;128:e1618–e1621. doi: 10.1542/peds.2011-0359. [DOI] [PubMed] [Google Scholar]
- 7.El Hajjar M, Vaksmann G, Rakza T, Kongolo G, Storme L. Severity of the ductal shunt: a comparison of different markers. Arch Dis Child Fetal Neonatal Ed. 2005;90:F419–F422. doi: 10.1136/adc.2003.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci USA. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham GG, Davies MJ, Day RO, Mohamudally A, Scott KF. The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology. 2013;21:201–232. doi: 10.1007/s10787-013-0172-x. [DOI] [PubMed] [Google Scholar]
- 10.Shetty N, Patil AK, Ganeshkar SV, Hegde S. Comparison of the effects of ibuprofen and acetaminophen on PGE2 levels in the GCF during orthodontic tooth movement: a human study. Prog Orthod. 2013;14:6. doi: 10.1186/2196-1042-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker KS, Matheson PJ, Galganski LA, Garrison RN, Downard CD. Application of prostaglandin E2 improves ileal blood flow in NEC. J Pediatr Surg. 2014;49:945–949. doi: 10.1016/j.jpedsurg.2014.01.029. discussion 949. [DOI] [PubMed] [Google Scholar]
- 12.Dang D, Wang D, Zhang C, Zhou W, Zhou Q, Wu H. Comparison of oral paracetamol versus ibuprofen in premature infants with patent ductus arteriosus: a randomized controlled trial. PLoS One. 2013;8:e77888. doi: 10.1371/journal.pone.0077888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu M, Wan J. COX-3: is it the target of acetaminophen? Prog Physiol Sci. 2010;41:40–42. [PubMed] [Google Scholar]
- 14.Le J, Gales MA, Gales BJ. Acetaminophen for patent ductus arteriosus. Ann Pharmacother. 2015;49:241–246. doi: 10.1177/1060028014557564. [DOI] [PubMed] [Google Scholar]
- 15.Allegaert K, Anderson B, Simons S, van Overmeire B. Paracetamol to induce ductus arteriosus closure: is it valid? Arch Dis Child. 2013;98:462–466. doi: 10.1136/archdischild-2013-303688. [DOI] [PubMed] [Google Scholar]
- 16.Oncel MY, Yurttutan S, Uras N, Altug N, Ozdemir R, Ekmen S, Erdeve O, Dilmen U. An alternative drug (paracetamol) in the management of patent ductus arteriosus in ibuprofen-resistant or contraindicated preterm infants. Arch Dis Child Fetal Neonatal Ed. 2013;98:F94. doi: 10.1136/archdischild-2012-302044. [DOI] [PubMed] [Google Scholar]
- 17.Nadir E, Kassem E, Foldi S, Hochberg A, Feldman M. Paracetamol treatment of patent ductus arteriosus in preterm infants. J Perinatol. 2014;34:748–749. doi: 10.1038/jp.2014.96. [DOI] [PubMed] [Google Scholar]
- 18.Sinha R, Negi V, Dalal SS. An interesting observation of PDA closure with oral paracetamol in preterm neonates. J Clin Neonatol. 2013;2:30–32. doi: 10.4103/2249-4847.109245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozdemir OM, Doğan M, Küçüktaşçı K, Ergin H, Sahin O. Paracetamol therapy for patent ductus arteriosus in premature infants: a chance before surgical ligation. Pediatr Cardiol. 2014;35:276–279. doi: 10.1007/s00246-013-0770-9. [DOI] [PubMed] [Google Scholar]
- 20.Terrin G, Conte F, Scipione A, Bacchio E, Conti MG, Ferro R, Ventriglia F, De Curtis M. Efficacy of paracetamol for the treatment of patent ductus arteriosus in preterm neonates. Ital J Pediatr. 2014;40:21. doi: 10.1186/1824-7288-40-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oncel MY, Yurttutan S, Erdeve O, Uras N, Altug N, Oguz SS, Canpolat FE, Dilmen U. Oral paracetamol versus oral ibuprofen in the management of patent ductus arteriosus in preterm infants: a randomized controlled trial. J Pediatr. 2014;164:510–514. doi: 10.1016/j.jpeds.2013.11.008. e.1. [DOI] [PubMed] [Google Scholar]