Abstract
Cervical cancer is the fourth leading cause of cancer mortality in females worldwide. Infection with high-risk human papillomavirus (HPV) is essential but insufficient to cause cervical cancer, and the clearance of HPV infection is mediated by the immune system. The deficit of molecules responsible for adhesion may play a role in the development of cervical cancer. E-cadherin is encoded by the cadherin 1 (CDH1) gene, and is involved in cell adhesion by forming adherens junctions. The aim of present study was to investigate the methylation pattern of the CDH1 promoter and to identify the association between CDH1 promoter hypermethylation, CDH1 gene expression and HPV infection in cervical specimens obtained from 93 patients with low-grade squamous intraepithelial lesions (SILs), high-grade SILs or squamous cell carcinomas, and from 47 patients with normal cervical cytology (HPV-negative). The methylation pattern of the CDH1 promoter was investigated by methylation-specific polymerase chain reaction and quantitative pyrosequencing. CDH1 gene expression was measured by relative quantification. CDH1 methylation was significantly higher in both types of lesions and in cervical cancer than in normal samples, and CDH1 gene expression was significantly reduced during SIL progression (P=0.0162). However, the influence of HPV infection or HPV E6 expression on the methylation pattern of the CDH1 gene or its gene expression levels could not be confirmed. The present results support that the methylation of the CDH1 gene is age-related in patients with cervical lesions (P=0.01085), and therefore, older patients could be more susceptible to cancer than younger patients. The important methylation of the CDH1 promoter occurred near the transcription factor binding sites on nucleotides −13 and +103, which are close to the translational start codon. These results suggest that methylation at these sites may be an important event in the transcriptional regulation of E-cadherin, and in patients harboring these methylated cytosines, this event may facilitate HPV-driven carcinogenesis.
Keywords: cervical cancer, CDH1 promoter, methylation, gene expression, pyrosequencing
Introduction
Cervical cancer is the fourth leading cause of cancer-associated mortality in females worldwide (1). The development of invasive cervical cancer is strongly associated with human papillomavirus (HPV) infection (2,3). The presence of high-risk HPV DNA, viral oncogene expression (E6 and E7) and interaction of viral oncoproteins with growth-regulating host-cell proteins has been established as the major risk factors for cervical cancer development (3). A well-known consequence of deregulated expression of E6 and E7 is chromosome instability, which contributes to the accumulation of aberrations in host cell genes over time (4,5). Following HPV infection, cervical cancer develops through a series of subsequent steps, including development of precancerous lesions, cervical intraepithelial neoplasia (CIN) grades 1–3 (CIN 1–3) and progression to cervical cancer (6). To overcome the limitations of morphological diagnosis, molecular diagnostic tests have been developed as a complementary form of testing, and HPV tests have been introduced into different testing algorithms, particularly in countries with underdeveloped health systems (7). However, it is known that only a small fraction of patients with high-risk HPV infection develop clinically relevant cervical lesions, and usually these viruses are eventually cleared from the tissue (7,8). Therefore, broad application of cervical cytology screening has been associated with a dramatic reduction in cervical cancer incidence and mortality (9). The Bethesda system, used to communicate accurately the risk of cervical cancer, enables to classify cytological samples into six categories (10). The second most common abnormal cytology results are low-grade squamous intraepithelial lesions (LSILs), and the risk of CIN 2–3+ at initial colposcopy following an LSIL result is 15–30% in the majority of studies (11,12). CIN 2 or 3 has been reported in ≥70% of women with cytology results of high-grade SILs (HSILs) (13). Since the sensitivity range of conventional cytology is very broad (30–70%), it has limited efficacy as a single screening method, and all abnormal cytology results must be evaluated by histopathology (11,12). The combination of cytology and HPV testing has markedly increased the sensitivity of early detection of cervical cancer (12).
Infection with high-risk HPV is not sufficient for cancer development, and the clearance of HPV infection is mediated by the hosts' immune system, particularly by migration of Langerhans cells (LCs) within the infected epithelium (14). LCs interact with keratinocytes trough E-cadherin-mediated contact (15), which is important for maintaining the immune response during chronic HPV infection (14). The deficit of molecules responsible for adhesion may be important in the development of cervical cancer (16). E-cadherin, encoded by the cadherin 1 (CDH1) gene, is a transmembrane glycoprotein localized at the surface of epithelial cells, and plays a pivotal role in cell-cell adhesion dependent on calcium ions (Ca2+) (17). E-cadherin is important for the maintenance of normal tissue architecture (18), and therefore, it is considered as a suppressor of invasiveness and metastasis (19).
Intensive studies on multiple types of human cancer detected reduced or lost expression of E-cadherin, thus, disturbance in E-cadherin expression may be one of the main events in the early and late steps of cancer development (20). It is known that the expression of numerous genes is affected by the presence of hypermethylation of cytosine residues within CpG islands of the promoter region, resulting in loss of function or inactivation of tumor suppressor genes (21,22). Aberrant methylation patterns have been described for a diverse number of tumor suppressor genes in CIN lesions and in cervical cancer (21–23). One of the most frequently methylated genes in transforming CIN lesions is CDH1 (24). The comparable CDH1 methylation frequency in primary breast tumors and paired sentinel lymph node metastases indicates its important role in the development of metastasis, which may be clinically used for patient prognosis and for predicting early regional metastases (19). CDH1 gene hypermethylation was detected in ~51.1% of cervical cancer tissue samples (24).
The aim of the present study was to investigate the methylation pattern of the CDH1 promoter in order to identify potential novel factors involved in cervical carcinogenesis using only cytological samples, which could contribute to an improved sensitive detection of early cervical cancer. However, the current study did not identify any association between CDH1 promoter hypermethylation, CDH1 gene expression or HPV infection in cytological cervical specimens obtained from patients with different stages of SIL or carcinoma.
Materials and methods
Patients and clinicopathological findings
Cervical specimens were obtained from 93 patients with cervical lesions who underwent colposcopy or surgical treatment of the cervical lesion at the Department of Obstetrics and Gynaecology of Jessenius Faculty of Medicine in Martin (Martin, Slovakia) between January 2010 and August 2013. Clinical diagnosis was verified by histological examination. Other cervical specimens were collected from 47 patients with normal cervical cytology (controls), who were HPV-negative and had no previous history of cervical lesion treatment. All patients agreed to be included in the study and signed the informed consent form, which was approved by the Regional Ethical Committee at the Jessenius Faculty of Medicine, Comenius University in Bratislava (Martin, Slovakia). Cervical samples were collected by Dacron™-tipped swabs (BD Biosciences, Franklin Lakes, NJ, USA), and transferred to the medium used for transportation. Cytological samples were classified according to the Bethesda classification of 2001 (10). The present study included 34 LSIL samples, 46 HSIL samples and 13 invasive squamous cervical cancer (SCC) samples. The presence of the HPV genotype was determined by a method described previously (25).
Nucleic acid extraction and bisulfite conversion
Nucleic acids were extracted from cervical cells using MasterPure Complete DNA and RNA Purification kits (Epicentre, Madison, WI, USA). RNA was treated with DNase to eliminate DNA contamination, and all samples were stored at −80°C. DNA was quantified by ultraviolet absorption, and 1–2 µg were used for bisulfite conversion, where unmethylated cytosines were converted to uracil using an EpiTect Bisulfite kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's protocol.
Methylation-specific polymerase chain reaction (PCR) (MSP)
The methylation status of the CDH1 promoter was investigated by MSP using a nested PCR approach. In the first step of the nested PCR, degenerated primers were used that flank the CpG-rich promoter region; do not discriminate between methylated and unmethylated nucleotides; and cover nucleotides −57 to +110 around the translational start region of CDH1. The PCR products of the first step were diluted 1:1,000 and subjected to the second step of MSP, which applied sets of specific primers for unmethylated or methylated DNA. The sequences of the primers used (which are shown in Table I) were previously published in the literature (26). The first step of the nested PCR was conducted in a 25-µl total reaction volume, containing Taq DNA polymerase (Roche Diagnostics, Indianapolis, IN, USA), 10X PCR buffer, 2.5 mmol/l MgCl2, 0.5 mmol/l of each of the four deoxynucleotides (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 10 pmol/l of each forward and reverse primer for methylated and unmethylated DNA. The PCR was performed at 95°C for 10 min, followed by 25 cycles of 95°C for 30 sec, 62°C for 30 sec and 72°C for 30 sec. PCR was performed in a thermocycler with an annealing temperature of 60°C. The PCR products were analyzed on a 1.75% agarose gel with ethidium bromide staining. The first-step PCR products were diluted 500-fold, and 1 µl of this dilution was added to the second PCR step in a 25-µl reaction volume, containing specific primers for methylated or unmethylated alleles. Amplification was performed in 25 cycles with an annealing temperature of 62°C. Similarly, 5 µl of PCR products were loaded onto 1.75% agarose gels with ethidium bromide staining for analysis.
Table I.
Specific primers used in MSP and pyrosequencing, and sizes of the PCR products.
| Type of primer | Sequence | Size (bp) | Number of analyzed CpGs |
|---|---|---|---|
| MSP primer setsa | |||
| External primer set | |||
| Forward | 5′-GTGTTTTYGGGGTTTATTTGGTTGT-3′ | 186 | |
| Reverse | 5′-TACRACTCCAAAAACCCATAACTAACC-3′ | ||
| Internal methylated primer set | |||
| Forward | 5′-TGTAGTTACGTATTTATTTTTAGTGGCGTC-3′ | 112 | |
| Reverse | 5′-CGAATACGATCGAATCGAACCG-3′ | ||
| Internal unmethylated primer set | |||
| Forward | 5′-TGGTTGTAGTTATGTATTTATTTTTAGTGGTGTT-3′ | 120 | |
| Reverse | 5′-ACACCAAATACAATCAAATCAAACCAAA-3′ | ||
| Pyrosequencing primer sets | |||
| PCR primer set | |||
| Forward | 5′-GATTGGTTGTGGTCGGTAGGTGAATTTT-3′ | 235 | 19 |
| Reverse | 5′-btn-ACTCCAAAAACCCATAACTAACC-3′ | ||
| Pyrosequencing primers | |||
| Sequencing primer 1 | 5′-GTAGGTGAATTTTTAGTTAATTAG-3′ | 7 | |
| Sequencing primer 2 | 5′-GTTTGCGGAAGTTAGTTTAGATT-3′ | 11 | |
| Sequencing primer 3 | 5′-GTGTTTTCGGGGTTTATTTGGTTGT-3′ | 5 |
Primers published in the literature (22). MSP, methylation-specific PCR; PCR, polymerase chain reaction; btn, biotin.
Quantitative pyrosequencing
Pyrosequencing was used to determine the percentage of methylation of 19 CpG islands within the minimal promoter region of the CDH1 gene (−68 to +124 bp relative to the transcription start site). In total, 20 ng of bisulfite-converted DNA was amplified using PyroMark PCR kit (Qiagen GmbH) and a primer set with biotin-labelled reverse primer. The PCR primer set and the sequencing primers (Table I) were designed using PyroMark Assay Design software version 2.0.1.15 (Qiagen GmbH), covering nucleotides from −57 to +116 around the CDH1 translational start site. Pyrosequencing was performed according to the manufacturer's protocol, using three assays with 4 pmol of the respective sequencing primer on a PyroMark Q96 ID System (Qiagen GmbH) with PyroMark Gold Q96 Reagents. Target CpGs were evaluated with the instrument software (PyroMark Q96 software version 2.5.8; Qiagen GmbH), which calculates the proportion of methylation at each CpG site as a C/T ratio according to the peak height. All replicates contained dilution series of control methylated DNA (0, 25, 50, 75 and 100%) mixed with unmethylated DNA following bisulfite conversion (Qiagen GmbH).
CDH1 gene expression analysis
The effect of CDH1 gene hypermethylation on CDH1 gene expression was evaluated by relative quantification. For CDH1 gene expression analysis, complementary (c)DNA was synthesized from 1 µg of total RNA using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) in a total volume of 20 µl. Multiplex quantitative PCR reactions were performed in triplicate in a final volume of 20 µl using 50 ng of cDNA, TaqMan® Gene Expression Master Mix (Thermo Fisher Scientific, Inc.) and TaqMan assays Hs01013959_m1 (CDH1) and Hs99999903_m1 (actin beta; ACTB; Thermo Fisher Scientific, Inc.). Relative gene expression of CDH1 was normalized to that of the endogenous control ACTB. Both assays (Hs01013959_m1 and Hs99999903_m1) were verified using the standard curve method and normalized to ACTB.
Statistical analysis
All statistical analyses were conducted in R version 3.2.1 (www.r-project.org), with the aid of libraries MASS (cran.r-project.org/web/packages/MASS/index.html), RVAideMemoire (cran.r-project.org/web/packages/RVAideMemoire/index.html; version 0.9–57), ridge (cran.r-project.org/src/contrib/Archive/ridge/; version 2.1–3), earth (cran.r-project.org/web/packages/earth/index.html; version 4.4.4), robustbase (cran.r-project.org/web/packages/robustbase/index.html; version 0.92–5), mgcv (cran.r-project.org/web/packages/mgcv/index.html), randomForest (cran.r-project.org/web/packages/randomForest/index.html) and Deducer (www.jstatsoft.org/article/view/v049i08). Plots were produced by R version 3.2.1 libraries ggplot2 (ggplot2.org/) and plotmo (cran.r-project.org/web/packages/plotmo/index.html; verions 3.1.4), and multiple comparisons with the binomial exact test with false discovery rate (FDR) correction were conducted. P<0.05 was considered to indicate a statistically significant difference.
Results
Patients and clinicopathological findings
The promoter hypermethylation status of the CDH1 gene was examined in 47 specimens of normal cervical squamous epithelium and 93 specimens of SIL or carcinoma. The average age of patients with normal cervical epithelium and cervical lesion was 52.4 years (range, 25–77 years) and 41 years (range, 18–75 years), respectively. HPV infection was detected in 67.6% (23/34) of LSIL, 84.8% (39/46) of HSIL and 84.6% (11/13) of SCC. Control specimens with negative cytology were also negative for HPV infection.
Detection of CDH1 promoter hypermethylation by MSP
In order to determine the methylation status of the CDH1 promoter, 93 samples from patients with cervical dysplasia and 47 control samples were analyzed by MSP. Methylation was detected in 0% (0/47) of normal cervical epithelium samples, and, of 93 cervical lesions, 20.6% (7/34) of LSIL, 21.7% (10/46) of HSIL and 46.2% (6/13) of SCC exhibited methylation of CpG islands in the CDH1 promoter. Using the non-parametric χ2 test, it was demonstrated that the methylation in LSIL, HSIL and SCC was significantly different from that in controls [P=0.01774 and 95% confidence interval (CI), 0.0172–0.3520 for LSIL; P=0.00914 and 95% CI, 0.0485–0.03438 for HSIL; and P=0.00011 and 95% CI, 0.1100–0.7635 for SSC]. However, multiple comparison by the binomial exact test with FDR correction supported the claim that the presence of CDH1 methylation in SCC is equally probable as its absence (presence of methylation, <50% of SCC; P=1.0000), which supports the evidence that methylation occurs rather randomly in these cells and is not a marker of SCC. The observed relative frequencies of methylation in LSIL and HSIL were half of the expected value, and the unequal probability was statistically significant (P=0.0012 and P=0.0005, respectively), which suggests that methylation in this lesions is not randomly, and may represent an early event.
The correlation of CDH1 methylation with age (threshold for age, 50 years) demonstrated a statistically significant difference in the control group and in the patients groups when χ2 test with Yates' continuity correction was used. CDH1 promoter hypermethylation in the control group was almost absent (P=0.5000; one-sided 95% CI, −1.0000 to 0.055573), thus demonstrating no correlation with patient's age. However, CDH1 methylation was more frequent in patients older than 50 years (P=0.01085; one-sided 95% CI, −1.0000 to −0.0514).
When the frequency of HPV infection was compared in cervical lesions with methylated and unmethylated CDH1 promoters, no significant difference between both groups was observed (χ2 test with Yates' continuity correction; P=0.6270 and one-sided 95% CI, for the probabilities of HPV infection −1.0000 to 0.2596). No change was observed when the cervical specimens were divided according to the severity of SIL (data not shown).
Detection of CDH1 promoter hypermethylation by pyrosequencing
To verify the presence of CDH1 promoter hypermethylation and to specify the affected nucleotides, quantitative pyrosequencing was used to assess the methylation level in 19 CpG islands of the CDH1 promoter region, which are covered by a previous MSP assay in 38 (all MSP-negative) control samples and in 43 (17 MSP-positive and 26 MSP-negative) samples from cervical lesions. Other samples were excluded from the analysis due to the low quality of the pyrosequencing data. The average of percentage methylation for each of the 19 CpG islands was calculated for cervical lesions and control samples. Certain level of basal methylation was detected in control samples that were unmethylated by MSP. However, the average methylation levels of CpG islands in cervical lesions were significantly higher (P<0.05; Table II) for all nucleotides, with the exception of nucleotides −52 and −36, compared with the control group. When cervical lesions were divided according to severity (LSIL, HSIL and SCC) and compared with control samples, the mean methylation status for each CpG island was significantly higher in cervical lesions for the nucleotides described in Table II with significant P-values (as marked by asterisks).
Table II.
Significance of the mean methylation level of investigated CpG islands according to the severity of the cervical lesion, compared with the mean methylation level of the control samples.
| Nucleotide | LSILa | HSILa | SCCa |
|---|---|---|---|
| −57 | 0.0133*; 0.0047 | 0.0226*; 0.0023 | 0.0961; −0.0125 |
| −52 | 0.4386; −0.0139 | 0.0384*; 0.0010 | 0.1943; −0.0299 |
| −45 | 0.0310*; 0.0019 | 0.0562; −0.0004 | 0.0259*; 0.0087 |
| −36 | 0.1371; −0.0096 | 0.2003; −0.0105 | 0.2236; −0.0150 |
| −13 | 0.1587; −0.8431 | 0.0177*; 0.0093 | 0.1098; −0.0125 |
| +6 | 0.0760; −0.0023 | 0.0483*; 0.0013 | 0.1890; −0.0137 |
| +9 | 0.0485*; 0.0002 | 0.0097*; 0.0053 | 0.0695; −0.0039 |
| +36 | 0.0303*; 0.0036 | 0.1038; −0.0053 | 0.1442; −0.0178 |
| +60 | 0.0371*; 0.0022 | 0.0587; −0.0013 | 0.1230; −0.0133 |
| +70 | 0.0300*; 0.0040 | 0.0302*; 0.0039 | 0.1380; −0.0179 |
| +75 | 0.0027*; 0.0121 | 0.0096*; 0.0073 | 0.1298; −0.0150 |
| +80 | 0.0033*; 0.0159 | 0.0317*; 0.0032 | 0.1056; −0.0118 |
| +84 | 0.0461*; 0.0004 | 0.0998; −0.0039 | 0.1767; −0.0262 |
| +90 | 0.0063*; 0.0129 | 0.0575; −0.0010 | 0.1123; −0.0142 |
| +93 | 0.0245*; 0.0027 | 0.0129*; 0.0038 | 0.2644; −0.0281 |
| +103 | 0.1904; −0.0161 | 0.0593; −0.0015 | 0.0867; −0.0098 |
| +107 | 0.0872; −0.0034 | 0.0900; −0.0036 | 0.1218; −0.0167 |
| +110 | 0.0050*; 0.0057 | 0.0079*; 0.0035 | 0.1123; −0.0087 |
| +116 | 0.0563; −0.0009 | 0.0716; −0.0027 | 0.1199; −0.0189 |
P-value and lower bound of the one-sided 95% confidence interval for the Welch's two sample t-test vs. the one-sided alternative hypothesis that the mean methylation in a lesion is higher than that in the control. *P<0.05. LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; SCC, squamous cell carcinoma.
The methylation level of MSP-methylated cervical lesions was also significantly higher in nucleotides −57, −45, −13, +9, +70, +75, +80, +93 and +110 compared with MSP-unmethylated control samples (Table III).
Table III.
Significance of the mean methylation of MSP-methylated cervical lesions compared with that of MSP-unmethylated control samples for the investigated nucleotides.
| Nucleotide | P-value and lower bound of 95% CI |
|---|---|
| −57 | 0.0409*; 0.0017 |
| −52 | 0.0648; −0.0025 |
| −45 | 0.0093*; 0.0107 |
| −36 | 0.1375; −0.0080 |
| −13 | 0.0196*; 0.0090 |
| +6 | 0.0654; −0.0017 |
| +9 | 0.0462*; 0.0005 |
| +36 | 0.1032; −0.0069 |
| +60 | 0.1086; −0.0078 |
| +70 | 0.0358*; 0.0033 |
| +75 | 0.0381*; 0.0021 |
| +80 | 0.0278*; 0.0053 |
| +84 | 0.0683; −0.0031 |
| +90 | 0.0671; −0.0029 |
| +93 | 0.0438*; 0.0010 |
| +103 | 0.1383; −0.0127 |
| +107 | 0.1963; −0.0153 |
| +110 | 0.0243*; 0.0034 |
| +116 | 0.1256; −0.0114 |
H0 (null hypothesis), the mean methylation level of sample with MSP methylated CDH1 is equal to the mean methylation level of MSP unmethylated control sample; HA, the mean for MSP methylated CDH1 promoter is higher than the mean of MSP unmethylated control sample. P-value for the Welch's two sample t-test and lower bound of the one-sided 95% confidence interval. CI, confidence interval; MSP, methylation-specific polymerase chain reaction.
Association between MSP and pyrosequencing results
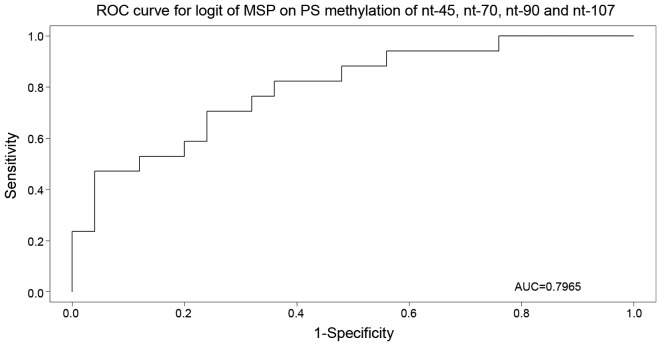
To validate the results obtained in MSP and pyrosequencing analyses, the results of both methods were processed in a random forest analysis with MSP as response. This analysis identified the most important nucleotides, and the realistic (out-of-bag) estimate of the misclassification error was observed to be 37.5%. Subsequent multivariate logistic regression and Akaike information criterion (AIC) model selection narrowed down the set of important nucleotides to nucleotides −45, +70, +90 and +107 (P=0.0122, 0.0819, 0.1749 and 0.1240, respectively). The selected model was used for the receiver operating characteristic curve construction (Fig. 1), with an area under the curve of 79.6%.
Figure 1.
Receiver operating characteristic curve for nucleotides −45, +70, +90 and +107 (P=0.0122, 0.0819, 0.1749 and 0.1240, respectively), with area under the curve equal to 79.6%. ROC, receiver operating characteristic; AUC, area under the curve; MSP, methylation-specific polymerase chain reaction; nt, nucleotide; logit, logistic regression; PS, pyrosequencing.
Effect of CDH1 hypermethylation on CDH1 gene expression
The observed methylation pattern of the CDH1 promoter region was compared with the relative CDH1 gene expression. The mean level of CDH1 gene expression was 2.4153 in the control group (negative for intraepithelial lesion or dysplasia), 0.7944 in LSIL, 1.3402 in HSIL and 1.5110 in SCC (Fig. 2). The Kruskal-Wallis rank sum test determined that the mean expression values were significantly different among the groups (P=0.0162).
Figure 2.
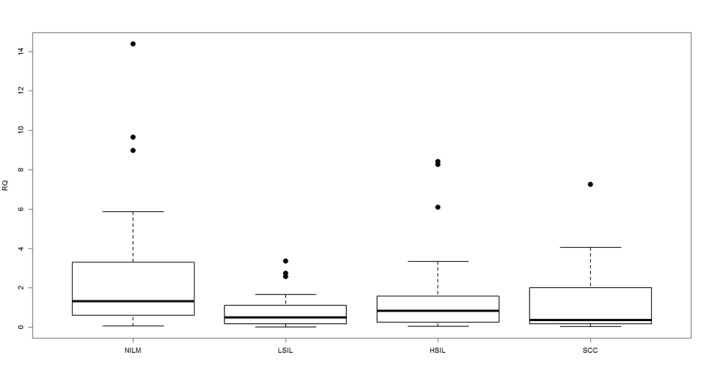
Mean level of cadherin 1 gene expression in the control group (negative for intraepithelial lesion or malignancy) compared with low-grade SIL, high-grade SIL and squamous cell carcinoma. Boxplots represent the interquartile range and the median. Kruskal-Wallis rank sum test revealed that the mean expression levels significantly differed among the groups (P=0.0162). NILM, negative for intraepithelial lesion or malignancy; low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; SCC, squamous cell carcinoma; RQ, relative quantification.
The present study also investigated the association between the mean level of CDH1 gene expression and the presence of HPV infection. It was observed that HPV infection had no significant effect on CDH1 gene expression (P=0.8117; 95% CI, -∞ to 1.0659). In HPV16 and 18-positive samples, information about the oncogene E6 expression was also available. Overall, E6 expression did not have a significant effect on CDH1 gene expression (P=0.3299; 95% CI, -∞ to 0.4519), although the presence of E6 expression was significantly associated with decreased CDH1 gene expression in the LSIL group (P=0.03596; 95% CI, -∞ to 0.0445).
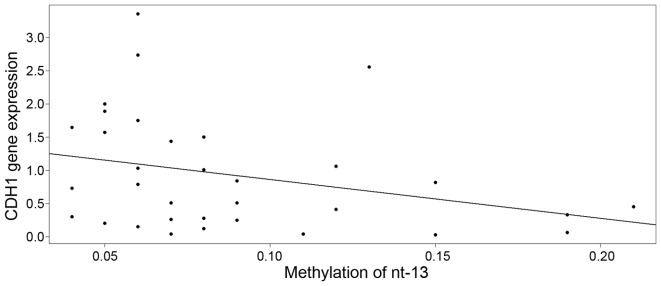
By means of multivariate regression model, the association between CDH1 gene expression and methylation of the nucleotides was explored separately for cervical lesions and for normal cervical epithelium. Upon model building (exploratory analysis, outlier detection, treatment of multicollinearity by ridge regression, model diagnostics, robust regression and model selection by AIC), it was observed that the expression of the CDH1 gene was decreased in cervical lesions with a high methylation level at nucleotide −13 (Fig. 3), with an estimate of descent of −5.8735 [95% CI, −12.4992 to 0.7522; standard error (SE)=3.2487] and intercept equal to 1.4494 (95% CI, −12.4992 to 0.7522; SE=0.3224). Although the slope was only weakly significant (P=0.0803), the P-value cannot be taken at face value due to the well-known post-model selection inferences issues (27).
Figure 3.
Cadherin 1 gene expression in cervical lesions decreases with an increase in the methylation level at nucleotide −13, with an estimate of descent of −5.8735 (95% CI, −12.4992 to 0.7522; SE=3.2487) and an intercept of 1.4494 (95% CI, −12.4992 to 0.7522; SE=0.3224). CDH1, cadherin 1; nt, nucleotide; SE, standard error; CI, confidence interval.
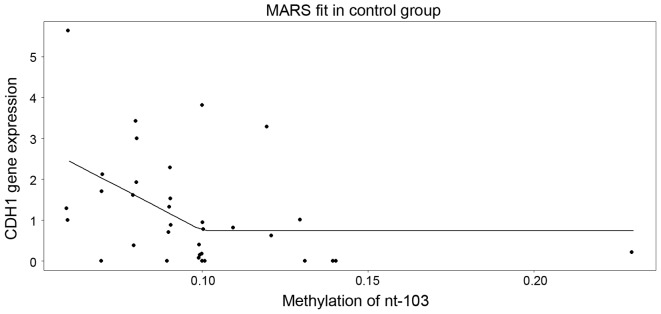
In control samples, model building with ridge regression revealed that nucleotides +103 and +107 are the only significant predictors. When fed into the multivariate regression analysis, solely nucleotide +107 was revealed to be statistically significant (P=0.04815), with a negative slope of −30.1142 (SE=14.7210) and an intercept of 2.5474 (SE=0.5961). As an alternative to the general linear model, the multivariate adaptive regression splines model was used, which among all the nucleotides, it selected solely nucleotide +103 (Fig. 4). Of note, this model suggested that the level of expression decreases as the methylation in nucleotide +103 increases up to ~10%, and above the cut-off, a saturation (plateau) appears.
Figure 4.
Multivariate adaptive regression splines model for nucleotide +103. The model suggests that the level of cadherin 1 gene expression decreases as the methylation in nucleotide+103 increases up to ~10%; above the cut-off, a saturation (plateau) appears. CDH1, cadherin 1; nt, nucleotide; SE, standard error; MARS, multivariate adaptive regression splines.
Discussion
In present study, the methylation status of the CDH1 promoter was investigated in cervical cells from precursor lesions, which represent a source for detecting biomarkers of relevance to cervical carcinogenesis. The CDH1 promoter was frequently reported to be methylated in numerous types of gynecological cancer, including breast (28), ovarian (29), endometrial (30) and cervical cancer. CDH1 gene hypermethylation is also detectable in the serum of patients with cervical cancer (31). The majority of studies to date have examined promoter methylation in tissue sections or cell lines, while studies on cervical cytology specimens were less frequent and had more different results for CDH1 methylation (21–23). The selected promoter was observed to be methylated in 58% of cervical cancer specimens and in 29% of HSIL, although, these results, in a survey of 51 studies, were observed to be dependent on the analysis method and the type of clinical material used (23). Similarly, the present study detected CDH1 methylation in 21.7% of HSIL, 46.2% of SCC and 20.6% of LSIL specimens. Previous studies investigating the CDH1 methylation status of LSIL specimens are uncommon; CDH1 methylation was not present (32,33) or was only detected in a small percentage of samples (11.3% of LSIL and 13.3% of normal cervical epithelium) (34). The unequal probability of CDH1 methylation in LSIL and HSIL was statistically significant (P=0.0012 and P=0.0005, respectively), which suggests that methylation in this lesions is not random and may represent an early carcinogenesis event, in contrast to the random methylation of CDH1 in SCC patients. The presence of CDH1 methylation in cervical lesions represents just one of all possible methylated targets. As it is generally known, different genes can be methylated in cervical cancer, while the combination of various methylated genes can act as a co-driver of carcinogenesis (21–24). As a consequence, and due to its specific role in cell adhesion, CDH1 should be considered.
It has been postulated that DNA methylation is age-related and usually occurs at age-related sites of the human genome (35). Cells have a lower threshold for malignant transformation and are more susceptible to cancer when acquire methylation at age-related sites (35). In the present study, CDH1 methylation was more frequent in patients older than 50 years (P=0.01085), indicating that the presence of promoter hypermethylation could be age-related, as it has been reported for other genes (36).
Cervical cancer is also associated with long-term persistence of HPV infection, which may induce progression of high-grade cervical dysplasia to cervical cancer, together with aberrant DNA methylation in the host genome (37). HPV infection with high-risk HPV types causes changes in the methylation status of cellular genes through upregulation of DNA methyltransferases (37). Viral oncogenes can induce tumor suppressor gene methylation (38), as well as expression of E7 and E6, which results in a further reduction in surface E-cadherin levels (39). A previous study by Flatley et al (40) reported that high-risk HPV infection may influence folate status and the frequency of promoter methylation of three tumor suppressor genes (CDH1, death-associated protein kinase and hypermethylated in cancer 1), which increased with the progression of cervical neoplasia. In the present study, HPV infection was not demonstrated to affect the methylation pattern of the CDH1 gene (P=0.627; 95% CI, −1.00 to 0.2596) or the E6 expression of high-risk HPV genotypes (P=0.3299; 95% CI, -∞ to −0.4519; data not shown). No correlation between the promoter methylation status of the CDH1 gene and the patients' clinicopathological parameters, including HPV infection, phenotypic distribution or stage of the disease, was observed in other studies (41,42). Thus, the impact of HPV infection on DNA methylation of the host genome still remains controversial.
To distinguish samples with methylated and unmethylated CDH1 promoter, MSP was used as the first step of the current study. Quantitative pyrosequencing was used for monitoring the ratio of methylated CpG islands in a nucleotide sequence. This provided additional information concerning the methylation of CpG islands. The level of DNA methylation within the CDH1 promoter was measured in normal cervical specimens and in cervical lesions with various stages of dysplasia. MSP is a widely used method to assess the methylation status of any group of CpG sites within a CpG island without the requirement for restriction enzymes, and exhibits a sensitivity of methylation detection of 0.1% of alleles (43). By pyrosequencing, the degree of methylation at several CpGs in close proximity can be quantitatively measured (44). The methylation at each CpG position in a sequence is determined from the ratio of T and C (44). However, the methylation values measured by pyrosequencing in the present study ranged from 0 to 6% in certain CpGs analyzed in unmethylated control DNA samples (data not shown). Various authors recommend the use of a ≥10% threshold of methylation to classify samples as methylated (45). However, the present study used statistical analysis to differentiate between significantly methylated and unmethylated CpGs in various types of cervical dysplasia compared with the mean methylation level at each CpG position in the control group (Table II). The results confirmed that certain CpGs in MSP-methylated samples had significantly higher methylation levels than those detected in the unmethylated controls. Following a detailed analysis of the CDH1 promoter sequence, two CpGs in the internal forward primer (nucleotides −13 and +9) and three CpGs in the internal reverse primer sequence (nucleotides +70, +75 and +80) were observed to have a significant influence on the result of MSP. When these nucleotides were used in the logistic regression for MSP an AUC of 61.18% was achieved. If, instead, a logistic regression model for MSP was constructed with use of all CpGs, the nucleotides −45, +70, +90 and +107 appeared to be most important, leading to an AUC of 79.6%. ROC curves for each nucleotide had weak AUC, and due to the low number of samples in the study, no analysis for prediction of high-grade lesion or cancer was performed. However, studies evaluating the ability of DNA methylation levels to identify cervical cancer cases usually use combination of genes and clinicopathological features to improve AUC and to increase the sensitivity and specificity of the test to identify cancer (46).
In the last part of the present study, the level of CDH1 gene expression was measured, and the association between CDH1 hypermethylation at each CpG island and CDH1 gene expression level was evaluated. The expression of E-cadherin, as a major adhesion component of epithelial cells, has been observed to be reduced or lost in epithelial tumor types by promoter hypermethylation mechanisms (47). It has been also reported that the presence and localization of cytoplasmic E-cadherin correlated with CIN grade (48). In other types of cancer, the degree of CpG methylation increased as the precancerous conditions progressed (49). The present study detected significantly reduced expression of the CDH1 gene in SILs or cancers (P=0.0162) compared with control samples. According to our observations, HPV infection had no effect on the relative quantity of E-cadherin (P=0.8117). However, E6 oncogene expression decreased the CDH1 gene expression only in HPV16 or 18-positive LSILs (P=0.0359). The HPV E6 protein has been shown to interact with cellular proteins; it creates a complex with p53 and mediates its degradation by the ubiquitin system (50). The E6 protein reduces the expression of cell-surface E-cadherin, and thus has also a function in the control of cell-surface E-cadherin expression and in the regulation of the cutaneous immune response in virus-infected skin (51). A previous study noticed that E-cadherin transcription regulation by E6 is independent of direct methylation of the E-cadherin promoter (39). Therefore, further studies are required.
In statistical analysis investigating the influence of methylation at each CpG position of the CDH1 promoter on CDH1 gene expression, the present study revealed that nucleotides +103 and +107 were the most influential ones on CDH1 gene expression in control samples. These nucleotides are localized near the CTCF binding site, and were observed to be methylated also in the control cell line HCT116 (39). In the present study in SILs, the main effect on CDH1 gene expression was exerted by nucleotide −13, which is localized near the Snail binding site. Regulation of E-cadherin gene expression in metastatic and non-metastatic cancer cells demonstrated that methylation states, chromatin constraint and Snail family transcription factors are important in the downregulation of E-cadherin gene expression (52). The presence of DNA methylation sites near the transcription factor binding sites could be required for efficient transcriptional regulation of E-cadherin and other tumor suppressor genes. However, further studies are required to elucidate the molecular interaction of E-cadherin promoter methylation and transcription factor binding (53).
In summary, E-cadherin expression during tumor progression was observed to be downregulated by several mechanisms, including genetic, epigenetic and transcriptional changes. The present study confirmed that epigenetic changes such as DNA methylation of the CDH1 promoter are frequent in LSILs. Similarly, CDH1 methylation was observed to be present in HSILs and in ~50% of cervical cancer specimens. CDH1 gene expression was reduced during SIL progression in the present study; however, the influence of HPV infection or HPV E6 expression on the methylation pattern of the CDH1 gene or its gene expression could not be confirmed. It was established that not all HPV infected cervical dysplasia develop into cancer, indicating that factors other than HPV viral proteins contribute to the progression to cervical cancer (54). The current findings support also the claim that methylation of the CDH1 gene is age-related, and therefore, older patients could be more susceptible to cancer than younger ones. The important methylation of the CDH1 promoter occurred near the transcription factor binding sites, which suggests that methylation at these sites may be an important event in the transcriptional regulation of E-cadherin and other genes, although additional studies are required to confirm this hypothesis. Inactivation of the E-cadherin system by multiple mechanisms, including genetic and epigenetic events, plays a significant role in both the early and late stages of multistep carcinogenesis (49).
Acknowledgements
The present study was supported by the projects ‘Increasing Opportunities for Career Growth in Research and Development in the Field of Medical Sciences’ of the Institute of Experimental Pharmacology and Toxicology (ITMS; Bratislava, Slovakia; grant no. 26110230067), VEGA (grant no., 1/0102/15) and ‘Molecular diagnosis of cervical cancer’ (ITMS; grant no. 26220220113), which were co-funded by the European Union and the European Social Fund (Brussels, Belgium).
References
- 1.Arbyn M, Castellsagué X, de Sanjosé S, Bruni L, Saraiya M, Bray F, Ferlay J. Worldwide burden of cervical cancer in 2008. Am Oncol. 2011;22:2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 2.Dürst M, Gissmann L, Ikenberg H, zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci. 1983;80:3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.zurHausen H. Papillomaviruses causing cancer: Evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000;92:690–698. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]
- 4.Korzeniewski N, Spardy N, Duensing A, Duensing S. Genomic instability and cancer: Lessons learned from human papillomaviruses. Cancer Lett. 2011;305:113–122. doi: 10.1016/j.canlet.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visnovsky J, Kudela E, Farkasova A, Balharek T, Krkoska M, Danko J. Amplification of TERT and TERC genes in cervical intraepithelial neoplasia and cervical cancer. Neuro Endocrinol Lett. 2014;35:518–522. [PubMed] [Google Scholar]
- 6.McCredie MR, Sharples KJ, Paul C, Baranyia J, Medley G, Jones RW, Skegg DC. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: A retrospective cohort study. Lancet Oncol. 2008;9:425–434. doi: 10.1016/S1470-2045(08)70103-7. [DOI] [PubMed] [Google Scholar]
- 7.Boone JD, Erickson BK, Huh WK. New insights into cervical cancer screening. J Gynecol Oncol. 2012;23:282–287. doi: 10.3802/jgo.2012.23.4.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunne EF, Markowitz LE. Genital human papillomavirus infection. Clin Infect Dis. 2006;43:624–629. doi: 10.1086/505982. [DOI] [PubMed] [Google Scholar]
- 9.Tjalma WA. The ideal cervical cancer screening recommendation for Belgium, an industrialized country in Europe. Eur J Gynaecol Oncol. 2014;35:211–218. [PubMed] [Google Scholar]
- 10.Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T., Jr The 2001 Bethesda system: Terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 11.Bentley J, Bertrand M, Brydon L, Gagne H, Hauck B, Mayrand MH, McFaul S, Power P, Schepansky A, Straszak-Suri M. Colposcopic management of abnormal cervical cytology and histology. J Obstet Gynaecol Can. 2012;34:1188–1202. doi: 10.1016/S1701-2163(16)35468-8. [DOI] [PubMed] [Google Scholar]
- 12.Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, Ratnam S, Coutlee F, Franco EL. Canadian Cervical Cancer Screening Trial Study Group: Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 13.Agramunt S, Checa MÁ, Gonzáles-Comadrán M, Larrazabai F, Arbós A, Alameda F, Mancebo G, Carreras R. High-grade squamous intraepithelial lesion could be managed conservatively in women up to 25 years: Results from a retrospective cohort study. J Low Genit Tract Dis. 2013;17:459–462. doi: 10.1097/LGT.0b013e3182838b7c. [DOI] [PubMed] [Google Scholar]
- 14.Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer. 2002;2:59–65. doi: 10.1038/nrc700. [DOI] [PubMed] [Google Scholar]
- 15.Tang A, Amagai M, Granger LG, Stanley JR, Udey MC. Adhesion of epidermal Langerhans cells to keratinocytes mediated by E-cadherin. Nature. 1993;361:82–85. doi: 10.1038/361082a0. [DOI] [PubMed] [Google Scholar]
- 16.Hubert P, Caberg JH, Gilles C, Bousarghin L, Franzen-Detrooz E, Boniver J, Delvenne P. E-cadherin-dependent adhesion of dendritic and Langerhans cells to keratinocytes is defective in cervical human papillomavirus-associated (pre)neoplastic lesions. J Pathol. 2005;206:346–355. doi: 10.1002/path.1771. [DOI] [PubMed] [Google Scholar]
- 17.Oda H, Takeichi M. Evolution: Structural and functional diversity of cadherin at the adherens junction. J Cell Biol. 2011;193:1137–1146. doi: 10.1083/jcb.201008173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pećina-Slaus N. Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int. 2003;3:17. doi: 10.1186/1475-2867-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sebova K, Zmetakova I, Bella V, Kajo K, Stankovicova I, Kajabova V, Krivulcik T, Lasabova Z, Tomka M, Galbavy S, Fridrichova I. RASSF1A and CDH1 hypermethylation as potential epimarkers in breast cancer. Cancer Biomark. 2011–2012;10:13–26. doi: 10.3233/CBM-2012-0230. [DOI] [PubMed] [Google Scholar]
- 20.Wijnhoven BP, Dinjens WN, Pignatelli M. E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg. 2000;87:992–1005. doi: 10.1046/j.1365-2168.2000.01513.x. [DOI] [PubMed] [Google Scholar]
- 21.Wajed SA, Laird PW, DeMeester TR. DNA methylation: An alternative pathway to cancer. Ann Surg. 2001;234:10–20. doi: 10.1097/00000658-200107000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: Epigenetics joins genetics. Trends Genet. 2000;16:168–174. doi: 10.1016/S0168-9525(99)01971-X. [DOI] [PubMed] [Google Scholar]
- 23.Wentzensen N, Sherman ME, Schiffman M, Wang SS. Utility of methylation markers in cervical cancer early detection: Appraisal of the state-of-the-science. Gynecol Oncol. 2009;112:293–299. doi: 10.1016/j.ygyno.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narayan G, Arias-Pulido H, Koul S, Vargas H, Zhang FF, Villella J, Schneider A, Terry MB, Mansukhani M, Murty VV. Frequent promoter methylation of CDH1, DAPK, RARB, and HIC1 genes in carcinoma of cervix uteri: Its relationship to clinical outcome. Mol Cancer. 2003;2:24. doi: 10.1186/1476-4598-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janusicova V, Mendelova A, Zubor P, Kapustova I, Svecova I, Kudela E, Burjanivova T, Lasabova Z, Danko J. mRNA expression in cervical specimens for determination of severe dysplasia or worse in HPV-16/18-positive squamous lesions. J Low Genit Tract Dis. 2014;18:273–280. doi: 10.1097/LGT.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 26.House MG, Guo M, Iacobuzio-Donahue C, Herman JG. Molecular progression of promoter methylation in intraductal papillary mucinous neoplasms (IPMN) of the pancreas. Carcinogenesis. 2003;24:193–198. doi: 10.1093/carcin/24.2.193. [DOI] [PubMed] [Google Scholar]
- 27.Leeb H, Pötscher BM. Model selection and inference: Facts and fiction. Econometric Theory. 2005;1:21–59. [Google Scholar]
- 28.Caldeira JR, Prando EC, Quevedo FC, Neto FA, Rainho CA, Rogatto SR. CDH1 promoter hypermethylation and E-cadherin protein expression in infiltrating breast cancer. BMC Cancer. 2006;6:48. doi: 10.1186/1471-2407-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhillon VS, Young AR, Husain SA, Aslam M. Promoter hypermethylation of MGMT, CDH1, RAR-beta and SYK tumour suppressor genes in granulosa cell tumours (GCTs) of ovarian origin. Br J Cancer. 2004;90:874–881. doi: 10.1038/sj.bjc.6601567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visnovsky J, Fiolka R, Kudela E, Slavik P, Krkoska M, Lasabová Z, Danko J. Hypermethylation of selected genes in endometrial carcinogenesis. Neuro Endocrinol Lett. 2013;34:675–680. [PubMed] [Google Scholar]
- 31.Abudukadeer A, Bakry R, Goebel G, Mutz-Dehbalaie I, Widschendter A, Bonn GK, Fiegl H. Clinical relevance of CDH1 and CDH13 DNA-methylation in serum of cervical cancer patients. Int J Mol Sci. 2012;13:8353–8363. doi: 10.3390/ijms13078353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gustafson KS, Furth EE, Heitjan DF, Fansler ZB, Clark DP. DNA methylation profiling of cervical squamous intraepithelial lesions using liquid-based cytology specimens: An approach that utilizes receiver-operating characteristic analysis. Cancer. 2004;102:259–268. doi: 10.1002/cncr.20425. [DOI] [PubMed] [Google Scholar]
- 33.Kim JH, Choi YD, Lee JS, Lee JH, Nam JH, Choi C. Assessment of DNA methylation for the detection of cervical neoplasia in liquid-based cytology specimens. Gynecol Oncol. 2010;116:99–104. doi: 10.1016/j.ygyno.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 34.McCormick TM, Canedo NH, Furtado YL, Silveira FA, de Lima RJ, Rosman AD, Filho GL Almeida, Mda G Carvalho. Association between human papillomavirus and Epstein-Barr virus DNA and gene promoter methylation of RB1 and CDH1 in the cervical lesions: A transversal study. Diagn Pathol. 2015;10:59. doi: 10.1186/s13000-015-0283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Z, Taylor JA. Genome-wide age-related DNA methylation changes in blood and other tissues relate to histone modification, expression and cancer. Carcinogenesis. 2014;35:356–364. doi: 10.1093/carcin/bgt391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung M, Pfeifer GP. Aging and DNA methylation. BMC Biol. 2015;13:7. doi: 10.1186/s12915-015-0118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonard SM, Wei W, Collins SI, Pereira M, Divaf A, Constandinou-Williams C, Young LS, Roberts S, Woodman CB. Oncogenic human papillomavirus imposes an instructive pattern of DNA methylation changes which parallel the natural history of cervical HPV infection in young women. Carcinogenesis. 2012;33:1286–1293. doi: 10.1093/carcin/bgs157. [DOI] [PubMed] [Google Scholar]
- 38.Woodman CB, Collins SI, Young LS. The natural history of cervical HPV infection: Unresolved issues. Nat Rev Cancer. 2007;7:11–22. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- 39.D'Costa ZJ, Jolly C, Androphy EJ, Mercer A, Matthews CM, Hibma MH. Transcriptional repression of E-cadherin by human papillomavirus type 16 E6. PLoS One. 2012;7:e48954. doi: 10.1371/journal.pone.0048954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flatley JE, McNeir K, Balasubramani L, Tidy J, Stuart EL, Young TA, Powers HJ. Folate status and aberrant DNA methylation are associated with HPV infection and cervical pathogenesis. Cancer Epidemiol Biomarkers Prev. 2009;18:2782–2789. doi: 10.1158/1055-9965.EPI-09-0493. [DOI] [PubMed] [Google Scholar]
- 41.Attaleb M, El hamadani W, Khyatti M, Benbacer L, Benchekroun N, Benider A, Amrani M, El Mzibbri M. Status of p16(INK4a) and E-cadherin gene promoter methylation in Moroccan patients with cervical carcinoma. Oncol Res. 2009;18:185–192. doi: 10.3727/096504009790217416. [DOI] [PubMed] [Google Scholar]
- 42.Kahn SL, Ronnett BM, Gravitt PE, Gustafson KS. Quantitative methylation-specific PCR for the detection of aberrant DNA methylation in liquid-based Pap tests. Cancer. 2008;114:57–64. doi: 10.1002/cncr.23258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylation-specific PCR: A novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tost J, Gut IG. DNA methylation analysis by pyrosequencing. Nat Protoc. 2007;2:2265–2275. doi: 10.1038/nprot.2007.314. [DOI] [PubMed] [Google Scholar]
- 45.Colella S, Shen L, Baggerly KA, Issa JP, Krahe R. Sensitive and quantitative universal Pyrosequencing methylation analysis of CpG sites. Biotechniques. 2003;35:146–150. doi: 10.2144/03351md01. [DOI] [PubMed] [Google Scholar]
- 46.Siegel EM, Riggs BM, Delmas AL, Koch A, Hakam A, Brown KD. Quantitative DNA methylation analysis of candidate genes in cervical cancer. PLoS One. 2015;10:e0122495. doi: 10.1371/journal.pone.0122495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Esteller M, Corn PG, Baylin SB, Herman JG. A gene hypermethylation profile of human cancer. Cancer Res. 2001;61:3225–3229. [PubMed] [Google Scholar]
- 48.Branca M, Giorgi C, Ciotti M, Santini D, Di Bonito L, Costa S, Benedetto A, Bonifacio D, Di Bonito P, Paba P, et al. HPV-PathogenISS Study Group: Down-regulation of E-cadherin is closely associated with progression of cervical intraepithelial neoplasia (CIN), but not with high-risk human papillomavisrus (HPV) or disease outcome in cervical cancer. Eur J Gynaecol Oncol. 2006;27:215–223. [PubMed] [Google Scholar]
- 49.Strathdee G. Epigenetic versus genetic alterations in the inactivation of E-cadherin. Semin Cancer Biol. 2002;12:373–379. doi: 10.1016/S1044-579X(02)00057-3. [DOI] [PubMed] [Google Scholar]
- 50.Thomas M, Pim D, Banks L. The role of the E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene. 1999;18:7690–7700. doi: 10.1038/sj.onc.1202953. [DOI] [PubMed] [Google Scholar]
- 51.Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153:333–339. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu YN, Lee WW, Wang CY, Chao TH, Chen Y, Chen JH. Regulatory mechanisms controlling human E-cadherin gene expression. Oncogene. 2005;24:8277–8290. doi: 10.1038/sj.onc.1208991. [DOI] [PubMed] [Google Scholar]
- 53.Kwon O, Jeong SJ, Kim SO, He L, Lee HG, Jang KL, Osada H, Jung M, Kim BY, Ahn JS. Modulation of E-cadherin expression by K-Ras; involvement of DNA methyltransferase-3b. Carcinogenesis. 2010;31:1194–1201. doi: 10.1093/carcin/bgq071. [DOI] [PubMed] [Google Scholar]
- 54.Syrjänen K, Kataja V, Yliskoski M, Chang F, Syrjänen S, Saarikoski S. Natural history of cervical human papillomavirus lesions does not substantiate the biologic relevance of the Bethesda system. Obstet Gynecol. 1992;79:675–682. [PubMed] [Google Scholar]